Cancer of the hypopharynx remains one of the most challenging chapters in head and neck oncology. The objective of this study is to ascertain the relevance of a transoral laser approach as a valid functional option for treatment of cancer of the hypopharynx in Portugal, and additionally, to confirm the reproducibility of survival and functional outcomes described in other reference centers.
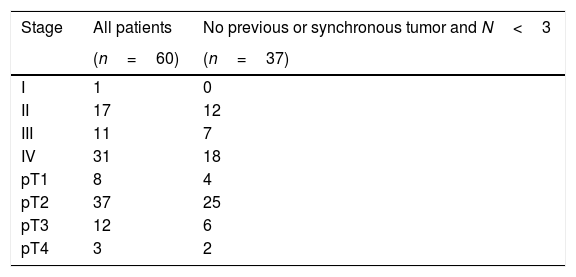
Subjects and methodsThe outcomes of 37 out of 60 patients presenting hypopharyngeal carcinoma primarily treated by TLM (transoral laser microsurgery) and neck dissection and or adjuvant treatment when needed, with curative intention in tertiary referral center, were retrospectively evaluated and compared with published results.
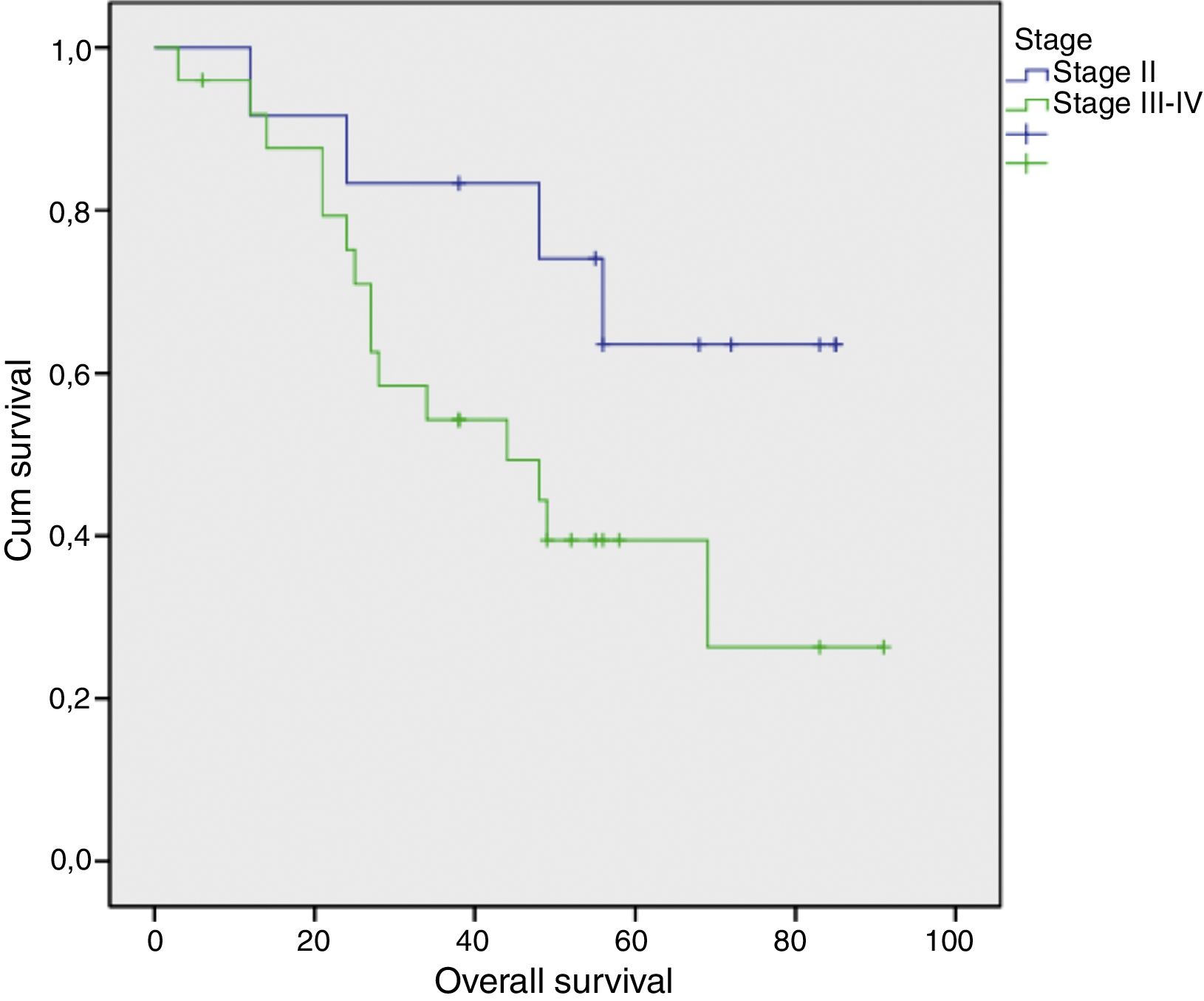
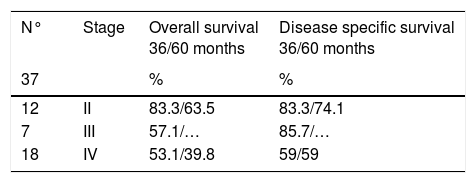
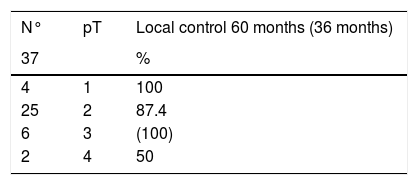
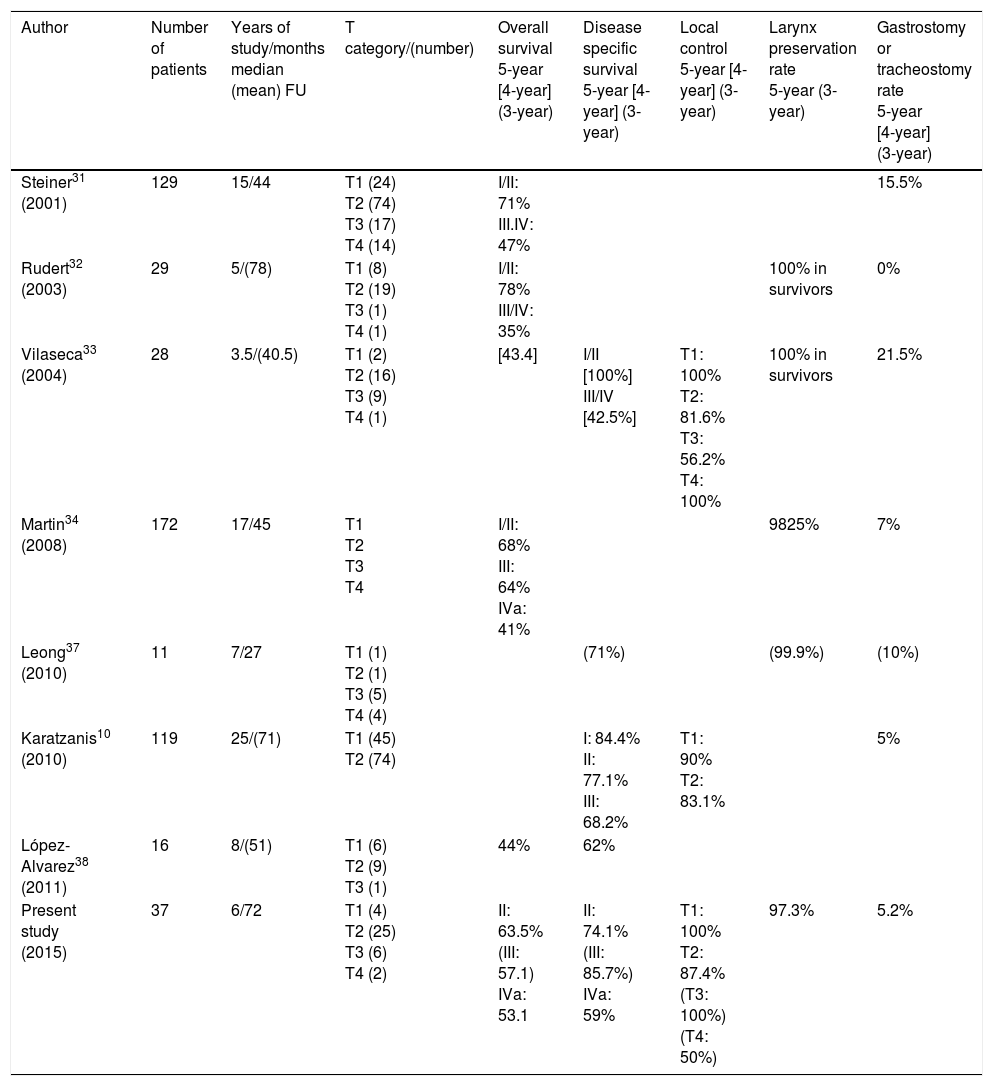
ResultsThere were no patients in stage I. Three-year and five-year overall survival (Kaplan–Meier) were 83.5% and 63.5% for stage II (n=12), 57.1% (only 3-year overall survival evaluable for this stage) for stage III (n=7), and 53.1% and 39.8% for stage IVa (n=18), respectively. Five-year local control rates were 90% for stage II and 87.5% for stage IVa, respectively; only three-year local control rates were possible to evaluate for stage III, with a 100% control rate. Five-year total larynx preservation rate was 97.3%.
ConclusionsTLM, alone or with neck dissection and adjuvant therapy, is a valid procedure for treatment of hypopharyngeal cancer in different stages. Furthermore, this kind of approach can be replicated in different oncologic centers with similar oncologic and functional results.
El cáncer de hipofaringe continúa siendo uno de los capítulos más difíciles en la oncología de cabeza y cuello. El objetivo del presente estudio es determinar la relevancia del abordaje con microcirugía transoral láser CO2 (MTL) como una opción válida para el tratamiento de cáncer de hipofaringe en un hospital terciario. Adicionalmente, se pretende comparar los datos obtenidos con los de otros centros de referencia en relación a la supervivencia y a los resultados funcionales.
Pacientes y Métodos37 pacientes de un total de 60 con diagnóstico de carcinoma hipofaríngeo han sido tratados con intención curativa con MTL sola o asociada á disección cervical y terapia adyuvante. Los resultados han sido evaluados retrospectivamente y comparados con los publicados en la literatura.
ResultadosNo hubo pacientes en estadio I. La supervivencia global a los 3 y 5 años (Kaplan-Meir) fue de 83.5% y 63.5% en el estadio II (n=12); 57.1% en el estadio III (n=7) (en este estadio sólo pudo ser evaluada la supervivencia global a los 3 años) y 53.1% y 39.8% para el estadio IVa (n=18) respectivamente. El porcentaje de control local a los 5 años fue de 90% en el estadio II y de 87.5% en el estadio IVa, respectivamente; en el estadio III, solamente ha sido posible evaluar el control local a los 3 años, que ha sido de 100%. El porcentaje total de preservación laríngea a los 5 años fue de 97.3%.
ConclusionesLa MLT, sola o asociada a la disección cervical y terapia adyuvante, es un procedimiento eficiente para el tratamiento del cáncer hipofaringeo en diferentes estadios. Esto confirma que este abordaje es una opción válida y reproducible en diferentes centros oncológicos.