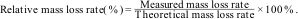Ultra-high temperature ceramic materials possess irreplaceable value in extreme environments, among which SiC–ZrC multiphase ceramics have emerged as a research focus due to their exceptional high-temperature performance. The synthesis of high-quality composite powders is pivotal for the fabrication of high-performance ceramics. To address the limitations of conventional carbothermal reduction methods—including impurity contamination from organic carbon sources, inadequate reaction stability, and the paucity of research on the oxidation behavior and biphase synergistic mechanisms of SiC–ZrC composite powders—this study developed a novel controlled carbothermal reduction process using high-purity graphite as the carbon source (argon atmosphere, 1400–1600°C). Systematic investigations were conducted to elucidate the regulatory effects of calcination temperature on the phase composition, microstructure, and elemental distribution of the powders. Additionally, high-temperature oxidation experiments under air atmosphere were performed to reveal the oxidation behavior characteristics and biphase synergistic mechanisms of the powders. The results demonstrate that high-purity SiC–ZrC composite powders with uniformly distributed elements can be successfully synthesized at 1600°C with a 1.5-h holding time. During high-temperature oxidation, SiC exhibits significantly superior oxidation resistance compared to ZrC:ZrC initiates oxidation to form ZrO2 as early as 800°C, while SiC retains excellent structural stability even at 1500°C. The two phases achieve synergistic oxidation enhancement through the formation of SiO2–ZrO2 composite oxide layers and ZrSiO4 phases.
Los materiales cerámicos de ultraalta temperatura presentan un valor irreemplazable en entornos extremos, entre los cuales las cerámicas multifásicas SiC-ZrC se han consolidado como foco de investigación gracias a su excepcional comportamiento a altas temperaturas. La síntesis de polvos compuestos de alta calidad es un factor crucial para la fabricación de cerámicas de alto rendimiento. A fin de abordar las limitaciones inherentes a los métodos convencionales de reducción carbotérmica —como la contaminación por impurezas derivadas de fuentes de carbono orgánico, la estabilidad insuficiente del proceso reactivo y la escasez de estudios sobre el comportamiento oxidativo y los mecanismos de sinergia bifásica de los polvos compuestos SiC-ZrC—, este trabajo ha desarrollado un novedoso proceso de reducción carbotérmica controlada basado en grafito de alta pureza como fuente de carbono (atmósfera de argón, 1400–1600°C). Se realizaron investigaciones sistemáticas para dilucidar los efectos reguladores de la temperatura de calcinación sobre la composición de fases, la microestructura y la distribución elemental de los polvos. Asimismo, se llevaron a cabo experimentos de oxidación a alta temperatura en atmósfera de aire para revelar las características del comportamiento oxidativo y los mecanismos de sinergia bifásica de los polvos estudiados. Los resultados demuestran que es posible sintetizar con éxito polvos compuestos SiC-ZrC de alta pureza con una distribución uniforme de elementos a 1600°C con un tiempo de mantenimiento de 1,5 horas. Durante la oxidación a alta temperatura, el SiC exhibe una resistencia a la oxidación significativamente superior a la del ZrC: mientras que el ZrC inicia su oxidación para formar ZrO2 ya a 800°C, el SiC mantiene una excelente estabilidad estructural incluso a 1500°C. Ambas fases logran una mejora sinérgica en la resistencia a la oxidación mediante la formación de capas de óxido compuesto SiO2-ZrO2 y fases de ZrSiO4.
Ultra-high-temperature ceramic (UHTC) materials exhibit exceptional properties, such as high melting points, exceptional hardness, strong chemical stability and excellent thermal shock resistance, while maintaining robust mechanical performance and oxidation resistance at elevated temperatures [1–3]. These materials are widely utilized across diverse fields, including metallurgy, electronics, energy, and aerospace, and in applications such as cutting tools. For example, they are used as linings for metallurgical furnaces and crucibles, where their resistance to corrosion and erosion from molten metals extends equipment lifespan. In electronics, UHTCs function as heat-dissipating components for high-power devices, leveraging their high thermal conductivity and temperature resistance to efficiently manage operational heat. In cutting tool applications, they facilitate the fabrication of durable tools that retain cutting efficiency and longevity under high-speed or dry machining conditions due to their exceptional hardness and thermal stability. Additionally, in the aerospace field, UHTCs are employed in thermal protection systems for spacecraft and engine nozzles [4]. These distinctive qualities render UHTC materials indispensable for applications operating in extreme environments [5–7].
SiC–ZrC composite ceramics are formed by combining two high-melting-point carbides: SiC (melting point 2700°C) and ZrC (melting point 3540°C). These ceramics combine the individual advantages of each component to form a synergistically reinforced system with high strength, high toughness, and exceptional high-temperature stability and mechanical properties [8–10]. Such composites meet the stringent requirements for high-performance materials under extreme operating conditions, positioning them as promising high-temperature structural materials with broad application prospects [11,12].
The key to preparing high-performance SiC–ZrC composite ceramics lies in the synthesis of high-quality SiC–ZrC ultrafine composite powders. These powders must exhibit uniform particle distribution, controlled morphology, small particle size, and high sintering activity. The primary methods for synthesizing ultrafine SiC–ZrC composite powders include self-propagating high-temperature synthesis (SHS), carbothermal reduction [13–16], sol–gel method [17–20], liquid precursor method [21,22] and chemical vapour deposition (CVD) [23]. The SHS method prepares SiC–ZrC composite powders through the exothermic reaction of Si–Zr–C mixed powders. However, this approach suffers from poor controllability of the combustion process, significant fluctuations in product purity, and difficulties in achieving scalable industrial production. The sol–gel method is limited by high raw material costs, long processing cycles, complex procedures, low production efficiency and scalability issues for industrial applications. CVD, while effective, requires substantial equipment investment and exhibits low deposition efficiency. By contrast, the carbothermal reduction method is distinguished by its low-cost raw materials, simple synthesis process, controllable reaction conditions, cost-effectiveness and suitability for large-scale production, all of which contribute to its widespread adoption in industrial chemical synthesis. Wang et al. [13] synthesized ZrC/SiBCN aerogels by using zirconium butoxide-modified polyborosilazane as a precursor through solvothermal reactions, freeze-casting, freeze-drying and pyrolysis. Subsequent pyrolysis-induced stepwise carbothermal reduction between oxygen-containing phases and free carbon resulted in the in situ formation of core–shell structured ZrC@SiC nanocrystals within an amorphous SiBCN matrix. This process effectively suppressed the crystallization and growth of SiC crystals. Liu et al. [14] prepared SiC–ZrC composite powders through microwave-assisted carbothermal reduction, using zircon and activated carbon as raw materials. Their thermodynamic analysis and investigation of reaction temperature, additive dosage (e.g., B2O3) and powder embedding conditions revealed that elevated temperatures and B2O3 addition facilitated synthesis, whereas embedding reactants in SiC powder inhibited the process. Lei et al. [15] employed a sol–gel method combined with boron/carbothermal reduction to fabricate ZrB2–SiC–ZrC (ZSZ) composite powders with C/(B+Zr+Si) molar ratios of 1.5, 1.9 and 2.4. Systematic studies on heat treatment temperatures (1100–1600°C) and holding times (1–2.5h) demonstrated that under optimized conditions (C/(B+Zr+Si)=2.4, 1500°C, 2.5h), the precursors were fully converted into ZSZ powders through solid-state reactions. The resulting powders exhibited a typical core–shell structure composed of ZSZ particles as the core and a low-crystallinity ZrB2 phase forming the outer shell.
While substantial efforts have been devoted to exploring the oxidation resistance mechanisms of SiC–ZrC bulk materials and coatings, investigations into the oxidation behavior of SiC–ZrC composite powders remain critically underexplored. Owing to their high specific surface area, these powders exhibit fundamentally distinct oxidation thresholds compared to dense bulk counterparts: bulk materials demonstrate delayed oxidation due to hindered oxygen diffusion pathways, whereas powders may undergo non-equilibrium oxidation at lower temperatures (e.g., anomalous oxidation rates, unstable phase composition of oxidation products) resulting from heightened surface reactivity and enlarged oxygen-contact interfaces. Therefore, this study focuses on elucidating oxidation behavior patterns in powdered states, with particular emphasis on clarifying synergistic mechanisms between phases. This work aims to address critical knowledge gaps in powder oxidation theory and provide foundational support for understanding oxidation characteristics in multiphase ceramic precursors.
Capitalizing on the industrial promise of carbothermal reduction and persistent voids in powder oxidation research, this study pursues three primary objectives: (1) To develop a controlled carbothermal reduction process using high-purity graphite as carbon source (argon atmosphere/1400–1600°C), eliminating impurity introduction and reaction instability associated with organic carbon sources; (2) To correlate calcination temperature with quantitative phase composition, microstructural characteristics (e.g., particle morphology, agglomeration), and size distribution; (3) To decipher biphase synergistic oxidation mechanisms via high-temperature air oxidation experiments, quantifying synergistic enhancement effects in SiC–ZrC powders to enable oxidation-resistant design paradigms for high-performance multiphase ceramics.
Experimental procedureGraphite (C, ≥99%, Sinopharm Chemical Reagent Co., Ltd., China), zirconium dioxide (ZrO2, ≥98.5%, Sinopharm Chemical Reagent Co., Ltd., China), and silica sol (mSiO2·nH2O, industrial grade, 27% SiO2 content, Changsha Water Glass Factory, Hunan, China) were selected as raw materials. The stoichiometric ratio of SiC to ZrC in the final product was set at 7:3 (molar ratio). According to the predetermined formulation, graphite, silica sol, and ZrO2 were weighed sequentially and placed in a ball mill jar. Zirconia milling balls and an ethanol–water solution (a small amount of ethanol was added to disperse graphite effectively) were introduced. The mass ratio among raw materials, milling balls, and liquid phase was set to 1:2:2, the jar was mounted on a planetary ball mill and mixed at 400rpm for 4h. The resulting slurry was transferred to a 100°C electric blast drying oven. The dried precursor powder was further refined using an agate mortar, and a portion of the processed powder was loaded into a ceramic boat, which was placed in a high-temperature tubular furnace. In argon atmosphere, the temperatures were set at 1400°C, 1450°C, 1500°C, 1550°C, and 1600°C for 1.5h (heating rate: ∼10°C/min) to synthesize SiC–ZrC composite powders. After cooling to room temperature in the furnace, the powder synthesized at 1600°C was weighed and placed in a corundum crucible. Oxidation tests were conducted in a high-temperature box furnace in air atmosphere at 800°C, 1000°C, 1200°C, and 1500°C for 30min each to evaluate its high-temperature oxidation resistance.
The mass of the SiC–ZrC composite powders before and after high-temperature calcination was measured using an electronic analytical balance to calculate the mass loss rate during synthesis and assess the extent of the synthesis reaction. Phase composition analysis was performed on powders synthesized at different calcination temperatures under an argon atmosphere, as well as on oxidation-treated products, using a Y-2000A X-ray diffractometer. Microstructural characterization of the powders was conducted with a Zeiss Sigma 500 scanning electron microscope coupled with an energy-dispersive X-ray spectroscopy (EDX) system to analyze localized elemental composition and distribution, and further elucidate the material's chemical homogeneity.
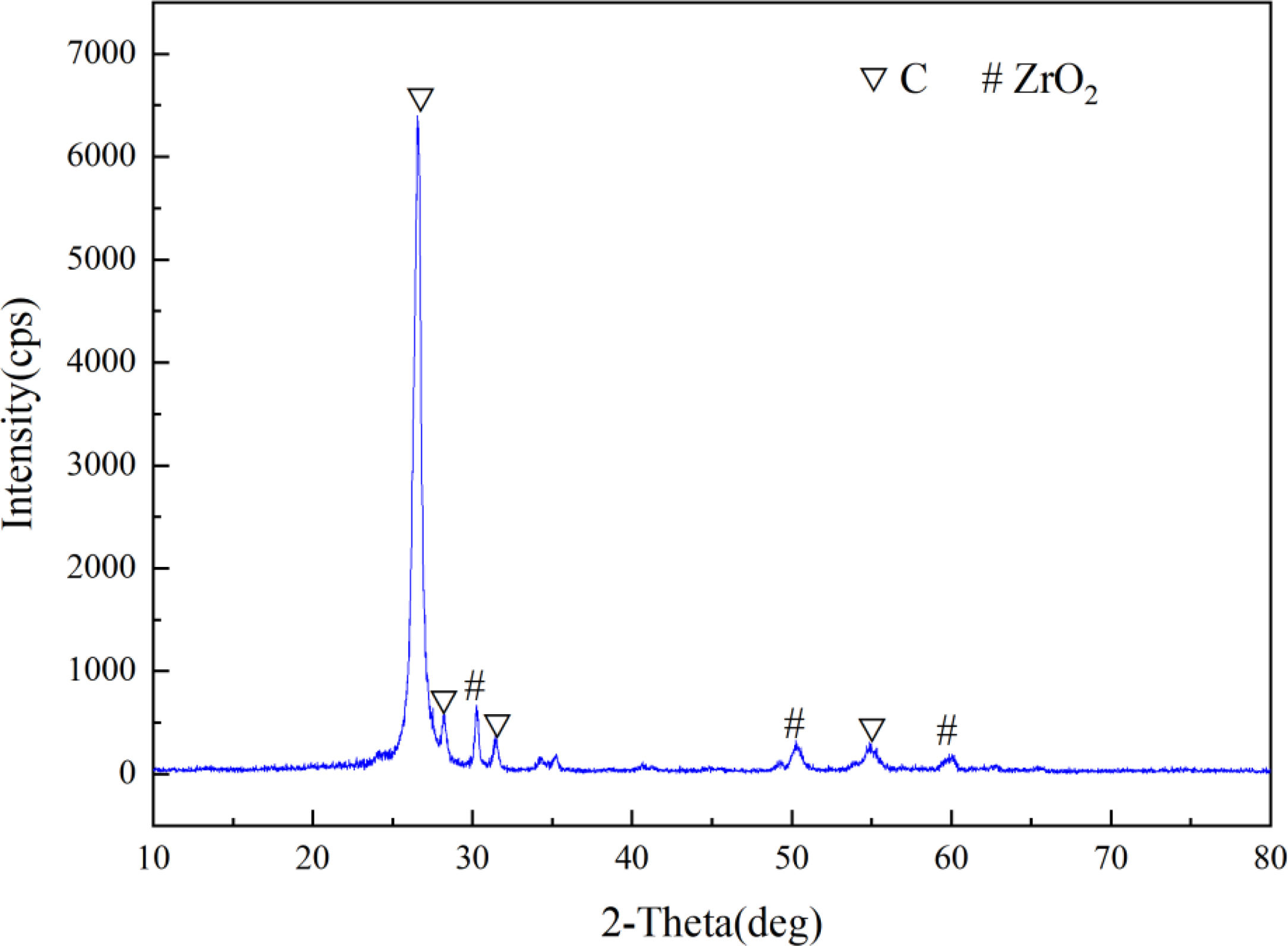
Results and discussionFig. 1 presents the X-ray diffraction (XRD) pattern of the dried precursor mixed powder in a Si/Zr molar ratio of 7:3. Only the characteristic diffraction peaks of C and ZrO2 were observed in the precursor, and no SiO2 peaks were observed. The absence of SiO2 diffraction signals is likely attributed to its amorphous state in the mixture under these conditions, which is consistent with the findings reported in [24].
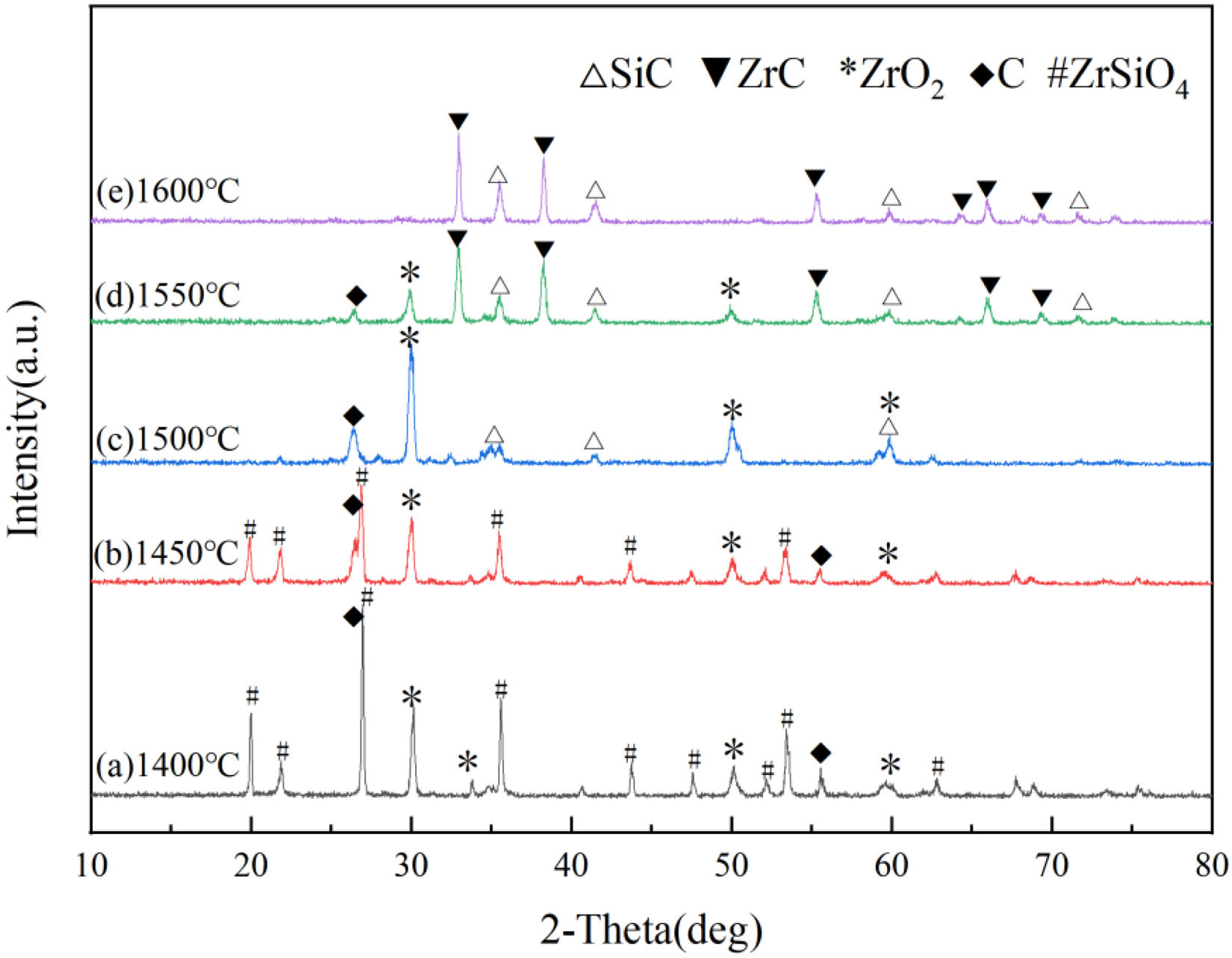
Fig. 2 presents the XRD patterns of powder samples synthesized under an argon atmosphere at calcination temperatures of 1400°C, 1450°C, 1500°C, 1550°C, and 1600°C with a holding time of 1.5h. Calcination temperature plays a decisive role in determining the phase composition of the synthesized powders. At 1400°C, the XRD pattern exhibits the characteristic peaks of ZrO2 and C and diffraction peaks corresponding to the intermediate product ZrSiO4. This result indicates that a solid-state reaction between ZrO2 and SiO2 occurred in the mixed powder under these conditions. As the temperature increases to 1450°C, the intensity of ZrSiO4 peaks diminishes considerably, suggesting the onset of its decomposition. Upon further heating to 1500°C, the ZrSiO4 peaks disappear completely, whereas weak SiC diffraction peaks emerge alongside residual ZrO2 and C peaks, which demonstrates the initiation of carbothermal reduction between SiO2 and C. At 1550°C, distinct SiC and ZrC peaks dominate the XRD pattern, though weak ZrO2 and C peaks persist, indicating an incomplete reaction. Finally, at 1600°C, only sharp SiC and ZrC diffraction peaks were observed, confirming a nearly complete carbothermal reduction and the successful synthesis of high-purity SiC–ZrC composite powders.
During the carbothermal reduction synthesis of SiC–ZrC composite powders, solid-state reactions between carbon (from graphite) and SiO2/ZrO2 generated gaseous products, such as CO, which escaped from the tubular furnace with the argon flow. The measured mass loss rate of the powder samples can be calculated using the formula:
where M1 and M2 represent the sample mass before and after calcination, respectively. The relative mass loss rate is then determined by:This parameter provides critical insights into the extent of reaction completion given that deviations from theoretical values reflect unreacted residues or incomplete gas evolution.
Table 1 summarizes the mass loss rate data during the synthesis of SiC–ZrC composite powders in an argon atmosphere at varying calcination temperatures. As shown in Table 1, the relative mass loss rate of the powder samples increases with calcination temperature. At 1400°C, the relative mass loss rate is 77%, which increases to 93% at 1500°C. Upon further heating to 1550°C, the rate exceeds the theoretical mass loss rate (100%), reaching 102%, and peaking at 108% at 1600°C. Combined with XRD analysis, these results confirm that the carbothermal reduction approaches completion when the temperature is maintained at 1600°C for 1.5h, enabling the synthesis of high-purity SiC–ZrC composite powders.
Mass loss rate during the synthesis of SiC–ZrC composite powders under an argon atmosphere at various calcination temperatures.
| No. | Calcination temperature (°C) | Holding time (h) | Measured mass loss rate (%) | Theoretical mass loss rate (%) | Relative mass loss rate (%) |
|---|---|---|---|---|---|
| 1# | 1400 | 1.5 | 37 | 49 | 77 |
| 2# | 1450 | 1.5 | 412 | 49 | 85 |
| 3# | 1500 | 1.5 | 45 | 49 | 93 |
| 4# | 1550 | 1.5 | 50 | 49 | 102 |
| 5# | 1600 | 1.5 | 53 | 49 | 108 |
Under synthesis conditions maintained at 1550°C and 1600°C for 1.5h, the relative mass loss rates of the powder samples (102% and 108%, respectively) exceeded the theoretical value (100%). This discrepancy primarily arises from the two-step reaction mechanism between C and SiO2 at elevated temperatures [25–27]: The gaseous SiO intermediate forms initially and is converted into SiC. During synthesis, some of the SiO escapes the system alongside CO through the argon flow, resulting in a gaseous product loss greater than the theoretically predicted loss.
Fig. 3 displays scanning electron microscopy (SEM) micrographs of the precursor mixed powder and the powder samples synthesized in an argon-protected atmosphere. Each was calcined at distinct temperatures with a 1.5h isothermal hold. As shown in Fig. 3a, the dried precursor exhibits diverse particle morphology, including quasi-spherical, irregularly shaped, and partially agglomerated particles. As for the sample calcined at 1450°C (Fig. 3b), the microstructure predominantly consists of irregular, flaky and rod-like particles with interparticle aggregation and minor quasi-spherical particles. As further confirmed by XRD analysis, these particles comprise residual ZrO2, C, and the intermediate product ZrSiO4. Additionally, fine flocculent structures (Fig. 3b) are hypothesized to consist primarily of C and ZrO2 phases derived from ZrSiO4 decomposition [28]. When the temperature increases to 1500°C (Fig. 3c), particle morphology shifts markedly to flaky and rod-like structures, and quasi-spherical particles are no longer detectable. At 1550°C (Fig. 3d), the microstructure diverges considerably. It is composed of sparse quasi-spherical and flaky particles, while abundant rod-like and whisker-shaped structures (diameters ∼100nm) emerge, forming entangled networks. In the 1600°C sample (Fig. 3e), whisker- and rod-like structures further proliferate. XRD analysis confirms these particles as SiC and ZrC crystals, indicating the progression of carbothermal reduction with increasing temperature, ultimately yielding the target SiC–ZrC composite powders.
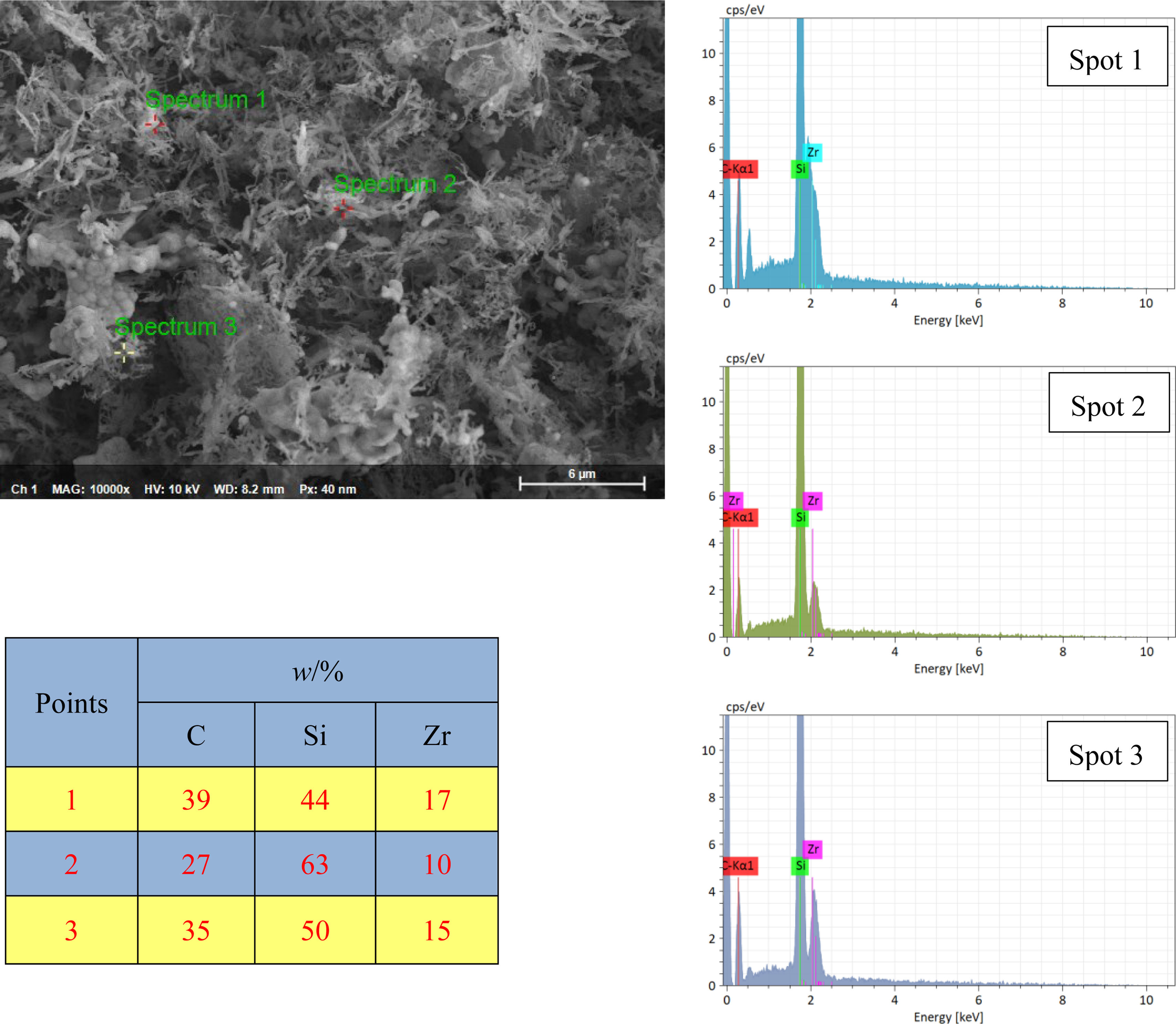
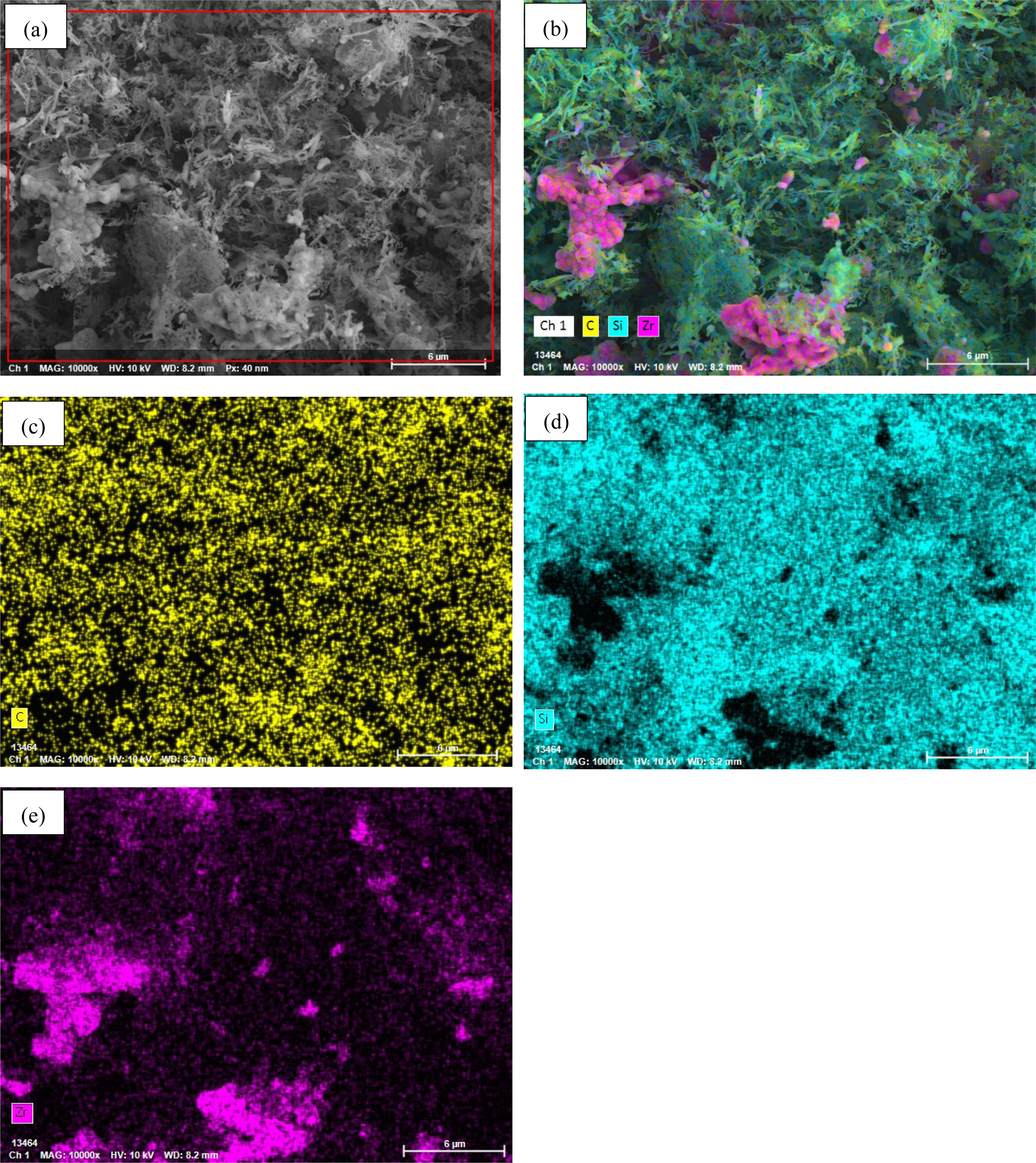
Figs. 4 and 5 present the EDX results of the powder synthesized at 1600°C for 1.5h. The powder is analyzed to accurately characterize its microstructural and chemical characteristics. Using point analysis mode (P-EDX) and elemental mapping mode (EDX mapping), quantitative elemental composition and spatial distribution patterns within the powder micro-regions were systematically investigated. The P-EDX spectra (Fig. 4) reveal the dominance of C, Si, and Zr. Their atomic ratios were precisely quantified. Meanwhile, the EDX mapping results (Fig. 5) visually demonstrate the homogeneous dispersion of C, Si, and Zr across the analyzed area, and no discernible elemental segregation was detected on the microscale. This uniform elemental distribution aligns with the phase composition identified through XRD analysis and the morphological features observed through SEM. Collectively, these multimodal characterization results confirm the successful synthesis of ultrafine SiC–ZrC composite powders under the specified experimental conditions.
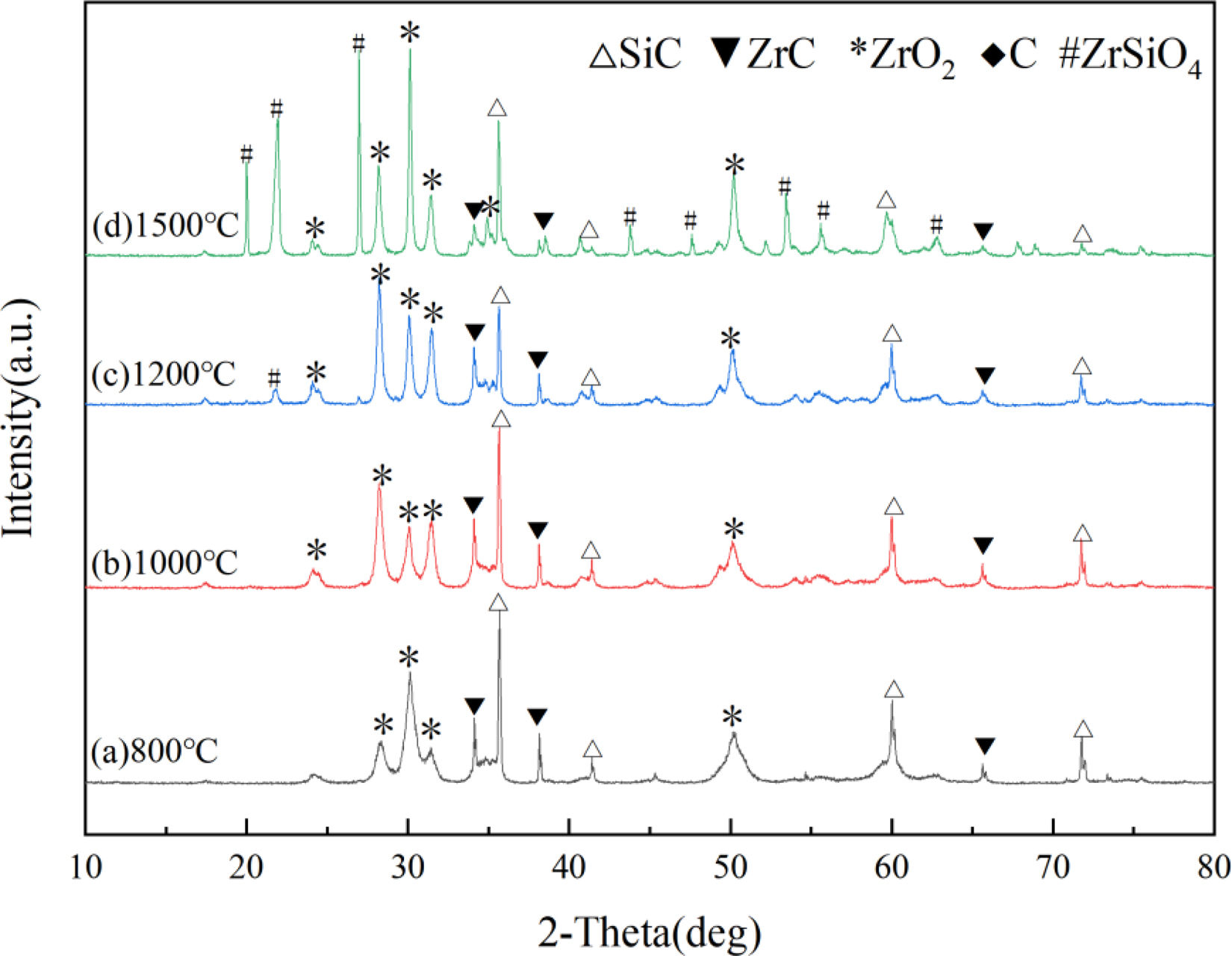
Fig. 6 displays the XRD patterns of SiC–ZrC composite powders after oxidation at 800°C, 1000°C, 1200°C, and 1500°C. As shown in Fig. 6, the sample oxidized at 800°C for 30min exhibits the characteristic diffraction peaks of SiC and ZrC, with additional distinct ZrO2 peaks, which indicate the partial oxidation of ZrC to ZrO2 at this temperature. When the oxidation temperature increases to 1000°C, the XRD pattern remains qualitatively similar, although the ZrO2 peak intensity shows slight enhancement, suggesting the progressive intensification of ZrC oxidation with increasing temperature. A phase evolution occurs at 1200°C. The diffraction intensities of SiC and ZrC markedly decrease, whereas the ZrO2 peaks intensify substantially. Notably, weak ZrSiO4 diffraction peaks emerge, implying the oxidation of trace SiC into SiO2, followed by solid-state reaction between SiO2 and ZrO2 to form ZrSiO4. At 1500°C, while SiC maintains strong diffraction intensity, ZrC peaks further diminish. Concurrently, ZrO2 and ZrSiO4 peaks exhibit continued enhancement. This progression of phase transformations demonstrates the superior oxidation resistance of SiC compared with ZrC. Even under extreme 1500°C oxidative conditions, SiC retains structural stability and oxidation resistance, while ZrC undergoes progressive degradation. Potential chemical reactions during air oxidation of the composite powders are summarized in Table 2.
Possible chemical reactions during the oxidation process of SiC–ZrC composite powder in an air atmosphere [29–32].
| No. | Reaction equation |
|---|---|
| (1) | ZrC(s)+2O2(g)=ZrO2(s)+CO2(g) |
| (2) | 2ZrC(s)+3O2(g)=2ZrO2(s)+2CO(g) |
| (3) | 2SiC(s)+3O2(g)=2SiO2(l)+2CO(g) |
| (4) | SiC(s)+2O2(g)=SiO2(l)+CO2(g) |
| (5) | 2CO(g)+O2(g)=2CO2(g) |
| (6) | SiO2(l)=SiO2(g) |
| (7) | ZrO2(s)+SiO2(s)=ZrSiO4(s) |
Fig. 7 presents the SEM characterization of SiC–ZrC composite powders after 30min of oxidation at varying temperatures. At 800°C (Fig. 7a), the oxidized microstructure predominantly consists of rod-like structures and whisker-like phases, and entangled particles exhibit intricate interlocking configurations. As oxidation temperature increases to 1000°C (Fig. 7b), a marked reduction in whisker content is observed, concurrent with the emergence of irregularly shaped particles that establish a diversified microstructural architecture. When the temperature reaches 1200°C (Fig. 7c), the whisker phase completely disappears and is replaced by a heterogeneous mixture of quasi-spherical, short rod-like, and irregularly faceted particles. As the temperature further increases to 1500°C (Fig. 7d), the powder exhibits pronounced sintering behavior characterized by substantial particle coarsening and intergranular bonding that generates agglomerated clusters.
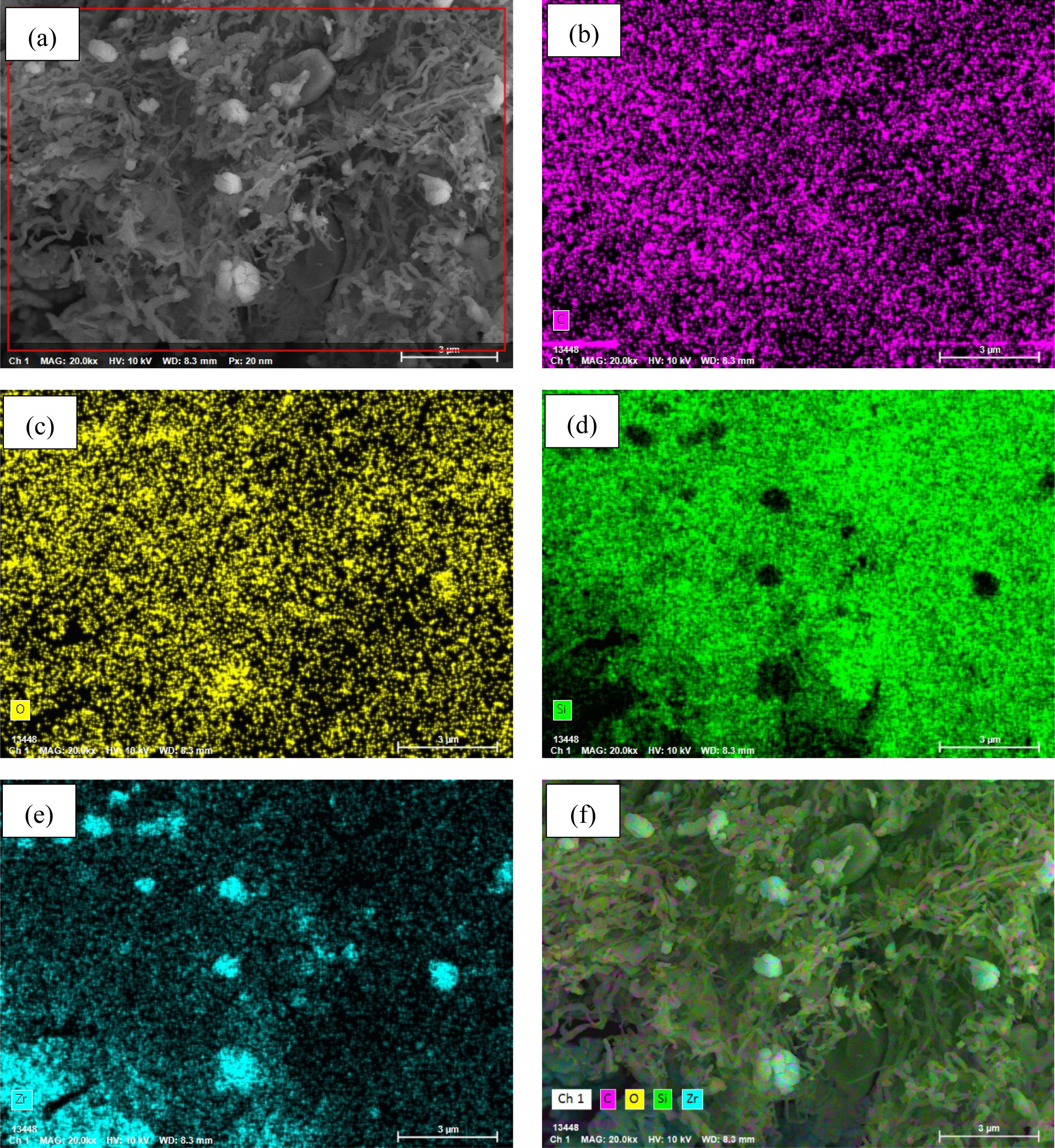
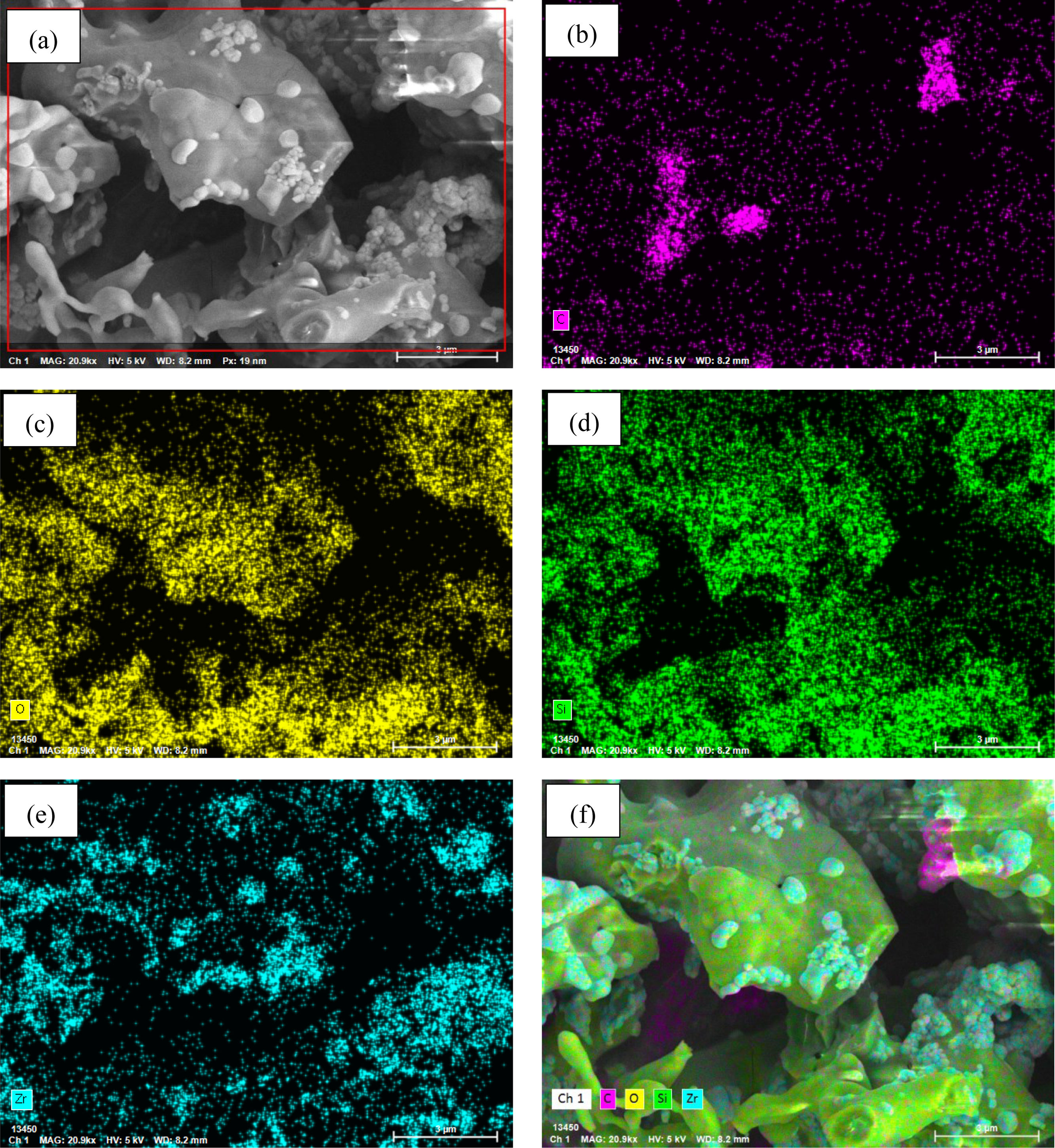
Figs. 8 and 9 display the elemental mapping micrographs of SiC–ZrC composite powders after 30min of oxidation at 1000°C and 1500°C. Both samples exhibit detectable concentrations of carbon (C), oxygen (O), silicon (Si), and zirconium (Zr), which confirm the partial oxidation of ZrC into ZrO2 at temperatures exceeding 1000°C. Comparative analysis of the C-distribution maps (Figs. 8b and 9b) reveals a marked reduction in carbon content within the 1500°C specimen. This observation indicates progressive oxidation behavior: At 1000°C, mild oxidation predominates, whereas intensified oxidation reactions are dominant at 1500°C. The EDX findings demonstrate analytical consistency with prior XRD phase identification and SEM morphological observations, all of which validate the temperature-dependent oxidation mechanisms in the composite system.
ConclusionIn this study, efficient synthesis of SiC–ZrC composite powders was successfully achieved by developing a controlled carbothermal reduction process based on high-purity graphite as the carbon source. The research clarified the decisive role of calcination temperature in powder synthesis: as the temperature increased from 1400°C to 1600°C, the intermediate product ZrSiO4 gradually decomposed, and the carbothermal reduction reaction progressively advanced. When the temperature reached 1600°C with a 1.5-h holding time, the reaction was nearly complete, yielding composite powders with high purity and uniform distribution of C, Si, and Zr elements. Analysis of relative mass loss rate further confirmed that the volatilization of gaseous SiO products at high temperatures is the key factor causing the mass loss to exceed the theoretical value, providing a quantitative basis for process optimization. Meanwhile, this study systematically revealed the high-temperature oxidation behavioral patterns of SiC–ZrC composite powders, filling the research gap in the differences in oxidation mechanisms between powders and bulk materials. Oxidation experiments showed that ZrC begins to oxidize to form ZrO2 at 800°C, while SiC maintains relatively high structural stability even at 1500°C. As the temperature increased from 800°C to 1500°C, the oxidation products gradually evolved from ZrO2 to ZrO2–ZrSiO4 composite phases. The composite oxide layer formed by the solid-state reaction between SiO2 and ZrO2 constitutes the core mechanism for achieving synergistic oxidation resistance.
FundingThis research was supported by the National Natural Science Foundation of China (Grant No. 52032011), the Natural Science Foundation of Hunan Province, China (Grant No. 2025JJ70346), and Scientific Research Project of the Education Department of Hunan Province, China (Grant No. 23C0387).
Conflict of interestNo potential conflict of interest was reported by the author(s).