To identify the significant predictors of locoregional recurrence in early stage squamous cell carcinoma (SCC) of buccal mucosa with pathologically clear surgical margins and negative neck.
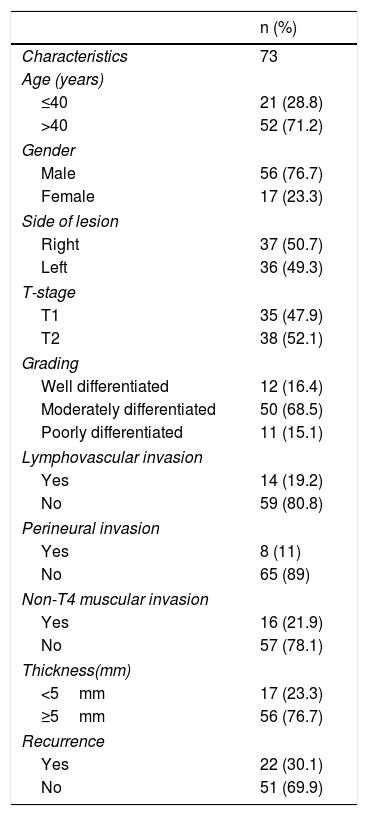
MethodSeventy-three patients who underwent per oral wide excision and supraomohyoid neck dissection for early stage buccal SCC with clear surgical margins (>5mm margins each) and negative neck (N0) were included. None of the patients received postoperative radiotherapy or chemotherapy. Univariate and multivariate analyses were used to identify independent predictors of locoregional recurrence.
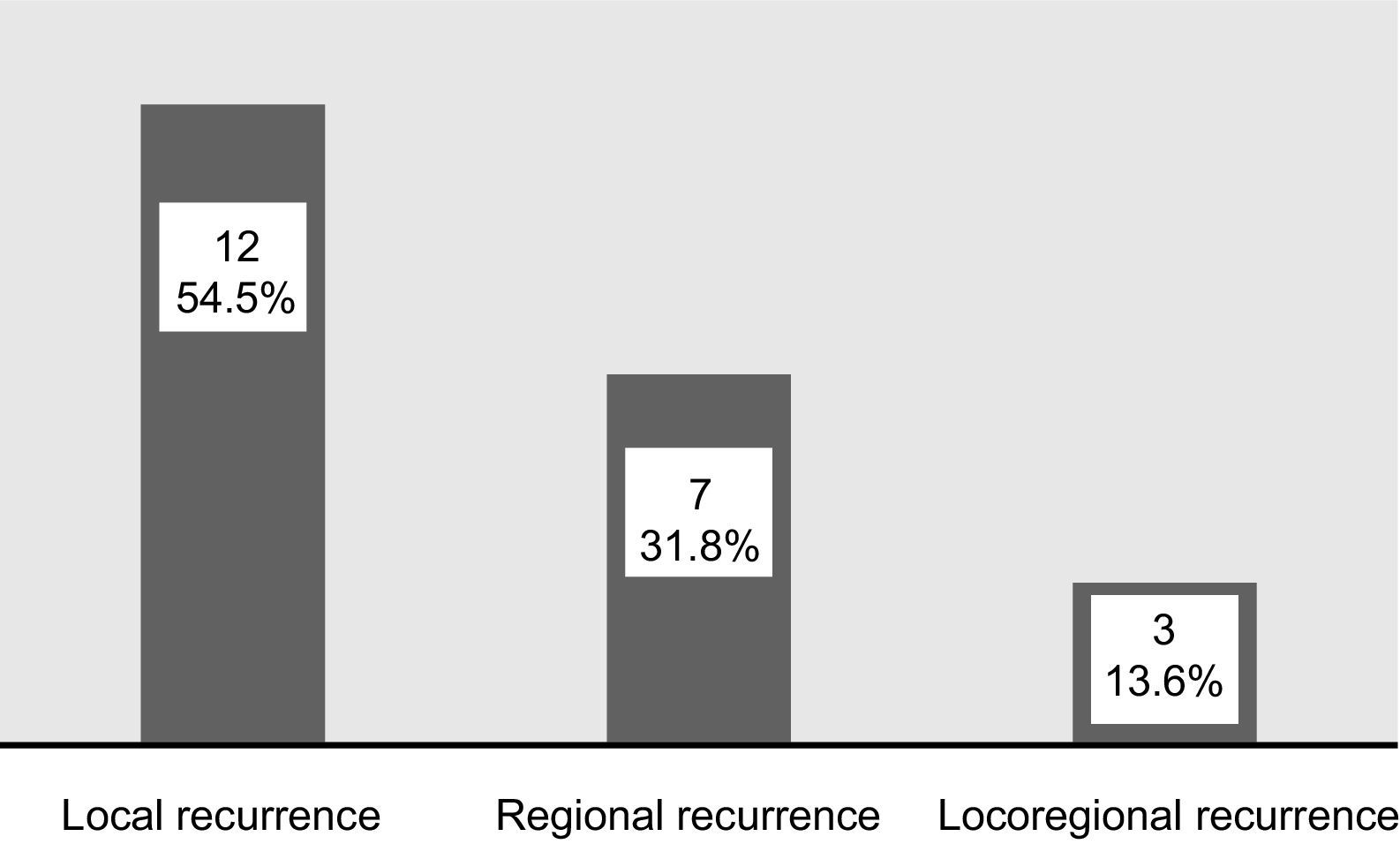
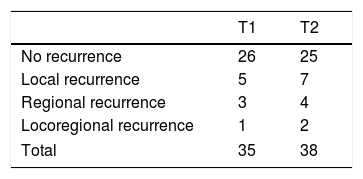
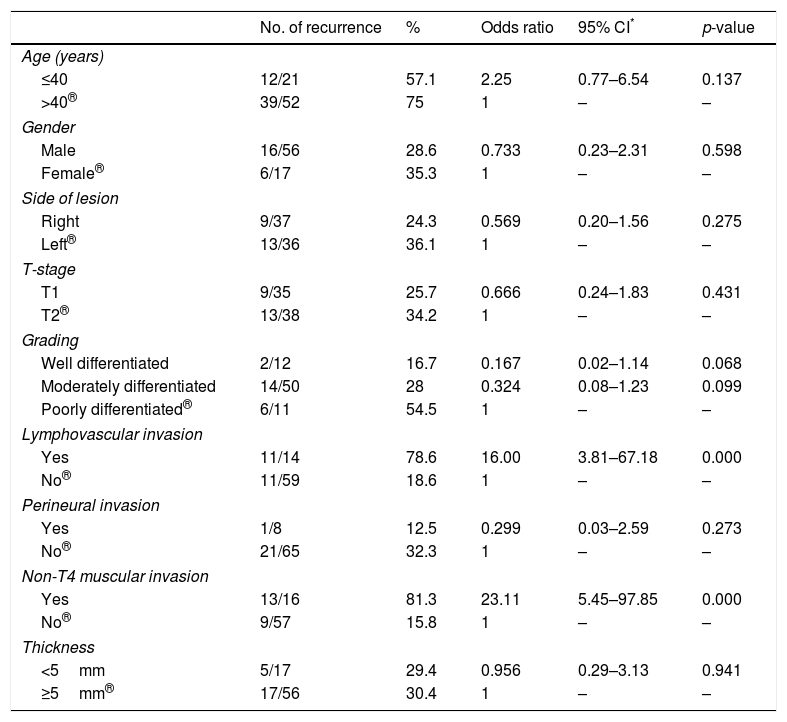
ResultsRecurrence was observed in 22 of 73 (30%) cases. Twelve had local, seven had regional and three developed locoregional recurrences. Both univariate and multivariate analyses demonstrated that lymphovascular invasion (LVI) and non-T4 muscular invasion (non-T4MI) were independent predictors affecting locoregional control.
ConclusionLymphovascular invasion (LVI) and non-T4 muscular invasion (non-T4MI) significantly increased the locoregional recurrence rate in early stage buccal SCC with clear surgical margins and negative nodal status. Adjuvant treatment with either radiation or chemoradiation should be considered when one or both of these factors present.
Identificar los predictores significativos de recidiva locorregional en el carcinoma de células escamosas (CCS) en estadios iniciales de la mucosa buccal, con los márgenes quirúrgicos patológico libres y el cuello negativo.
MétodoSe incluyeron en el estudio 73 pacientes sometidos a extirpación tumoral y disección supraomoioidea de cuello con cáncer bucal en estadios iniciales con márgenes quirúrgicos libres (margen de 5mm cada uno) y cuello negativo (N0). Ninguno de los pacientes recibió radioterapia postoperatoria o quimioterapia. Se utilizaron análisis univariantes y multivariantes para identificar los factores predictivos independientes de recidiva locorregional.
ResultadosLa recidiva se observó en 22 de 73 casos (30%). Doce tenían recidivas locales, 7 regionales y 3 desarrollaron recidivas locorregionales. Tanto los análisis univariantes como multivariantes demostraron que la invasión linfovascular (LVI) y la invasión muscular no T4 (non-T4MI) fueron predictores independientes que afectaron al control locorregional.
ConclusiónLa LVI y la non-T4MI aumentaron significativamente la tasa de recurrencia locorregional en el CCS bucal precoz con márgenes quirúrgicos libres y estado nodal negativo. El tratamiento adyuvante con radiación o quimiorradiación debe considerarse cuando se presentan uno o ambos de estos factores.