In recent years, the integration of artificial intelligence (AI) has emerged as a transformative force in various domains of healthcare, revolutionizing traditional practices and enhancing patient care. One particularly impactful area is critical care medicine, where rapid decision-making and precise interventions are paramount for patient outcomes. This paper delves into the integration of AI within critical care medicine and elucidates its profound significance. Specifically, it explores the concept of Augmented Critical Care Ecosystems (ACCEs), where AI technologies are seamlessly integrated into existing clinical workflows to enhance efficiency, accuracy, and ultimately, patient outcomes.
Decision-making process in critical careOne of the biggest challenges in critical care medicine is the complexity of the decision-making process. ICU patients often have complex medical problems requiring ongoing assessment and treatment. Physicians must consider various factors, including the patient's age, medical history, current condition, and other risk factors. The decisions made in the ICU can have life-or-death consequences, and the consequences of a wrong decision can be severe.
If we add to this the intrinsic complexity of the cancer patient in the ICU, the number of variables to be included and their multicollinearity is magnified, with a high risk of error and bias that may lead to overtreatment or undertreatment in light of the new realities of oncology patients and their new therapies and outcomes.
Another challenge is the need for standardized protocols for patient care. Each ICU may have its own set of protocols and guidelines, and physicians may have different approaches to managing the same patient. This can lead to negative impact in the safety and quality attention of the critical care patient and make it difficult to compare outcomes between different ICUs.
Finally, the sheer amount of data generated by ICU patients can be overwhelming. Physicians must process and analyze large amounts of data from multiple sources, including lab results, imaging studies, and vital signs. This can be a time-consuming and error-prone process, and there is a risk that important information may be missed or misinterpreted.
These challenges highlight the potential benefits of using AI and predictive analytics in critical care medicine. By leveraging advanced algorithms and machine learning models, physicians can better predict patient outcomes and make more informed treatment decisions. Additionally, standardized protocols and guidelines could be developed based on AI-generated insights, helping reduce care inconsistencies and improve ICU patient outcomes.
Clinicians do not consistently apply care based on the best evidence. For example, critical care clinicians need to consistently apply mechanical ventilation's widely acknowledged life-saving potential to patients with acute lung injury.1
Morris counted 236 variable categories being considered by the intensive care unit clinicians. These variables are required to make decisions regarding sepsis-induced acute respiratory distress syndrome that might involve mechanical ventilation, arterial oxygenation, circulatory, renal, pharmacological, and intravenous infusion variables, in addition to multiple consultant suggestions.2
Therefore, it is necessary to adapt traditional ICU protocols to context-sensitive protocols and simultaneously transform the intensive care unit (ICU) model to that of Augmented Critical Care Ecosystems (ACCEs). We must effectively incorporate artificial intelligence and exponential technologies into the daily workflow through the above-mentioned strategies to achieve this.
Incorporation of AI into critical care medicineThe clinical outcomes of critically ill patients have reached significant and unprecedented improvements as standards of care have improved. Critical care medicine has seen the arrival of advanced monitoring systems and various invasive and non-invasive treatment strategies to provide timely intervention to critically ill patients. However, conventional intensive care practice still has limitations in understanding the degree of acuity required to care for patients, managing extreme individual heterogeneity, anticipating deterioration, and providing early treatment strategies before decompensation.
The recent exponential growth in computing power and portability increases the power of artificial intelligence (AI), making it available for many fields, including critical care medicine, where data is vast, abundant, and complex.3,4 The idea of AI in the ICU is to allow computers to find patterns in a complex environment of multidimensional and multi-domain data, with the prerequisite that such patterns would not otherwise be recognized.4
AI models provide helpful solutions in detecting, phenotyping, predicting, and characterizing conditions that could alter the course of critical illnesses. At the same time, they can lead to more certain decision-making about optimal and individualized treatment strategies, especially when options are multiple.
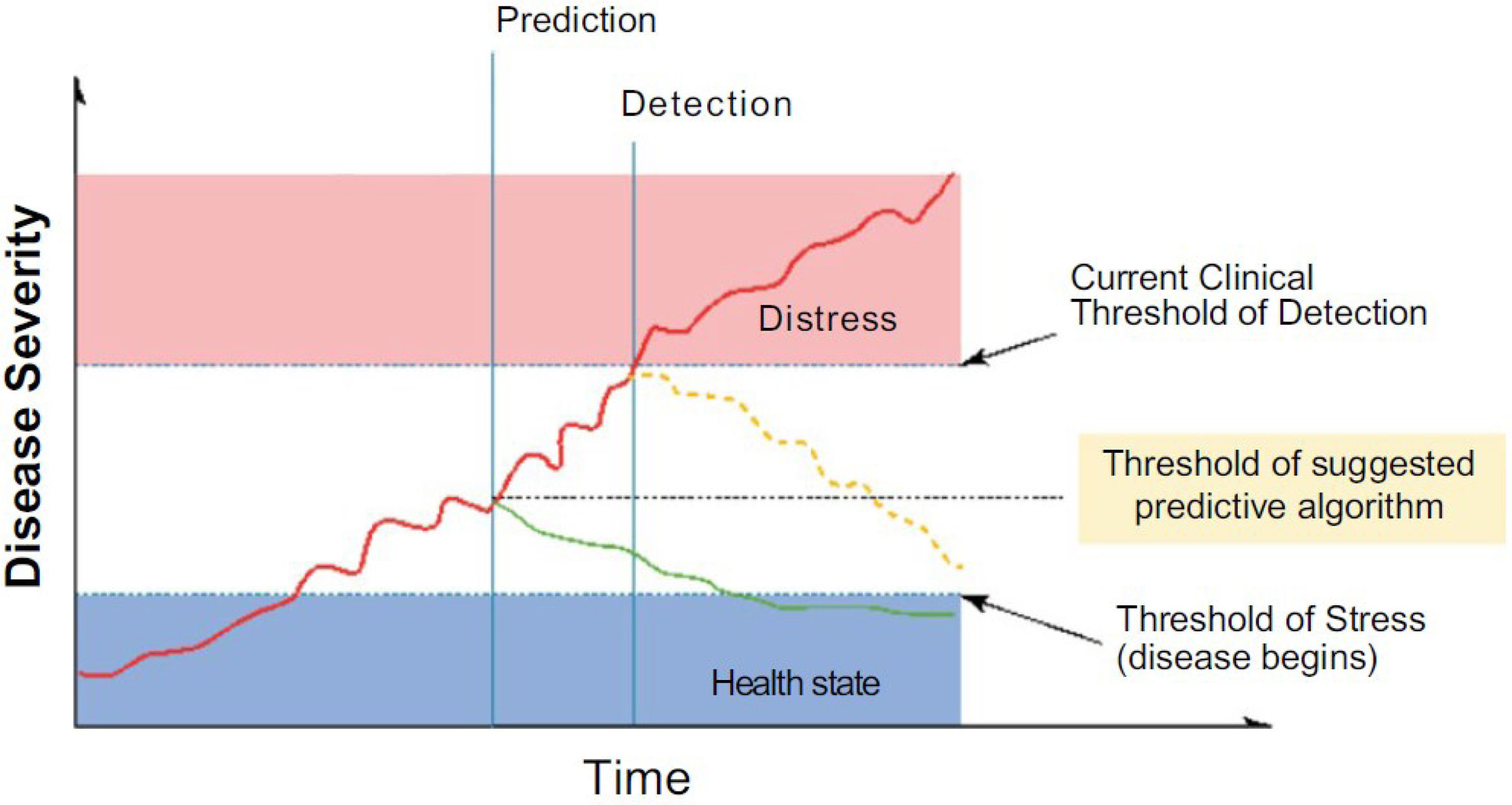
Finding the root cause of clinical deterioration from an exhaustive list of differential diagnoses is challenging due to the insidious nature of early disease progression or coexisting conditions that mask the main problem. In Fig. 1, the conceptual role of AI can be seen, as applied algorithms and models allow for early detection and prediction, favoring timely, efficient, effective, and appropriate care of critically ill patients (point indicated by the green line), as opposed to late stages of conventional strategies (point indicated by yellow dotted line).5
Conceptual role of predictive analysis in disease progression based on AI.
Source: Yoon et al.3.
The challenges in developing and implementing AI solutions are many. First, generalizing information with adequate databases, including de-identification and standardization, is more straightforward. Second, AI models are not always robust; they can have suboptimal performance compared to reporting standards; they can also have a high risk of bias, lack of reproducibility, and in many cases, need adequate external validation with open data and transparent model architecture. Third, with opacity and a probabilistic approach, AI models could lead to unforeseen ethical dilemmas. Despite the wide variety of obstacles and an indeed rudimentary understanding of AI, research is increasing, and models are being built that, even when they are not yet a central component of daily clinical practice, are leveraged to deepen the search for hidden disease patterns among extremely heterogeneous and noisy clinical data sets.
Successful implementation of AI in future clinical practice requires collaborative research efforts with plans for data standardization (medical ontologies) and exchange, development of advanced models to determine data safety, real-time application, and quality control. Examples of ICU data standardization are eICU6 project and MIMIC-III that is an extensive, freely-available database comprising de-identified health-related data associated with over forty thousand patients who stayed in critical care units of the Beth Israel Deaconess Medical Center between 2001 and 2012. The database includes demographics, vital sign measurements made at the bedside (∼1 data point per hour), laboratory test results, procedures, medications, caregiver notes, imaging reports, and mortality (including post-hospital discharge).7
MIMIC supports various analytic studies spanning epidemiology, clinical decision-rule improvement, and electronic tool development. It is notable for three factors: it is freely available to researchers worldwide; it encompasses a diverse and vast population of ICU patients; and it contains highly granular data, including vital signs, laboratory results, and medications.8
In complex cases such as sepsis, where single solutions do not work well, research has not improved the outcome of septic shock with different treatment regimens.9 AI could address, at least partially, the extreme heterogeneity of septic shock, the various underlying conditions, and the different host responses, to provide individualized solutions through reinforcement learning. The algorithm in reinforcement learning is designed to detect numerous variables in a given state and build a model of action, which then learns from the reward or penalty of the results of that action. By applying this to the affected population, reinforcement learning could provide optimal solutions for sequential decision-making in sepsis treatments, showing AI's potential impact in generating accurate personalized responses.10
Another topic of application is the use of vital sign signals for predictive purposes as opposed to signals that recognize a patient's clinical state. Physiological signals have limitations because they are often confusing, highly heterogeneous, can be interpreted in numbers, frequencies, wave characteristics, and artifacts, have an amplitude between what is observed and what can be interpreted, are unlabelled data, and their interpretation is often statistical. AI and machine learning models, through time series analysis and supervised and unsupervised analysis, seek confidential data in physiological vital sign signals to achieve a transition in analysis, offering added value – prediction – which is nothing more than the search for a model that can be used as a guide for daily routine.
Most studies in the context of critical patients still do not show satisfactory results that allow predicting clinical deterioration and consider factors such as heterogeneity between different pathologies with which patients enter and vital signs among different age groups.11 Additionally, studies carried out with vital sign signals have mostly not been able to be put into real-time clinical practice, and not all algorithms found in the literature have been sufficiently reported, are not available as models to be tested or used as a starting point and are only found as results of theoretical situations.12,13
These examples demonstrate AI's role in guiding critical decision-making for critically ill patients. The future design of the ICU should adopt the functionalities of AI solutions to allow physicians to react earlier to potential deteriorations. Research should be extensive so that specialists and researchers can build better models using more complete, available, highly accurate, and reliable data for primary care physicians.
Challenges for AI in critical care medicineThere are three significant challenges to implementing AI in critical care medicine. The first is the explainability and interpretability of models: overcoming black-box effect and achieving clinical team adherence. This is related to the development of strategies that facilitate explainability, such as SHAP graphs that stands for “Shapley Additive Explanations,” a method for assigning contributions to the prediction of each input feature. The SHAP plot shows how each feature affects the model's output and helps to understand and explain the model's decisions in a more interpretable way.
The second challenge is to have robust enough models to be included in the daily workflow of the ecosystem, but this requires knowledge and evaluation frameworks to evaluate the strength of the algorithms to be implemented properly and the robustness of the data from which they have been constructed.
Moreover, the third challenge is the bioethical component related to data privacy, security, and accountability for decisions derived from the models, and ultimately respect for autonomy and shared decision-making in patients under the special conditions of the ICU.3
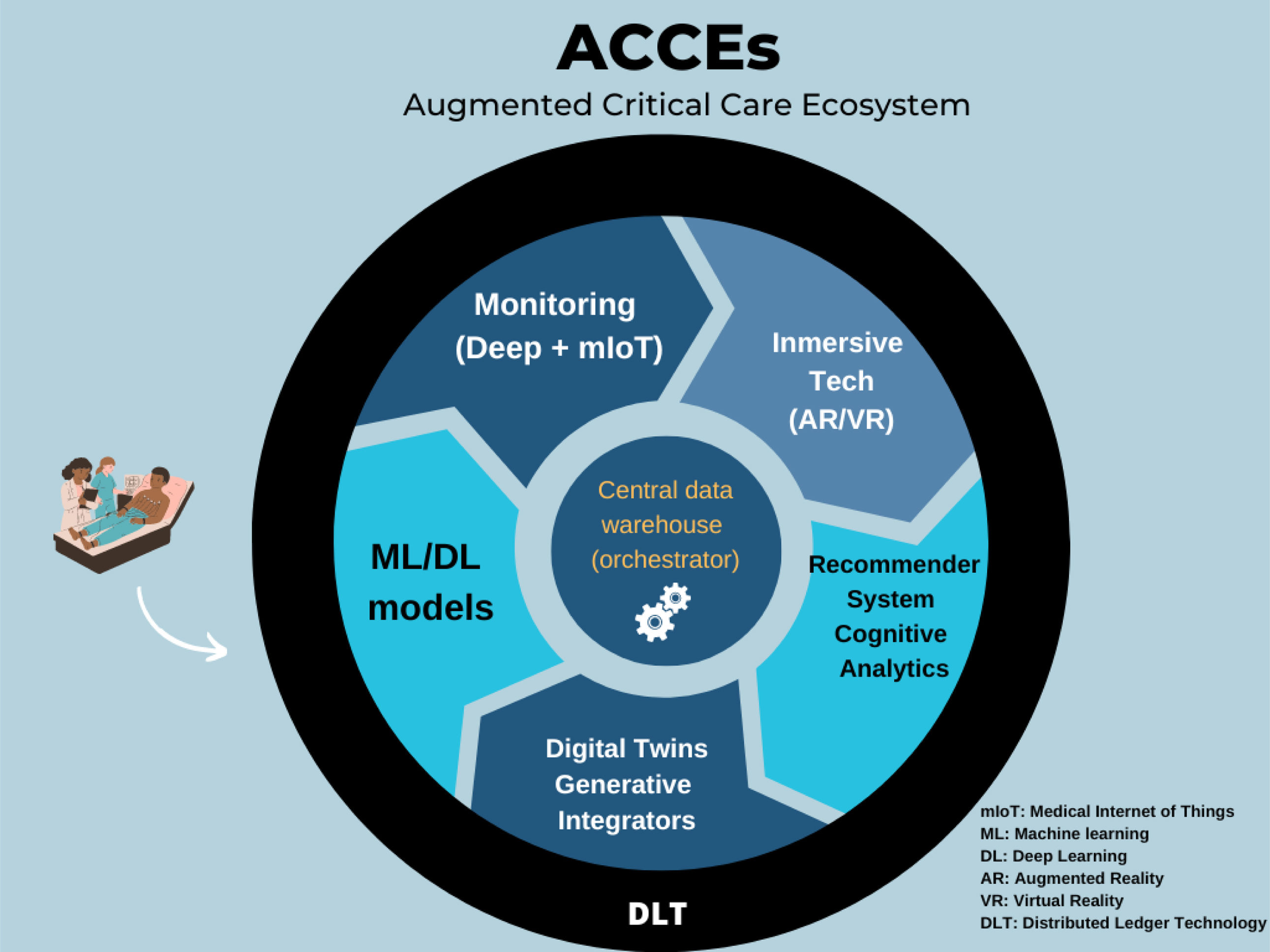
Augmented Critical Care Ecosystems (ACCEs)The progressive transformation of the ICU concept into Augmented Critical Care Ecosystems (ACCEs) is proposed. This requires an enveloping healthcare model that allows for the integration of machine learning models that optimize critical care team decisions with other technologies, such as IoT-connected monitoring and adaptive systems. Similarly, with the arrival of generative models, they will have to be integrated as elements that facilitate data capture and optimize the output flow for intelligent recommendation systems. Fig. 2 shows a critical patient's basic journey, the augmented intelligence's clinical operations integrated into the workflow, and some support technologies for creating the enveloping model in the ecosystem that would transform the concept into ACCEs.
To successfully implement AI into clinical practice in the future, collaborative efforts with plans for digital health competencies, data standardization and sharing, advanced model development to ascertain data security, real-time application, and quality control are required.3 This will be the basis for ACCEs. However, the process will be incremental, through the summative implementation of capabilities, initially with the hosting of high-quality AI models and deep monitoring systems, these data will be hosted in a standardized data warehouse that functions as an orchestrator of algorithms that, in turn, feed critical care recommendation systems, and finally, exponential capabilities such as digital twins, immersive technologies such as extended reality, and ultimately, the entire ecosystem will be connected in an adaptive control tower that closes the ecosystem, ideally with distributed ledger technologies to ensure the highest levels of security and anonymization of data and transactions. The future of the intensive care unit is a fundamental transformation to an enveloping model.
CRediT authorship contribution statementAll authors have contributed equally to the design of the intellectual content and have approved the manuscript.
FundingNone declared.
Conflict of interestThe authors have no conflict of interest to declare.