Auditory steady state responses to continuous amplitude modulated tones at rates between 70 and 110Hz, have been proposed as a feasible alternative to objective frequency specific audiometry in cochlear implant subjects. The aim of the present study is to obtain physiological thresholds by means of auditory steady-state response in cochlear implant patients (Clarion HiRes 90K), with acoustic stimulation, on free field conditions and to verify its biological origin.
Methods11 subjects comprised the sample. Four amplitude modulated tones of 500, 1000, 2000 and 4000Hz were used as stimuli, using the multiple frequency technique. The recording of auditory steady-state response was also recorded at 0dB HL of intensity, non-specific stimulus and using a masking technique.
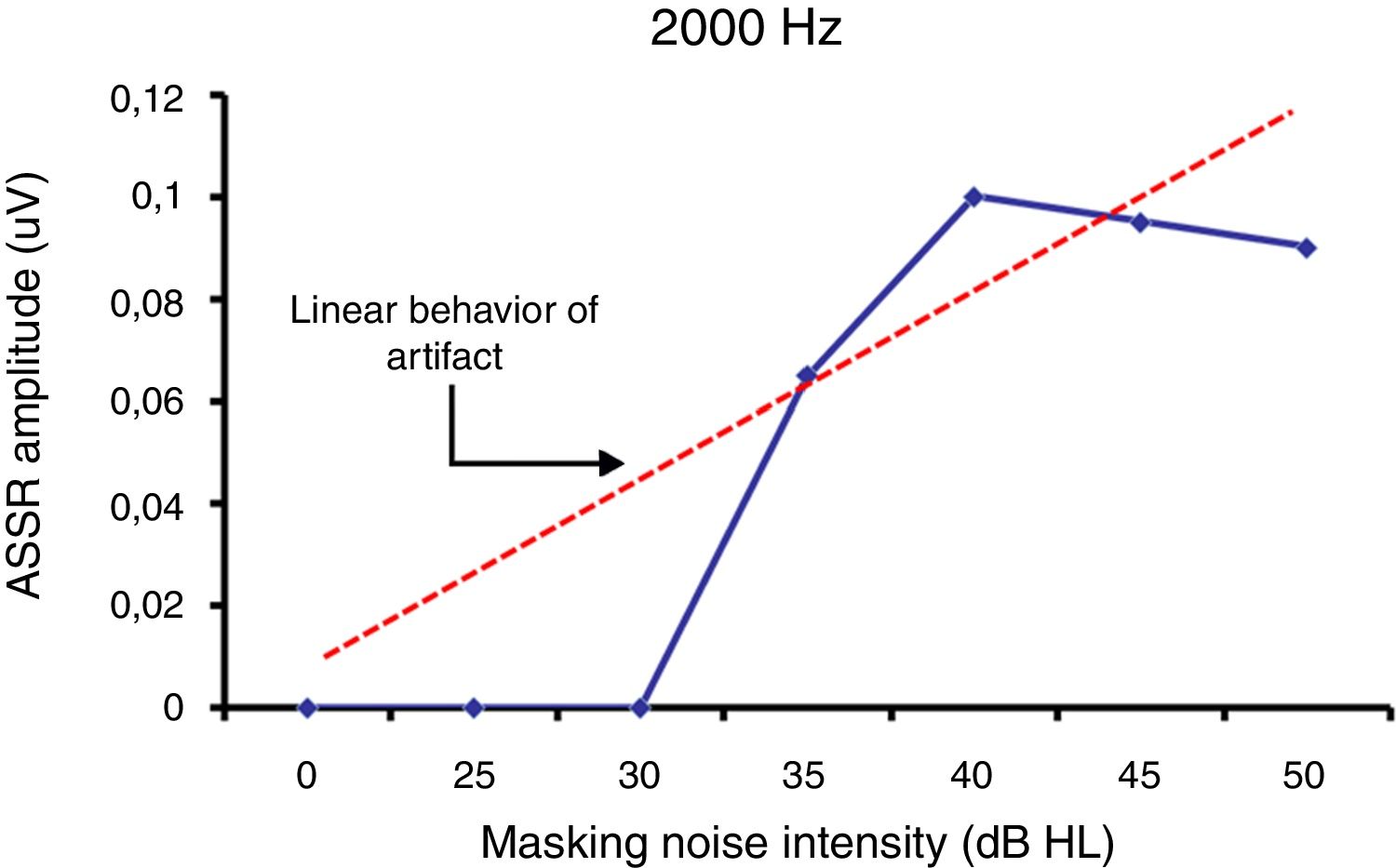
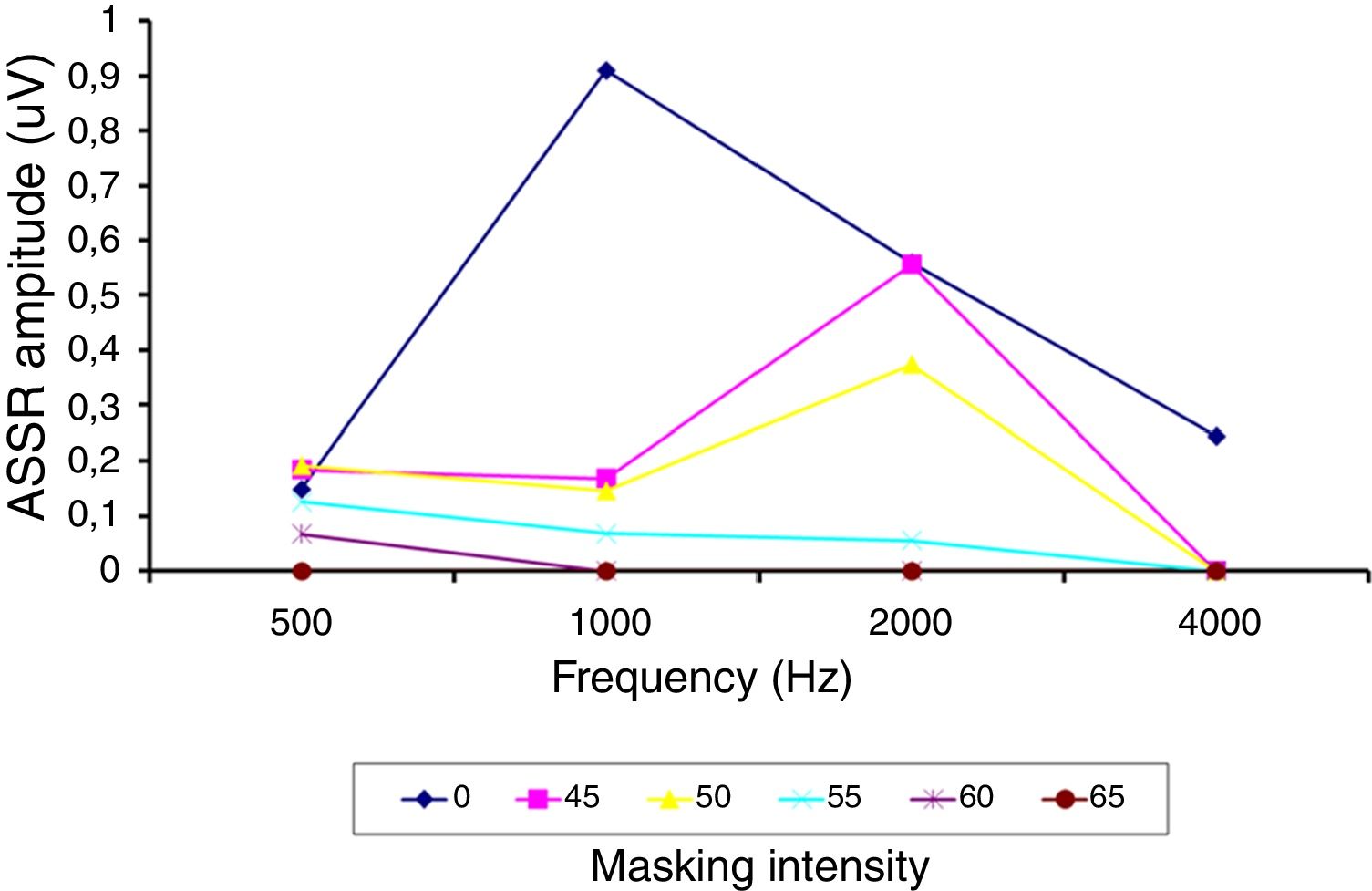
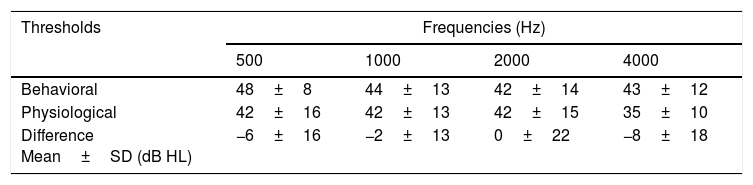
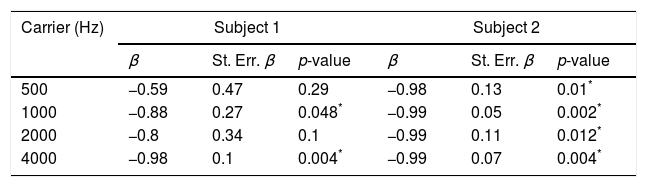
ResultsThe study enabled the electrophysiological thresholds to be obtained for each subject of the explored sample. There were no auditory steady-state responses at either 0dB or non-specific stimulus recordings. It was possible to obtain the masking thresholds. A difference was identified between behavioral and electrophysiological thresholds of −6±16, −2±13, 0±22 and −8±18dB at frequencies of 500, 1000, 2000 and 4000Hz respectively.
ConclusionsThe auditory steady state response seems to be a suitable technique to evaluate the hearing threshold in cochlear implant subjects.
Los potenciales evocados auditivos de estado estable (PEAEE) por estimulación con tonos modulados en amplitud entre 70 y 110Hz han sido propuestos como una alternativa factible para realizar una audiometría objetiva en pacientes con implante coclear. El objetivo del presente estudio es verificar el origen biológico de los umbrales auditivos obtenidos mediante PEAEE por estimulación acústica y en condiciones de campo libre, en pacientes con implante coclear (Clarion HiRes 90K).
MétodosLa muestra constó de 11 pacientes. Cuatro tonos modulados en amplitud con frecuencias portadoras de 500, 1.000, 2.000 y 4.000Hz y presentados simultáneamente fueron empleados como estímulo. Se registraron series de intensidad hasta alcanzar el umbral auditivo, así como registros a 0dB HL, con estímulos no específicos y empleando técnicas de enmascaramiento.
ResultadosEl estudio permitió obtener los umbrales electrofisiológicos par cada paciente de la muestra explorada. No hubo respuesta de estado estable ni a 0dB ni al emplear estímulos no específicos. Fue posible obtener los umbrales de enmascaramiento. Se identificó una diferencia entre los umbrales conductuales y electrofisiológicos de −6±16dB, −2±13dB, 0±22dB y −8±18dB a las frecuencias de 500, 1.000, 2.000 y 4.000Hz, respectivamente.
ConclusionesLos PEAEE pueden constituir una técnica apropiada para evaluar el umbral auditivo en sujetos con implante coclear.