The main objective is to transfer to clinical practice a new smoking cessation application (“Vive sin Tabaco” a) in all health centers of the public Basque Health Service.
DesignAn implementation study of a smoking cessation program previously validated. After implementation, a retrospective study has been carried out to evaluate its use under normal conditions.
SiteThe process of transfer to clinical practice has been held in several phases; first a pilotage in four health centers of Alava and subsequently, when all reported incidents were resolved, it was extended to all health centers of the Basque Health Service.
Intervention and main measurementDevelopment of “Vive sin Tabaco”; a corporate tool for smoking cessation, and its transfer to clinical practice. All interested health care workers received training on how to use the application. User manuals for both patients and professionals were developed. Smoking cessation rates at 12 months during implementation were also collected.
ResultsThe percentage of patients of post pilot phase who quit smoking at 12 months was 14.1%.
ConclusionsThe conception of “Vive sin tabaco” as a corporate tool for smoking cessation, available in all health centers of Basque Health Service, has been long and arduous, and has required the participation of health professionals and patients as end-users in order to obtain a tool that adapts to their expectations and guarantees greater usability and satisfaction. This application is being effective as an adjuvant tool to health advice.
El objetivo principal es transferir a la práctica clínica una herramienta corporativa para deshabituación tabáquica («Vive sin Tabaco») en la red sanitaria pública del País Vasco.
DiseñoEstudio de implementación de un programa de deshabituación tabáquica previamente validado. Posteriormente se llevó a cabo un estudio retrospectivo para evaluar su efectividad en condiciones de práctica clínica.
EmplazamientoLa transferencia a la práctica clínica se ha realizado en varias fases; primero se realizó un pilotaje en 4 centros de salud de Álava y, posteriormente, tras resolver todas las incidencias notificadas, se extendió al resto de centros de salud de la red sanitaria pública vasca.
Intervención y principales medidasDesarrollo de una aplicación móvil corporativa para dejar de fumar «Vive sin Tabaco», y transferencia a la práctica clínica. Todo el personal sanitario interesado recibió formación sobre el uso de la aplicación. Se elaboraron manuales de uso para pacientes y profesionales. Se recogieron las tasas de abandono del tabaco a los 12 meses.
ResultadosEl porcentaje de pacientes de la fase pospilotaje que dejó de fumar a los 12 meses fue del 14.1%.
ConclusionesLa concepción de «Vive sin Tabaco» como herramienta corporativa para la deshabituación tabáquica, ha sido larga y ardua, y ha requerido la participación de los profesionales sanitarios y de los pacientes para conseguir una herramienta que se adapte a sus expectativas, y garantice una mayor usabilidad y satisfacción. Esta aplicación está siendo eficaz como herramienta coadyuvante del consejo sanitario.
Tobacco is a major risk factor for chronic diseases and the most important preventable cause of death worldwide. Tobacco kills more than 8 million people each year.1 People who smoke are more likely to suffer from a wider range of diseases. Smokers have higher rates of absenteeism from work2,3 and longer absences from work than non-smokers.
To avoid smoking-related diseases, however, it is not enough to reduce smoking. Among people who significantly reduce smoking by up to 50%, the risk of myocardial infarction or chronic obstructive pulmonary disease is closer to those who continue smoking than those who have quit,4 so the aim of health services should be to promote smoking cessation.
In Spain, there has been an upturn in daily tobacco consumption, as published in the 12th Survey on Alcohol and Other Drugs in Spain (EDADES) 2019–2020, the prevalence of smoking is 32.3%, a Fig. 1 similar to that of 2005, before approval of the anti-smoking (32.8%).5
The morbidity caused by smoking generates an increase in healthcare costs, as it involves a greater use of resources. The annual health care cost for smokers is 864.64 euros, compared to 474.71 euros for non-smokers (82% more).2
Currently there are different approaches to smoking cessation treatment such as more or less intensive motivational counseling-based interventions, pharmacological therapy and group intervention with different success rates depending on the therapy used.6 There are also patients who decide to quit smoking without any help, with success rates varying between 3 and 8% after 6 months.7–8
Smoking cessation treatment is not only clinically effective but also cost-effective,9–11 in fact, it is one of the most cost-effective interventions in health care.9 On the other hand, health advice is considered one of the most cost-effective interventions in the treatment of smoking,10–11 however, the changes promoted by health advice do not last long,10 so it is necessary to establish reinforcement mechanisms, including ICT (information and communication technologies), and more specifically mHealth, for which there is ample evidence in the treatment of smoking.12–15
Mobile technology has changed the way we live, work and communicate. The use of mobile technologies has the potential to transform health care delivery worldwide.16 It is an emerging and rapidly developing field, which has the potential to play a key role in transforming healthcare to increase the quality and efficiency of healthcare, and whose mission is to complement rather than replace traditional healthcare.17
There is a wide range of mobile health applications (app) available for iOS and Android devices, but the level of trust they deserve is a widely debated issue.18,19 Moreover, the quality of these apps is highly variable,17 so it is necessary to involve both healthcare professionals and the patients in the design to ensure higher quality and usability.20
On the other hand, it is estimated that the use of mobile apps could improve the efficiency of patient healthcare and minimize up to 30% the time spent accessing and analyzing information, with an economic saving of 15% of healthcare utilization costs through remote monitoring via mobile apps.16,21
Mobile apps have great potential to support patients in healthcare, and to encourage healthy behavioral changes, such as smoking cessation. However, it is the characteristics of the apps that define patients’ attitudes toward their use and success. A recent study showed that motivational messages have a good rate of satisfaction,22–24 although it is necessary to take into account the technical characteristics of mobile devices that may difficult the reception of messages.22
Moreover, since the launch of mobile networks in the 1980s, the use of mobile phones has grown exponentially. The penetration rate of mobile telephony now exceeds 100%.25
In view of the above, it seems more than evident that it is necessary to explore different strategies that adapt these tools to the needs and preferences of smokers. The objective of this paper is to make known the whole process of development and transfer to clinical practice of a mobile application for smoking treatment “Vive sin tabaco”, and to evaluate the preliminary results of its implementation.
Material and methodsThe main objective is to transfer to clinical practice a new smoking cessation application (“Vive sin Tabaco” a) in all health centers of the public Basque Health Service.
A pilot study in a four primary care health centers was also been carried out before its widespread implementation in all public health centers of Osakidetza, to detect possible incidents in the handling of application. Smoking cessation rates at 12 months during implementation phase were also collected in a cohort of people user of “Vive sin tabaco” application since October 2019.
This project started in January of 2012 with the support of a grant from the Industry Department of the Basque Government, and subsequently it has been funded by Carlos III Health Institute.
PopulationThis application is addressed to motivated smoker population who is thinking about making an attempt to quit smoking, and especially through the use of the new technologies. Patients attending primary care consultations for a variety of reasons were asked about their smoking status, and about their intention to quit smoking. Those smoker patients who wanted to quit tobacco, and start an attempt within 15–30 days were informed about the different alternatives available, including the application. Patients who choose “vive sin tobacco application” were included in the program. It has estimated that around 65,831 men and 9564, women could choose “Vive sin Tabaco” as treatment for smoking cessation in Basque country. This estimate is based on the prevalence of smoking in the population aged 15 years and older (20.1% in men and 13.5% in women in the Basque Country-4), the percentage of mobile phone owners (67%26), the percentage of smartphones (73%26), the percentage of patients who seek health information through the mobile phone (25%27), and the percentage of patients who download health applications (58.23%28).
Intervention and implementation strategyFirst phase was to assess the effectiveness of SMSalud®A Short Message Service (SMS) program designed to be used as an adjuvant tool that reinforces motivational counseling provided in smoking cessation programs in primary care consultations (by nurses or general practitioners), and it was evaluated between 2014 and 2016 through a randomized clinical trial (RCT) carried out in 320 motivated smoker people, achieving smoking cessation rates of 16.25% versus 5.6% with motivational counseling only at 12 months from beginning.11 To assess quitting rate, we used a breath CO (carbon monoxide) test considering the result negative for levels of 0–6ppm, and positive for higher levels.
Second phase: Transformation a previously tested SMS program SMSalud® into “Vive sin tabaco” applicationIn order to offer this program to all smoker potential users of this technology in the Basque Public Health System, at a lower cost, SMSalud® has been transformed into a corporate mobile application named “Vive sin Tabaco”. Among functionalities of “Vive sin tabaco” may be mentioned the automatic sending of motivational messages, as well as the sending of messages in case of a punctual relapse, and anxiety states, at the patient's request. In addition, the application provides information about the money saved since the “D”-day “day the patient decides to quit smoking”, number of cigarettes not smoked, and life-time gained.
“Vive sin tabaco” application is integrated with the Osakidetza electronic medical record, and must be prescribed by healthcare professionals (nurses or general practitioners) to allow patients to activate it. Once prescribed, it can be downloaded from the corresponding stores (iOS or Android). If application is downloaded by patients without the previously intervention of the healthcare professional, a message appears in the mobile phone prompting the patient to contact their health center for prescription. From the application, data on smoking cessation at 3, 6 and 12 months, as well as falls, if any, and the dates of these, are dumped in real time to the electronic medical record. In addition, there is an intermediate console that collects all the information provided by the patient to the application and allows the healthcare professionals (nurses or general practitioners) to monitor the patient's smoking cessation process more exhaustively.
Third phase: Pilot study in smoker patientsTo test “Vive sin tabaco” in a real-life setting in smoking patients, a pilot project began in four primary care centers of Vitoria-Gasteiz. For this purpose, face-to-face training sessions were held in each of these four health centers for all the professionals interested in using the application. This application was offered to all smoker people visiting their nurse or general practitioners between February 2019 and April 2020. 55 smoker people agreed to quit smoking using the app, requesting them to report any incident related to the application to their health professional of reference.
This pilot project allowed healthcare professionals and patients to detect failures and options of improvement of application. In this way, an attempt was made to involve end-users in the design of the application, an essential requirement for improving its usability and satisfaction. Among the failures or incidents reported by users were the regular disconnection of the application, which was mainly due to low usage, and the non-reception of messages, aspects that were solved.
Fourth phase: widespread implementation in the rest of the health centers of OsakidetzaAfter incorporating the solutions to the incidences detected into the application, the implementation continued in the rest of Osakidetza health centers since May of 2020. Training courses in the use of the mobile application were given to more than 100 healthcare professionals from the different Osakidetza Integrated Health Organizations.
Outcomes and statistical analysisAspects such as number of smokers using the application, quitting rate (considered an ex-smoker if patient has been smoke-free for 12 months since “D” day), adherence to the application measured through the visualization of the messages within the application itself, and number of fall and anxiety messages requested were evaluated. All data were collected retrospectively and in a pseudonymized manner.
Main end-point (quitting rate) was collected as patient self-reported information at 12 months, through the application. As the half-life of CO in the body is around 5h, in 24h nearly all CO is cleared from the body. This may lead to false negatives if patients have gone a whole day without smoking. For this reason, smoking cessation has been measured as self-information report of patients.
Patients who activated the application but did not read any prevention message were also included in the study, in order to make an intention to treat analysis. All patients who asked to leave program were considered failures.
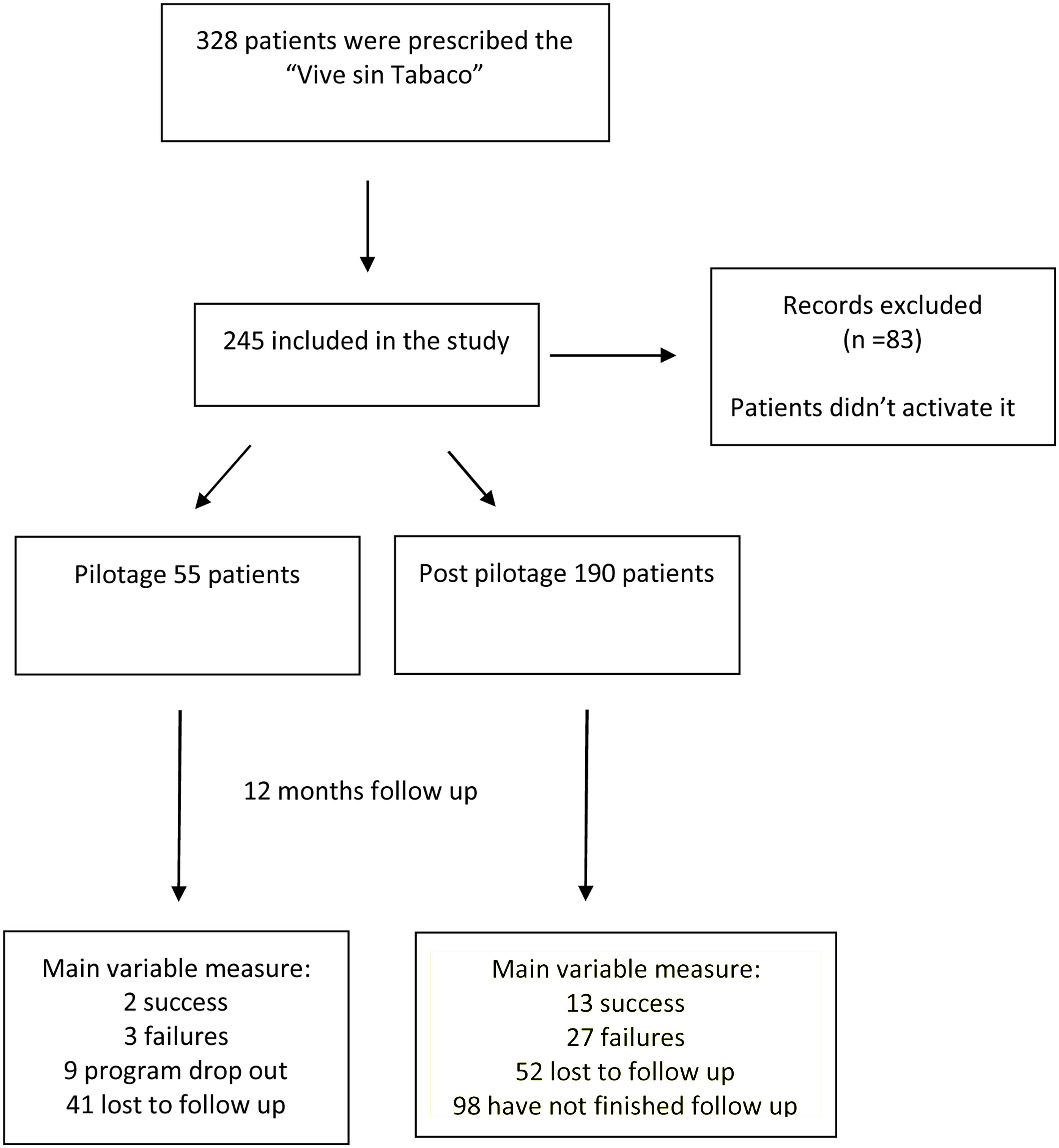
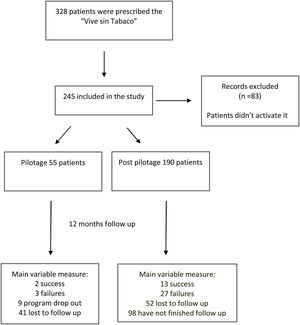
ResultsFrom 25 October 2019 to 11 March 2022, 245 patients with a mean age of 47.1 (standard deviation-SD 11.4) years old have activated the app in 65 different primary care centers of and 114 different primary care consultations. The 55.1% percent were women (n=135) (Table 1),
Features of all patients.
| Variable | N=245N (percentage) |
|---|---|
| Age (mean; standard deviation) | 47.1 (11.4) |
| Gender | |
| Woman | 135 (55.1%) |
| Man | 110 (44.9%) |
| Region | |
| Álava | 138 (56.8%) |
| Guipúzcoa | 31 (12.8%) |
| Vizcaya | 74 (30.5%) |
| Prevention messages viewed (median; interquartile range) | 27 (4–74) |
| Relapse messages requested | |
| 1–3 | 35 (14.3%) |
| >3 | 14 (5.7%) |
| 0 | 196 (80%) |
| Anxiety messages requested | |
| 1–3 | 49 (20%) |
| >3 | 13 (5.3%) |
| 0 | 183 (74.7%) |
| Messages therapeutic adherence | |
| <25% | 135 (55.1%) |
| 25–50% | 34 (13.9%) |
| 50–75% | 70 (28.6%) |
| ≥75% | 6 (2.4%) |
Among the 245 patients, 147 patients have finished program due to have completed the one year follow up period or to have failed. Among these, 15 patients (10.2%) had had given up smoking at 12 months, 80% (n=12) in Álava and 20% (n=3) in Vizcaya, 9 patients asked to leave the program, and 30 failed in their attempt. The rest (98 patients) have not completed treatment at the time of analysis. No statistically significant differences being observed in relation to sex and age between those who have given up tobacco and those who have not given up tobacco (p>0.05) (Table 2), and although patients who quit smoking displayed a greater number of messages than those who did not quit smoking, the difference was not statistically significant (p=0.076)(Table 2).
Features of patients according to the success of failure.
| Variable | Category | Success | Failure | p value |
|---|---|---|---|---|
| (n=15) | (n=30) | |||
| N (percentage) | ||||
| Age (mean; standard deviation) | 50.2 (11.9) | 48.3 (12.9) | 0.634 | |
| Gender | ||||
| Woman | 9 (60%%) | 17 (56.7%) | 0.831 | |
| Man | 6 (40%) | 13 (43.3%) | ||
| Region | ||||
| Álava | 12 (80%) | 16 (53.3) | No valorable | |
| Guipúzcoa | – | 4 (13.3%) | ||
| Vizcaya | 3 (20%) | 10 (33.3%) | ||
| Prevention messages viewed | 86 (70–89) | 72 (25-87) | 0.076 | |
| Relapse messages requested | ||||
| 1–3 | 1 (6.7%) | 7 (23.3%) | No valorable | |
| >3 | – | 5 (16.7%) | ||
| 0 | 14 (93.3%) | 18 (60%) | ||
| Anxiety messages requested | ||||
| 1–3 | 4 (26.7%) | 7 (23.3%) | No valorable | |
| >3 | 1 (6.7%) | 5 (16.7%) | ||
| 0 | 10 (66.7%) | 18 (60%) | ||
Among 147 patients who have finished follow up, 10.2% (n=15) have quit tobacco, and 12 of them achieved an adherence to message visualization above 50% (80%) (Table 3).
Features of patients according to the therapeutic adherence in patients who have finished follow up.
| Variable | Category | Therapeutic adherence | p value | |
|---|---|---|---|---|
| ≤50% (n=83 | >50% (n=46) | |||
| N (percentage) | ||||
| Age (mean; standard deviation) | 49.3 (12) | 47.9 (10.7) | 0.453 | |
| Gender | ||||
| Woman | 46 (50%) | 28 (50.9%) | 0.915 | |
| Man | 46 (50%) | 27 (49.1%) | ||
| Prevention messages viewed (median; IQarange) | 12 (3–28) | 86 (75–90) | 0.000 | |
| Pilot patient | ||||
| Yes | 42 (45.7%) | 13 (23.6%) | 0.008 | |
| No | 50 (54.3%) | 42 (76.4%) | ||
| Smoking cessation | ||||
| Yes | 3 (17.6%) | 12 (42.9%) | 0.082 | |
| No | 14 (82.4%) | 16 (57.1%) | ||
Furthermore, out of these 147 patients, 92 belong to post-piloting, moment from which application began to work better, and among these, the 14.1% (n=13) of patients quit smoking. The remaining patients are still in follow-up (n=98).
Adherence to the messages visualization was bigger in post piloting patients (median; interquartile range) 50; 11–85 versus 16; 4–61; p=0.018. Patients who have requested at least one message for occasional relapse have less probability of success (1/13 versus 14/32); p=0.034.
DiscussionSmoking cessation is a complicated process influenced by different factors, sometimes requiring up to 30 attempts.29 Smoking abstinence rates also changes depending on the type of therapy used and can range from 10% with motivational counseling to 33% with pharmacological therapy (varenicline). Drugs are not free of side effects, and there are patients who avoid taking them, so it is necessary to explore different strategies adapted to patients’ expectations and preferences.
Since the launch of mobile phone networks in the 1980s, their growth has been exponential, reaching a level of penetration in the population that has already exceeded the 100%.25 Mobile technology has changed the way we live, work and communicate, and has enabled easier access to healthcare to increase the quality and efficiency of healthcare, and its mission is to complement rather than replace traditional healthcare.17
“Vive sin tabaco” app is a corporate tool available in all health centers in the public health system of the Basque Country, and it is used as an adjuvant tool to reinforce the motivational counseling provided by health care workers to quit smoking. The health professional must be present to provide motivation and support to the patient at all times. The app reinforces this advice provided by the healthcare professional. All the information provided by the patient through the application is downloaded in real time to the electronic medical records. This program is also cost-effective, with an incremental cost-effectiveness ratio (ICER) of 7.4 euros for men and 1327 euros for women from a health perspective,30 far from the 22,000 euros threshold calculated for the Spanish health system.31 These results that agree with obtained by other research groups,30 have been published previously.32
Smoking cessation rate at 12 months from program beginning, and including pilot and no pilot patients was 10.2%. Taking only post-pilot patients into account, abstinence rate was 14.1%. Adherence to the program was low, 37.4% of patients who have finished follow up had an adherence less than 25%, but in post piloting phase was bigger. In general, it is estimated that in case of chronic disorders the 45% of patients do not adhere to therapeutic prescriptions, and if the treatment also involves a change in habits or lifestyle,33 as in the case of smoking, non-adherence exceeds these percentages.39 The lack of adherence can be partially explained by the whole process of continuous improvement that the app has undergone since its initial development, and by the continuous adaptations, it has had to deal with, due to changes in the corresponding operating systems (iOs or Android). The implementation of the program in the territory of Alava before the rest of regions of the Basque Country allowed us to collect incidences and the opinion of professionals and patients to improve the tool, satisfaction and usability.20 However, despite the low rates of adherence, the rate of smoking cessation in the clinical practice in the post piloting phase is very encouraging.
Despite the fact that the application has been running for two years, since October 2019 very few patients have chosen the application as method for smoking cessation. However, we have we to take into account two important aspects: first) from October 2019 to May 2020 a pilotage in a few Health centers of Álava, has been carried out, and second) since May 2020 the widespread implementation started in the rest of Health centers of public health system of the Basque, coinciding with the outbreak of the pandemic throughout the Basque Country, being one of the most affected autonomous communities from the beginning. This caused that professionals didn’t know the existence and the use of the application because the main indication given to health professionals was to treat and be prepared for covid patients, relegating other non-priority actions to a secondary plan.
The effectiveness of mobile applications to quit smoke is currently assessed. Our group published a meta-analysis in 202035 evaluating de effectiveness of these type of tool for smoking cessation, obtaining a pooled Risk Relative after combining results of 0.90 (95% CI: 0.57–1.423) versus others type of interventions. Similar results have been published by other groups of researchers.36 At the moment where these meta-analysis was carried out, more clinical trials were on going. Some of them have recently published as the Pallèja-Millan et al.,37 who observed that participants in the intervention group who used the app regularly and correctly were more likely to be non-smokers at 12 months (OR 7.20; 95% CI 2.14–24.20) than control participants. Other prospective, interventional, multicenter, single-arm study, evaluating the long-term abstinence effect of a novel smartphone app in patients with nicotine dependence, found a continuous abstinence rate from weeks 9 to 24, was the 64%, concluding that mobile applications added to usual smoking cessation therapies resulted in high abstinence rates.38
Smartphones are intelligent devices with the ability to assume autonomously and independently a multitude of tasks, in a similar way to artificial intelligence. An act performed repeatedly by users is “learned” by the phone. A simple act such as “scrolling a pop-up notification to the right” repeatedly is understood as a rejection of that notification by the phone, turning it off and not receiving it again.
Another interesting aspect, related to notifications, was the fact that short notifications (with a low number of characters) could be read on the smartphone screen itself without having to access the app itself. As a result, the notification was considered not viewed by the patient. Since the notifications received by the patient are all less than 160 characters, the probability of being displayed on the smartphone screen was very high, so the patient did not see the need to access the app. We believe this had an impact on the low adherence observed.
These type of tools should be evaluated and maintained constantly in order to detect incidents which can be resolved immediately, and that do not interfere with the patients’ treatment. The continuous changes in the operating systems of smartphones make it necessary to review continually the functionalities of applications for a correct running. In this regard, our application has been integrated in the protocol of maintenance and improvement of OSAKIDETZA applications. On the other hand, in order to assess the acceptability, feasibility, usability, validity, reliability or potential effects on health variables, among other aspects, the tool for evaluating mhealth-based technologies for mobile applications, from the Ministry of Health, has been used, obtaining a standardized score of 57–89% in the different domains.
This study suffers from weaknesses, the main of them is that it is un observational prospective study. In spite of this, it is the last phase of a process where a multidisciplinary team who look out for health prevention have been working for more than 10 years, and the transfer to clinical practice is the culmination of the this work.
Conclusions“Vive sin tabaco” application has had to overcome many obstacles and has undergone continuous modifications and adaptations over the years, with a negative impact on adherence, being one of the main reasons for the results obtained.
Nowadays, the Basque Public Health System has a corporate application for smoking cessation, which is available to all patients who wish to quit smoking. It is a tool that has been validated through a clinical trial, and it is also cost-effective.
It must be prescribed by health professional to activate it, and it is considered a tool to reinforce the motivational advice provided in primary care consultations.
It is necessary to continue to deepen and analyze the characteristics of smartphones, to understand them, and to co-design useful tools with their end-users. It is important to bear in mind, when considering developments of this type, that the process will be long and arduous, and that they need a process of continuous improvement and adaptation in order to function correctly.
- •
Mobile apps have great potential to support patients in healthcare, and to encourage healthy behavioral changes, such as smoking cessation.
- •
End-users need to be involved throughout the process to ensure greater satisfaction and usability.
- •
It is advisable to carry out economic evaluations to justify the opportunity for transfer to clinical practice.
- •
The development process of a health application is dynamic and has to adapt to the continuous changes in the operating systems of the terminals.
- •
Smoking cessation rates in the implementation phase are 14.1% per year.
- •
Among people who request occasional relapse messages there is a lower probablity of success.
The authors declare that they have no conflicts of interest.