The objective of the study was to identify clinical and demographic factors predictive of hospitalization in primary healthcare patients diagnosed with suspected COVID-19 at the beginning of the pandemic.
MethodsA retrospective cohort study design was used. Patients attended in Casanova primary healthcare centre (CAP) (Barcelona, Spain) for symptoms compatible with possible or confirmed SARS-CoV-2 infection between February 24 and May 30, 2020, were included. Data was collected through the electronic medical record and by telephone interview.
Results518 patients were included, of whom 283 (54.6%) were female. The median age was 50.2 years and 19.3% were aged ≥65 years: 79% were followed on an outpatient basis while the rest were hospitalized. Predictive factors for hospital admission were male sex, older age, a history of ischemic heart disease and the presence of dyspnoea, haemoptysis, nausea and vomiting, with a sensitivity of 48% and a specificity of 95.4%. Odynophagia and nasal congestion were predictors of a good prognosis. Mortality was 2.3% and 25% of deaths did not occur in hospital.
ConclusionsNearly 80% of primary healthcare patients received only outpatient care. Male sex, older age, a history of ischemic heart disease and symptoms like dyspnoea, haemoptysis, nausea and vomiting could lead to a greater risk of an unfavorable evolution during COVID-19. Patients with at least one of the above factors, which correlate with a higher hospital admission rate, should receive a closer follow-up to early detect when they can benefit from a hospital evaluation based on their clinical evolution.
El objetivo del estudio fue identificar factores clínicos y demográficos predictivos de hospitalización en pacientes de atención primaria con diagnóstico de sospecha de COVID-19 al inicio de la pandemia.
MétodosCohorte retrospectiva, con pacientes atendidos en el centro de atención primaria (CAP) de Casanova (Barcelona, España) por síntomas compatibles con infección por SARS-CoV-2, entre 24 de febrero y 30 de mayo de 2020. Los datos se recogieron de la historia clínica electrónica y mediante entrevista telefónica.
ResultadosSe incluyeron 518 pacientes. 283 (54.6%) fueron mujeres. La edad media fue 50.2 años. Un 19.3% tenían 65 años o más. El 79% se siguieron de forma ambulatoria y el resto ingresaron en el hospital. Los factores predictivos de ingreso fueron sexo masculino, edad, antecedentes de cardiopatía isquémica y la presencia de disnea, hemoptisis, náuseas o vómitos, con una sensibilidad del 48% y una especificidad del 95.4%. Odinofagia y congestión nasal fueron factores predictores de buen pronóstico. La mortalidad fue del 2.3%. El 25% de los pacientes que fallecieron no lo hicieron en el ámbito hospitalario.
ConclusionesCasi el 80% de los pacientes atendidos en Atención Primaria recibieron únicamente atención ambulatoria. Sexo masculino, edad avanzada, antecedente de cardiopatía isquémica y síntomas como disnea, hemoptisis, náuseas y vómitos, podrían conllevar un mayor riesgo de evolución desfavorable. Los pacientes con al menos uno de los factores anteriores, deben recibir un seguimiento más estrecho para detectar precozmente cuándo pueden beneficiarse de una evaluación hospitalaria en función de su evolución clínica.
The first case of COVID-19 (Coronavirus Disease 2019) was reported by the Chinese authorities in December 20191 and has spread at breakneck speed, with 103,597,957 cases reported in 192 countries at the time of writing.2 SARS-CoV-2, which causes the disease, is the seventh known coronavirus that infects human cells. The most common symptoms, according to a systematic review and meta-analysis,3 are fever (88.7%), cough (57.6%) and dyspnoea (45.6%), although studies show a range of incidence, and some report fatigue is also a common symptom4,5,6. However, as the infection has spread and research has grown, numerous clinical presentations have been associated, ranging from mild conditions (conjunctivitis,7 anosmia,8 skin manifestations9–12) to severe presentations (septic shock, pulmonary thromboembolism, acute respiratory distress syndrome, acute renal failure and myocardial involvement).13,14 Studies suggest that clinical deterioration, when it occurs, happens around the eighth day of disease,4 and is associated with a poor evolution and requires a thorough initial follow-up in all patients.
Clinical and epidemiological health strategies carried out since the onset of the pandemic differ substantially between countries. In Spain, primary healthcare had to adapt care to the circumstances imposed by the pandemic,15 starting from an initial scenario marked by the lack of diagnostic methods available to primary healthcare and by the problems of physicians' access to patients by prioritizing telephone care to the detriment of face-to-face visits. Studies16–22 have shown that analysis of the sociodemographic and clinical characteristics of patients with a presentation compatible with COVID-19 provides useful information for decision-making, making it essential to develop models predictive of an unfavorable evolution23–26 based on the differences between patients requiring hospitalization and those treated in primary healthcare.
This study was carried out in the first wave of the pandemic in order to combine information to identify primary healthcare patients with a clinical suspicion of COVID-19 who require referral for hospital assessment.
The objective of this study was to identify clinical and demographic factors predictive of hospitalization, comparing a cohort of patients treated by our primary healthcare centre diagnosed with suspected or confirmed COVID-19 who required hospital admission and those who did not.
MethodsStudy designA retrospective study was made of patients treated by CAP Casanova for possible or confirmed COVID-19 disease between 24 February 2020 and 30 May 2020. The suspected diagnóstico was made when patients presented symptoms of acute respiratory infection, such as fever, cough or dyspnoea, or other atypical symptoms including odynophagia, anosmia, ageusia, myalgia, diarrhea, chest pain or headache, among others, according to the case definition criteria determined by the Public Health Agency of Catalonia as of March 15, 2020.27 A confirmed diagnóstico was defined as positive PCR for SARS-CoV-2, detection of positive antibodies using enzyme-linked immunosorbent assay (ELISA) or both, at any time during the study period.
Inclusion criteriaAll Casanova basic health area patients aged ≥15 years with a confirmed diagnóstico of SARS-CoV-2 infection and all patients with suspected COVID-19 were included, so that outpatients with mild–moderate symptoms were also represented. Patient identification was based on official diagnostic codes of suspected or confirmed infection according to the International Classification of Diseases, 10th revision (ICD-10).28
Exclusion criteriaThere were no exclusion criteria. All patients who met the inclusion criteria were included, in order to obtain as representative a sample as possible.
Data collection and measurement of variablesPatients were recruited in June 2020. Data was initially collected through the medical record, which includes primary healthcare visits, emergency visits, hospital discharge records, and complementary tests. Subsequently, during July 2020, each patient meeting the inclusion criteria was phoned to obtain oral informed consent to participate in the study, and the data not recorded were completed.
The baseline and sociodemographic variables collected were age, sex, place of birth, body mass index, smoking, alcohol use and other drugs, and adjusted morbidity groups (AMG)29 scale, a clinical grouping method based on comorbidities and clinical risk to stratify patients from 1 to 4, where 1 is a low clinical risk and 4 a high risk. This method establishes the clinical risk of each patient based on the typology of their disease (acute, chronic or oncological). In the case of chronic disease, it identifies whether there is multimorbidity. The morbidity groups generated are: healthy population, pregnancy or childbirth, acute pathology, chronic disease in one system, chronic disease in two or three systems, chronic disease in ≥4 or more systems, and neoplasia. Each morbidity group is then divided into 5 levels of complexity based on the care needs of the users.
Other variables included were occupation (essential workers/non-essential workers during the study period); family support (cohabiting relatives/no cohabiting relatives/no relatives); and social support (formal support/no formal support). All diagnoses of chronic conditions and pharmacological treatments recorded in the medical record were collected, as were all signs and symptoms related to SARS-CoV-2 infection.
Variables collected related to possible transmission pathways were exposure to suspected or confirmed cases of COVID-19 (yes/no) and, if yes, whether contact occurred in the family or at work.
Statistical analysisIn the descriptive analysis, categorical variables were described using absolute and relative frequencies, expressed in percentages, and quantitative variables as means and standard deviation (SD). A bivariate analysis was carried out to study the relationship between hospital admission and sociodemographic, comorbidity and clinical variables. The chi-square test was used for categorical variables and the Student's t-test for quantitative variables with a normal distribution.
A logistic regression model was constructed to predict hospital admission. Sociodemographic characteristics, symptoms, medical history, and chronic drug treatments associated with hospitalization in the bivariate analysis (with a p < 0.05) were included as possible explanatory variables in the model. The best model was selected using the stepwise method with the Akaike information criterion. The statistical results obtained were expressed by calculating the odds ratio (OR) and the 95% confidence intervals (95%CI). The database was randomly split into two equally sized sets: training and testing. The first set was used to create the model and the second set to validate it. To study the predictive capacity of the model on the test dataset, indicators such as reliability, sensitivity, specificity, positive predictive value, and negative predictive value were calculated. A p value <0.05 was considered statistically significant. The R statistical package was used for the analysis.
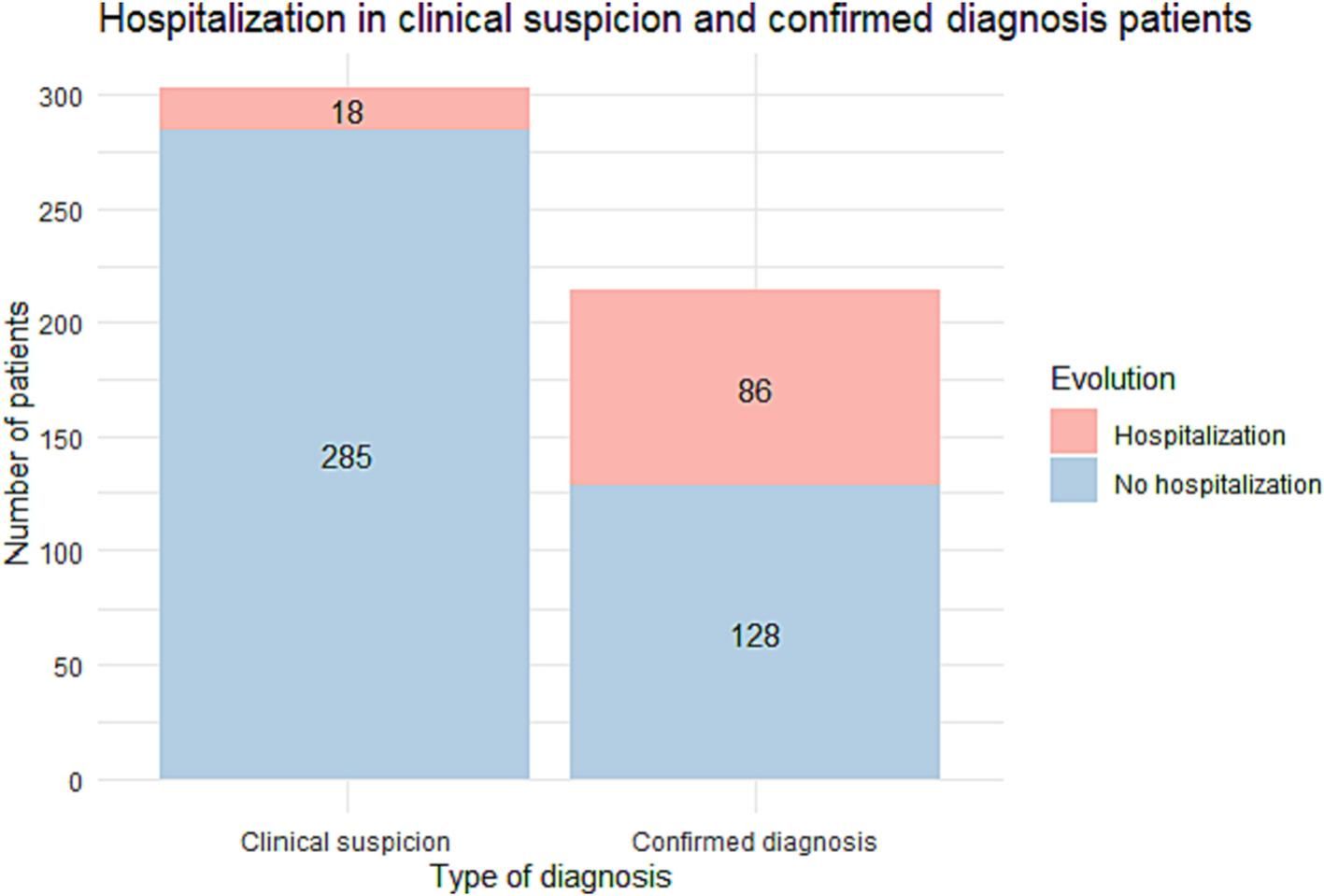
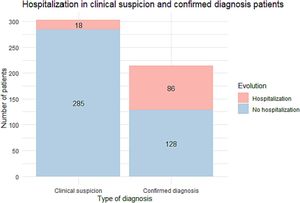
ResultsWe included 518 patients: a diagnóstico was confirmed by PCR or serology in 215 (41.5%) while 303 (58.5%) were classified as clinical suspicion of COVID-19 without diagnostic confirmation. Most patients with clinical suspicion were followed on an outpatient basis whereas the percentage of hospitalized patients was high in those with a confirmed diagnóstico (Fig. 1).
The characteristics of the population are displayed in Table 1. Patients who required hospital admission were most often male, aged 65–80 years, and obese or overweight, with an AMG of 4, who did not work face-to-face during the study period. No differences were found in terms of country of origin, smoking, alcohol use or drug use (Table 1). Twelve (2.3%) patients died, including three outpatients and 8 hospitalized patients.
Patient characteristics in total population and according to evolution (primary healthcare follow up or hospitalization).
| Total Population | Evolution | |||
|---|---|---|---|---|
| Variables | n = 518 | Primary care follow-up (n = 413) | Hospitalization (n = 104) | p valuea |
| Age in years - Mean ± SD | 50.2 ± 16.8 | 46.9 ± 15.5 | 63 ± 15.6 | <0.001* |
| Age in years - n (%) | <0.001* | |||
| 15–30 | 68 (13.1) | 65 (15.7) | 3 (2.9) | |
| 31–49 | 207 (40) | 184 (44.6) | 23 (22.1) | |
| 50–64 | 143 (27.6) | 119 (28.8) | 24 (23.1) | |
| 65–80 | 69 (13.3) | 30 (7.3) | 38 (36.5) | |
| >80 | 31 (6) | 15 (3.6) | 16 (15.4) | |
| Sex - n (%) | 0.013* | |||
| Male | 235 (45.4) | 176 (42.6) | 59 (56.7) | |
| Female | 283 (54.6) | 237 (57.4) | 45 (43.3) | |
| BMI - Mean ± SD | 25.7 ± 5.2 | 25.2 ± 5 | 27.8 ± 5.2 | <0.001* |
| BMI - n (%) | <0.001* | |||
| <18.5 | 17/418 (4.1) | 14/327 (4.3) | 3/90 (3.3) | |
| 18.5–24.9 | 175/418 (41.9) | 155/327 (47.4) | 20/90 (22.2) | |
| 25–29.9 | 149/418 (35.6) | 110/327 (33.6) | 38/90 (42.2) | |
| 30–34.9 | 49/418 (11.7) | 28/327 (8.6) | 21/90 (23.3) | |
| 35–39.9 | 17/418 (4.1) | 12/327 (3.7) | 5/90 (5.6) | |
| > = 40 | 11/418 (2.6) | 8/327 (2.4) | 3/90 (3.3) | |
| Smoking - n (%) | 0.074 | |||
| Ex-smoker | 101/462 (21.9) | 72/364 (19.8) | 29/97 (29.9) | |
| Smoker | 71/462 (15.4) | 60/364 (16.5) | 11/97 (11.3) | |
| Nonsmoker | 290/462 (62.8) | 232/364 (63.7) | 57/97 (58.8) | |
| Alcohol consumption - n (%) | 0.970 | |||
| Low risk consumption | 313/461 (67.9) | 244/360 (67.8) | 69/100 (69.0) | |
| High risk consumption | 9/461 (2) | 7/360 (1.9) | 2/100 (2.0) | |
| No consumption | 139/461 (30.2) | 109/360 (30.3) | 29/100 (29.0) | |
| Drug abuse - n (%) | 7/394 (1.8) | 7/313 (2.2) | 0/80 (0.0) | 0.381 |
| Adjusted morbidity groups (AMG)26 | <0.001* | |||
| 1 | 186 (35.9) | 170 (41.2) | 16 (15.4) | |
| 2 | 179 (34.6) | 146 (35.5) | 33 (31.7) | |
| 3 | 108 (20.8) | 74 (17.9) | 33 (31.7) | |
| 4 | 45 (8.7) | 23 (5.6) | 22 (21.2) | |
| Occupation - n (%) | 0.002* | |||
| No exposure | 314/484 (64.9) | 236/382 (61.8) | 77/98 (78.6) | |
| Healthcare worker or essential | 170/484 (35.1) | 149/382 (38.2) | 21/98 (21.4) | |
| Death - n (%) | 12 (2.3) | 3 (0.7) | 8 (7.7) | <0.001* |
Hospitalization was significantly more frequent in patients with fever, chills, general discomfort, haemoptysis, dizziness, dyspnoea, chest pain and vomiting or nausea (Table 2). However, fewer patients with odynophagia and nasal congestion were hospitalized compared with those without. Hospitalization was non-significantly higher in patients with coughing and diarrhea than in those without.
Hospitalized patients among patients presenting and not presenting several symptoms.
| Symptoms | Hospitalization in patients presenting the symptom n/N (%) | Hospitalization in patients not presenting the symptom n/N (%) | p valuea |
|---|---|---|---|
| Fever | 92/387 (23.8) | 10/124 (8.1) | <0.001* |
| Chills | 30/120 (25.0) | 56/355 (15.8) | 0.033* |
| Cough | 79/366 (21.6) | 20/142 (14.1) | 0.073 |
| Headache | 39/247 (15.8) | 48/236 (20.3) | 0.237 |
| Fatigue | 67/326 (20.6) | 27/171 (15.8) | 0.243 |
| General discomfort | 82/350 (23.4) | 16/150 (10.7) | 0.002* |
| Sore throat | 16/152 (10.5) | 73/336 (21.7) | 0.005* |
| Muscle and joint pain | 55/260 (21.2) | 36/229 (15.7) | 0.154 |
| Expectoration | 22/108 (20.4) | 68/378 (18) | 0.674 |
| Nasal congestion | 13/114 (11.4) | 75/370 (20.3) | 0.045* |
| Hemoptysis | 6/14 (42.9) | 85/475 (17.9) | 0.044* |
| Conjunctivitis | 6/30 (20.0) | 84/456 (18.4) | 1.000 |
| Dizziness | 19/68 (27.9) | 67/412 (16.3) | 0.031* |
| Dyspnea | 77/195 (39.5) | 21/306 (6.9) | <0.001* |
| Chest pain | 28/108 (25.9) | 60/380 (15.8) | 0.023* |
| Anosmia/Dysgeusia | 32/182 (17.6) | 54/301 (17.9) | 1.000 |
| Diarrhea | 43/182 (23.6) | 50/307 (16.3) | 0.060 |
| Sickness/Vomiting | 24/81 (29.6) | 64/402 (15.9) | 0.006* |
| Petechiae | 3/9 (33.3) | 84/473 (17.8) | 0.444 |
| Urticaria | 5/21 (23.8) | 84/463 (18.1) | 0.713 |
| Acrocyanosis | 2/3 (66.7) | 87/481(18.1) | 0.156 |
| Other skin lesions | 13/29 (44.8) | 75/453 (16.6) | <0.001* |
| Paresthesia/Hemiparesis | 4/17 (23.5) | 85/468 (18.2) | 0.808 |
| Pharyngitis | 1/8 (12.5) | 81/214 (37.9) | 0.278 |
| Tonsilitis | 0/2 (0) | 82/220 (37.3) | 0.725 |
| Tachycardia | 14/25 (56) | 68/197 (34.5) | 0.061 |
| Tachypnea | 25/29 (86.2) | 57/193 (29.5) | <0.001* |
| Anormal respiratory auscultation | 66/97 (68.0) | 16/125 (12.8) | <0.001* |
With respect to comorbidities, hospitalization was significantly higher in patients with COPD, diabetes, hypertension, ischemic heart disease, cerebrovascular disease, chronic kidney disease and obesity, among others (Table 3).
Hospitalization in patients presenting or not various medical conditions.
| Medical condition | Hospitalization in patients presenting the medical condition - n/N (%) | Hospitalization in patients not presenting the medical condition - n/N (%) | p valuea |
|---|---|---|---|
| Any condition | 80/316 (25.3) | 24/201 (11.9) | <0.001* |
| COPD | 9/14 (64.3) | 95/503 (18.9) | <0.001* |
| Diabetes mellitus | 20/48 (41.7) | 84/469 (17.9) | <0.001* |
| Hypertension | 45/99 (45.5) | 59/418 (14.1) | <0.001* |
| Ischemic heart disease | 13/20 (65.0) | 91/497 (18.3) | <0.001* |
| Cerebrovascular disease | 8/13 (61.5) | 96/504 (19.0) | 0.001* |
| Viral hepatitis | 3/8 (37.5) | 101/509 (19.8) | 0.429 |
| Cancer | 16/44 (36.4) | 88/473 (18.6) | 0.009* |
| Chronic kidney disease | 10/19 (52.6) | 94/498 (18.9) | 0.001* |
| Chronic pulmonary embolism | 2/4 (50.0) | 102/513 (19.9) | 0.384 |
| Obesity | 32/92 (34.8) | 72/425 (16.9) | <0.001* |
| Chronic liver disease | 5/9 (55.6) | 99/508 (19.5) | 0.024* |
| Asthma | 4/33 (12.1) | 100/484 (20.7) | 0.337 |
| Atrial fibrillation | 5/8 (62.5) | 99/509 (19.4) | 0.010* |
| Autoimmune disease | 4/18 (22.2) | 100/499 (20) | 1.000 |
| HIV | 1/11 (9.1) | 103/506 (20.4) | 0.588 |
| Other sexually transmitted infections | 0/3 (0.0) | 104/514 (20.2) | 0.881 |
| Pregnancy | 1/9 (11.1) | 103/508 (20.3) | 0.795 |
| Vitamin D deficiency | 4/23 (17.4) | 100/494 (20.2) | 0.946 |
| Psychiatric disorder | 1/13 (7.7) | 103/504 (20.4) | 0.435 |
| Depression or Anxiety | 11/38 (28.9) | 93/479 (19.4) | 0.230 |
| Other | 17/41 (41.5) | 87/476 (18.3) | 0.001* |
The bivariate analysis between hospitalization and medication showed a higher probability of hospitalization in patients receiving any type of treatment compared with those who were not (Table 4).
Hospitalization in patients receiving or not various medical treatments.
| Treatment | Hospitalization in patients receiving the medical treatment - n/N (%) | Hospitalization in patients not receiving the treatment - n/N (%) | p value |
|---|---|---|---|
| Any treatment | 64/233 (27.5) | 40/284 (14.1) | <0.001* |
| Angiotensin-converting enzyme inhibitors | 16/47 (34) | 88/470 (18.7) | 0.021* |
| Angiotensin-II receptor antagonists | 23/40 (57.5) | 81/477 (17) | <0.001* |
| Vitamin K antagonists | 3/6 (50) | 101/511 (19.8) | 0.185 |
| Oral antidiabetics | 12/22 (54.5) | 92/495 (18.6) | <0.001* |
| Novel anticoagulants | 2/4 (50) | 102/513 (19.9) | 0.384 |
| Heparin | 1/2 (50) | 103/515 (20) | 0.863 |
| Non-steroidal anti-inflammatory drugs | 2/8 (25) | 102/509 (20) | 1.000 |
| Antiplatelet agents | 15/32 (46.9) | 89/485 (18.4) | <0.001* |
| Antiretroviral therapy | 2/13 (15.4) | 102/504 (20.2) | 0.936 |
| Immunosuppressive therapy | 5/11 (45.5) | 99/506 (19.6) | 0.082 |
| Corticosteroids | 4/9 (44.4) | 100/508 (19.7) | 0.156 |
| Hydroxychloroquine | 0/1 (0) | 104/516 (20.2) | 1.000 |
| Influenza vaccine in the last year | 23/65 (35.4) | 81/452 (17.9) | 0.002* |
| Pneumococcal vaccine | 13/39 (33.3) | 91/478 (19) | 0.053 |
| BCG vaccine | 2/9 (22.2) | 102/508 (20.1) | 1.000 |
| Inhaled corticosteroids | 8/27 (29.6) | 96/490 (19.6) | 0.308 |
| Other | 26/99 (26.3) | 78/418 (18.7) | 0.119 |
27% of patients consulted primary healthcare services on the day of symptom onset, 43.3% consulted between the second and fifth days and 29.7% later.
In the logistic regression model, the independent predictive variables for hospitalization were male sex, age, a history of ischemic heart disease and the presence of dyspnoea, haemoptysis and nausea or vomiting. The coefficients obtained in the predictive model and the OR and 95% CI are shown in Table 5.
Variables predicting hospitalization selected using the Aikake Information Criterion.
| Variable | Coefficient | OR | 95% CI of OR | p value |
|---|---|---|---|---|
| Constant | −7.807 | <0.001* | ||
| Male | 1.358 | 3.89 | (1.19–14.83) | 0.034* |
| Age | 0.074 | 1.08 | (1.03–1.13) | 0.001* |
| Hemoptysis | 2.094 | 8.12 | (0.76–72.36) | 0.062 |
| Dyspnea | 1.748 | 5.74 | (1.85–20.23) | 0.003* |
| Sickness/Vomiting | 1.369 | 3.93 | (0.97–16.21) | 0.052 |
| Ischemic heart disease | 2.452 | 11.61 | (1.42–253.2) | 0.045* |
The reliability of the predictive model was 86.4%. The negative predictive value was 88.7%, and the positive predictive value was 71%. The sensitivity of the model was 48% and the specificity was 95.4%.
DiscussionIn our cohort of primary healthcare patients with a presentation compatible with COVID-19, the risk factors for hospitalization were male sex, older age, a history of ischemic heart disease and dyspnoea, haemoptysis, nausea or vomiting. During the study period, no COVID-19 diagnostic tools were available in primary healthcare. Due to this, at first the diagnóstico was only confirmed in patients hospitalized at some point during the infection and in essential healthcare workers. Subsequently, thanks to increased accessibility to diagnostic techniques and the development of serologies for the detection of antibodies to SARS-CoV-2, the diagnóstico could be confirmed in more patients. This study aimed to evaluate the ability of primary healthcare to diagnose and manage these patients.
Regarding comorbidities, several studies23,30–35 have found cardiovascular risk factors such as diabetes, hypertension, obesity or cardiovascular disease as the most common comorbidities found in patients diagnosed with COVID-19, as well as renal disease or chronic pulmonary disease. Similarly, our study found that diabetes, high blood pressure, COPD, ischemic heart disease and cerebrovascular disease were added risk factors for COVID-19 hospitalization, as were obesity and chronic kidney disease.
With respect to chronic medication, the bivariate analysis showed that patients receiving ACE inhibitors, ARA II or oral antidiabetics were more frequently hospitalized than those who were not, although the differences disappeared in the multivariate analysis. This suggests that these drugs are not a risk factor in themselves but are associated with diseases such as high blood pressure, ischemic heart disease and diabetes for which they are used. Our study, like others,36–38 helps refute the statements made at the beginning of the pandemic that patients receiving ACE inhibitors had a risk of an adverse evolution due to the mechanism of action of the drug and the pathogenesis of the virus.39,40
With respect to chronic corticosteroid treatment, paradoxically, patients who were receiving them, and who had severe comorbidities that could result in an unfavorable evolution, were not significantly more often hospitalized than those who were not. This may be because, contrary to what was initially thought, patients with active SARS-CoV-2 infection may benefit from corticosteroid therapy by potentially mitigating the inflammatory cascade developed by patients with severe disease, as suggested in some studies.41–44
With regard to the clinical manifestations of Covid-19, different studies3–6,30,45 have suggested dyspnoea, cough or fever as the most prevalent symptoms, as well as myalgia or fatigue, among others. In our study, fever, chills, general discomfort, haemoptysis, and dyspnoea resulted in an increased risk of hospitalization compared with patients without these symptoms. However, we also identified nausea and vomiting as risk factors for hospitalization, unlike other studies which did not.3,4,46 On the other hand, and contrary to what is suggested in reports,3–6,30,45 cough was not a risk factor for hospitalization, while nasal congestion and odynophagia seemed to have a slight protective effect against hospitalization.
The mortality rate was 2.3%, including 7.7% of hospitalized patients and 0.7% of outpatients. The outpatients who died were at high clinical risk due to age and comorbidities. This highlights the value of primary healthcare in the adequacy of therapeutic measures and in ensuring the comfort and home care of patients who require it.
The logistic regression model had a sensitivity of 48%, and a specificity of 95.4%. This suggests that this tool helps identify patients who should be referred for hospital care. However, some patients with a negative test also might benefit from hospital care. In this group, the decision on referral should be based on the clinical criteria of the attending family physician. The formula would gain validity if used in another cohort of primary healthcare patients to predict the probability of hospitalization.
This study analyzed a sample of patients attended in primary care, presenting a wide range of clinical manifestations of COVID-19, and not only severe cases, unlike most published studies, which describe hospitalized patients. Also, because it was conducted in the first wave of the pandemic it provides a description of the care provided by Spanish family doctors with very limited access to diagnostic tests for COVID-19. Furthermore, the model developed makes it possible to identify some risk factors that are worth taking into account to guide clinical decisions in the care of patients with COVID-19. We conducted a review in which we talked with every patient included and did not rely only on the medical record.
However, the study has some limitations. As it is a retrospective cohort based on electronic medical records, there is a recording bias, which we tried to minimize by conducting a telephone interview with all patients during the month after patient recruitment was concluded. Despite this limited period there may also have been minimal memory bias. In addition, the sample is of limited size as it is restricted to patients in a single health centre as we prioritized immediacy in conducting the study given the pandemic situation. In addition, it should be taken into account that due to the exceptional situation, many patients with admission criteria remained at home instead. Also, the evolution of the severity in some cases could be influenced by external factors such as the lack of access to hospital resources due to the collapse of the health system, as well as the difficulty in maintaining the isolation or overcrowding of patients in residences.
In the first pandemic wave, 20% of patients treated for COVID-19 in primary healthcare required hospital admission, most during the first seven days after symptom onset. As determined by the logistic regression model, male sex, older age, a history of ischemic heart disease, and dyspnoea, haemoptysis, nausea or vomiting may be factors for a higher risk of an unfavorable evolution during COVID-19, and these patients should be evaluated carefully. Patients with at least one of the above factors, which correlate with a higher hospital admission rate, should receive a closer follow-up in order to early detect when they may benefit from a hospital evaluation based on their clinical evolution.
Ethical considerationsThe study was conducted in accordance with Spanish legislation (Biomedical Research Act, 14/2007, 3 July) and international norms (Helsinki Declaration and Nuremberg Code), guaranteeing the confidentiality of patient data in accordance with Organic Law on the Protection of Personal Data (3/2018, 5 December) and Law 41/2002, of 14 November, regulating patient autonomy and rights and obligations in matters of information and clinical documentation. The study was approved by the research bioethics committee of the Hospital Clínic de Barcelona (Reg. HCB/2020/0616).
FundingThis study received a research grant from the Commission de Recerca ad hoc COVID-19 of the Hospital Clínic de Barcelona and the Fundació Clínic per a la Recerca Biomèdica.