Coronavirus disease 2019 (COVID-19) is the most critical issue in nowadays medicine. We aimed to evaluate the use and therapeutic outcomes of oseltamivir, an antiviral drug for patients with COVID-19.
Materials and methodIn an observational study conducted at Imam Khomeini Hospital in Amol, Iran, data for 544 patients with laboratory and CT scan result confirmed COVID-19 were retrospectively collected between February 24th and April 13th 2020. To compare the characteristics of patients based on gender, the chi-square test was used. Logistic regression was used to evaluate the effect of oseltamivir on the outcome of treatment. Logrank test were used to compare the length of hospital stay in people treated with oseltamivir and drugs other than oseltamivir.
ResultsKaplan–Meier and logrank test showed no significant reduction in hospitalization time and survival rate following treatment with oseltamivir. However, a significant increase in lymphocytes count and reduction of C-reactive protein (CRP) level detected.
ConclusionAdministration of oseltamivir for patients with COVID-19 didn't show any improvement in hospitalization duration and survival rate.
la enfermedad por coronavirus 2019 (COVID-19) es el tema más crítico en la medicina actual. Nuestro objetivo fue evaluar el uso y los resultados terapéuticos de oseltamivir, un medicamento antiviral para pacientes con COVID-19.
Materiales y métodoen un estudio observacional realizado en el Hospital Imam Khomeini en Amol, Irán, los datos de 544 pacientes con resultados de laboratorio y tomografía computarizada confirmados de COVID-19 se recopilaron retrospectivamente entre el 24 de febrero y el 13 de abril de 2020. Para comparar las características de los pacientes en función del género se utilizó la prueba de chi-cuadrado. Se utilizó regresión logística para evaluar el efecto de oseltamivir en el resultado del tratamiento. Se utilizó la prueba de rango logarítmico para comparar la duración de la estancia hospitalaria en personas tratadas con oseltamivir y otros fármacos distintos del oseltamivir.
ResultadosKaplan–Meier y la prueba de rango logarítmico no mostraron una reducción significativa en el tiempo de hospitalización y la tasa de supervivencia después del tratamiento con oseltamivir. Sin embargo, se detectó un aumento significativo en el recuento de linfocitos y una reducción del nivel de proteína C reactiva (PCR).
Conclusiónla administración de oseltamivir para pacientes con COVID-19 no mostró ninguna mejora en la duración de la hospitalización y la tasa de supervivencia.
Corona virus disease 2019 (COVID -19) is the most challenging pandemic diseases in the century 21.1 COVID-19 was diagnosed in Wuhan, China in December 2019, and then spread out in all world during some months.2 To date, several millions people have been confirmed with positive COVID-19 in worldwide.3 Furthermore, a large numbers of people have been died because of severe side effects associated with COVID-19.4 Epidemiological studies suggest that most people with COVID-19 may show mild or moderate symptoms.5,6 However, side effects with this disease can be killer in some patients, especially aged patients and patients with underlying medical conditions like patients with kidney diseases and hypertension, diabetes, and also patients that suffer from cardiac and lung diseases.7–11 Mortality rate for patients with COVID-19 is very different dependent on sex, age, and underlying diseases. It has been reported that COVID-19 may cause death in 0.03 patients without underlying health conditions and 0.12 patients with underlying health conditions. Furthermore, mortality rate has a direct relation with age.12–14
Iran was one of the first countries that confirmed outbreak of COVID-19.6,15 The numbers of infected people with COVID-19 are growing and now approved vaccine are available for control of this pandemic.16–18 Furthermore, there is no certain cure for COVID-19. Scientists in worldwide trying to reduce mortality of this disease via alleviation of its side effects and also recommending some antiviral drugs. Oseltamivir (phosphate) is one of common antiviral drugs that is prescribed for patients with flu.19 Oseltamivir is able to attack the flu virus directly.20 It inactivates neuraminidase enzyme, a critical enzyme which is expressed virus and facilitate its migration.21,22 By inhibition of this pathway, oseltamivir can prevent infection of new cells by flu virus and also alleviate symptoms associated with type A and B flu viruses.23
According to the Iranian national guidelines, oseltamivir was part of the treatment protocol for patients with COVID-19, which was subsequently removed from the national protocol. In the current retrospective cohort study, we assessed the possible effect of oseltamivir for patients with positive COVID-19. This is one of the few studies that investigated the effect of oseltamivir for COVID-19 patients. For detection of lung pneumonitis, we used computed tomography (CT) scan. As reported by previous study, CT scan has a high sensitivity for detection of lung changes and its sensitivity may be higher than real-time polymerase chain reaction (RT-PCR) method.24,25 CT scan has a higher sensitivity for the detection of COVID-19, however, some clinical evaluations suggest that it has a lower specificity compared to RT-PCR.26 Recently, some studies have shown that CT scan can be used to detect infection and also severity of pneumonia in patients with COVID-19.27–30 A high resolution of CT scan can help to find and evaluate the condition of patients with COVID-19.31–33
MethodsEthics approvalThe study was reviewed by the ethics committee of Mazandaran University of Medical Sciences and was approved with the code of IR.MAZUMS.REC.1399.074.
Study settingThis retrospective cohort study was performed at Imam Khomeini Hospital in Amol, Iran. This hospital is one of the few centers for the treatment of COVID-19 in northern Iran (Mazandaran province). The medical records of patients were retrospectively collected between February 24th and April 13th 2020.
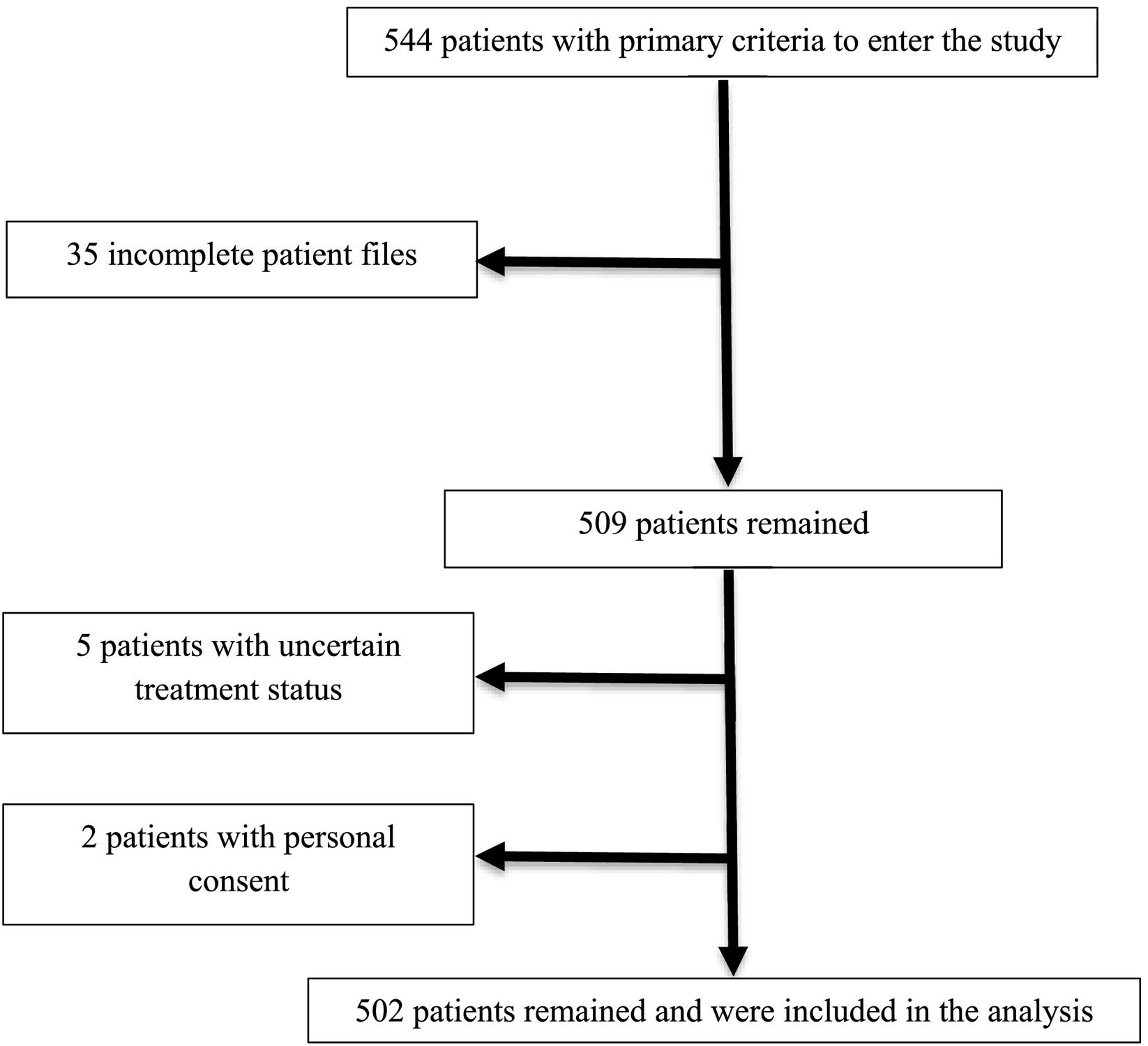
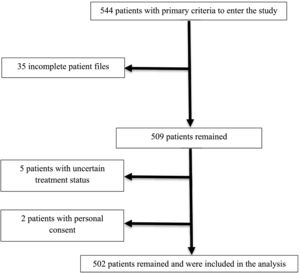
ParticipantsThe main criterion for entering the study for all people of different ages was referring to Imam Khomeini Hospital in Amol city and proving their COVID-19 with CT scan or Reverse Transcription Polymerase Chain Reaction (RT-PCR) assay of nasopharyngeal swab specimens. The participants had not received the COVID-19 vaccines. Completing the course of treatment and determining the individual's disease status were other criteria for entering the study. Personal satisfaction and discharge from the hospital and the incompleteness of the patient's file and not knowing the individual's disease status were also exclusion criteria. Sampling was done by non-probability method and Convenience. Data were collected for 544 patients during the study period (Fig. 1). No patient received vaccination before infection with COVID-19.
Data collectionDemographic, epidemiological, laboratory, pharmacological information, vital and clinical signs, length of hospital stay, and outcome of treatment (in the form of recovery, partial recovery, and death) were obtained using a standardized checklist from patients' files. Complete relief of symptoms including cough, fever and chills, shortness of breath, etc. was considered as recovery and partial improvement of these symptoms was considered as partial recovery. Data obtained were reviewed by statisticians, pharmacists, hematologists, radiologists and nurses, and then the discrepancies in the files were resolved in consultation with infectious disease specialists. Underlying health conditions, common medications, smoking history, patient addiction, and hospitalization history for any reason were also obtained using self-reporting.
Statistical analysisTo describe the data, Mean ± SD was used for quantitative variables and frequency tables were used for qualitative variables. To compare the characteristics of patients based on gender, the chi-square test was used. Logistic regression with controlling the intervening variables was used to evaluate the effect of oseltamivir on the outcome of treatment. Kaplan–Meier and logrank test were used to compare the length of hospital stay in people treated with oseltamivir and drugs other than oseltamivir. SPSS software version 25 was used for analysis and the significance level was considered 0.05.
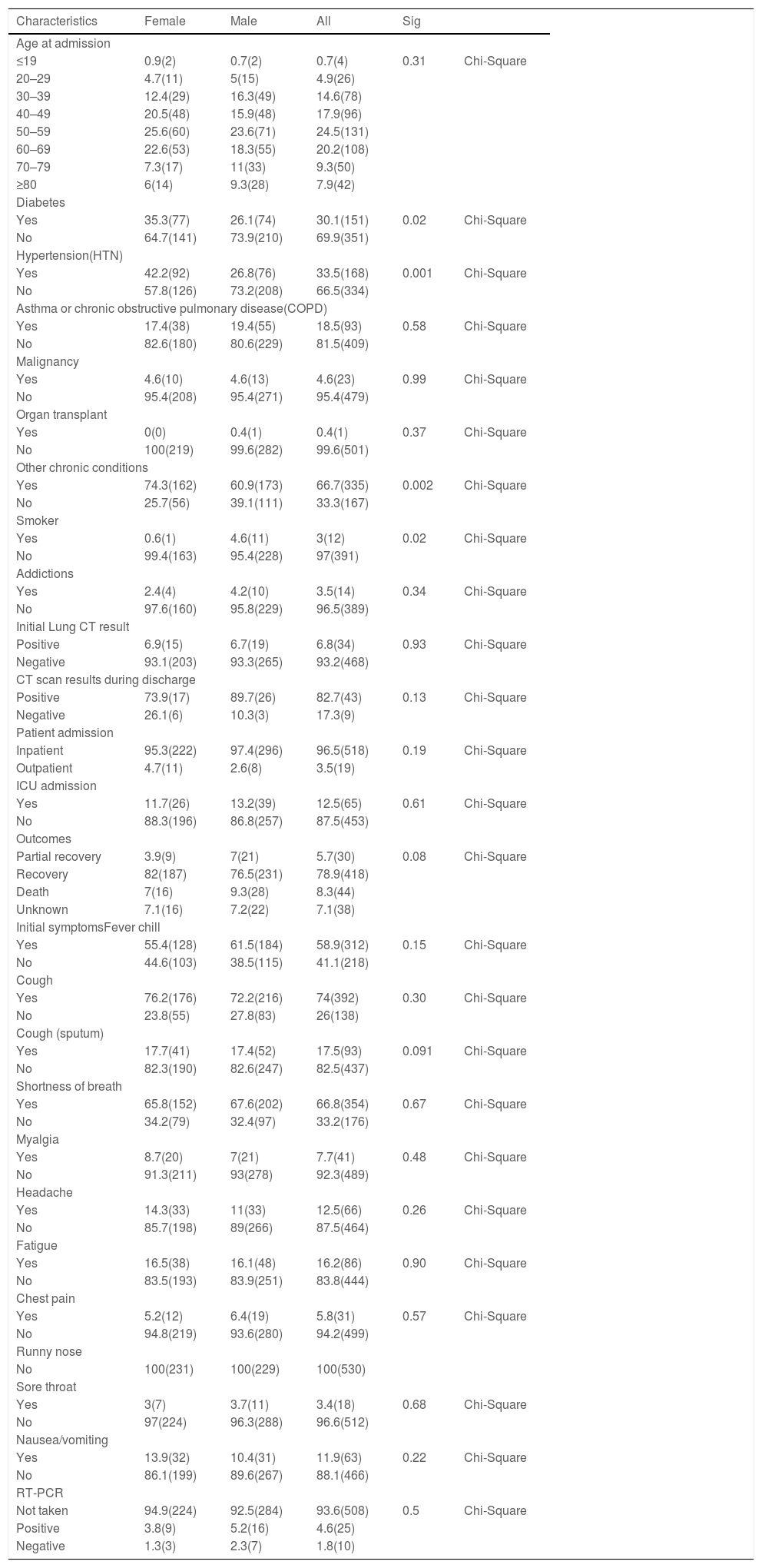
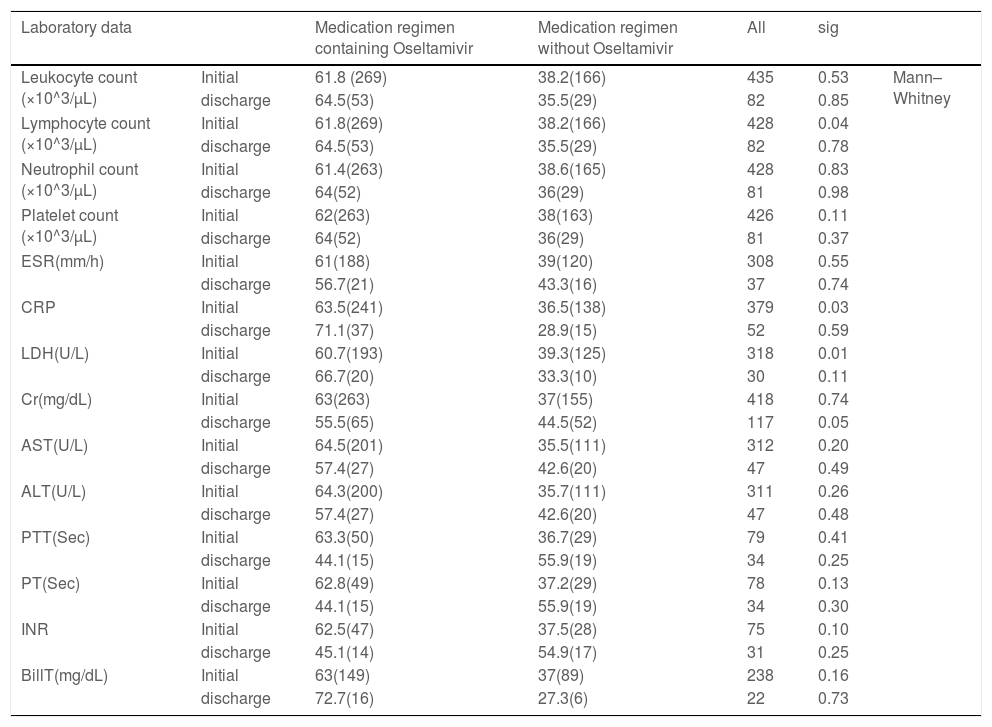
ResultsThe Chi-square test was used to compare the patient's characteristics (risk factors, symptoms, and demographic characteristics) by gender. As we showed in the Table 1, approximately 30% of patients were diabetic, which was higher in women (P value = 0.02). In the case of blood pressure, 33.5% of patients had hypertension, which was 42% in women (P value = 0.01). In other chronic diseases, it was more common in women (P value = 0.001). Smoking in men was more than women ((P value = 0.02). In other cases, as shown in the Table 1 no significant difference was observed between men and women. The medication regimen of most patients contained hydroxychloroquine, azithromycin, and lopinavir/ ritonavir. The Chi-square test was used to compare the group receiving oseltamivir or not receive oseltamivir. No significant difference was observed between the two groups in terms of treatment (recovery, partial recovery, and death) and CT scan results. Also, no significant difference was observed in laboratory data except for lymphocytes and CRP level, which a significant increase in lymphocytes count and reduction of C-reactive protein (CRP) level detected (Table 2).
Characteristics of patients with Covid-19 based on gender. Values are expressed as percentages (numbers).
| Characteristics | Female | Male | All | Sig | |
|---|---|---|---|---|---|
| Age at admission | |||||
| ≤19 | 0.9(2) | 0.7(2) | 0.7(4) | 0.31 | Chi-Square |
| 20–29 | 4.7(11) | 5(15) | 4.9(26) | ||
| 30–39 | 12.4(29) | 16.3(49) | 14.6(78) | ||
| 40–49 | 20.5(48) | 15.9(48) | 17.9(96) | ||
| 50–59 | 25.6(60) | 23.6(71) | 24.5(131) | ||
| 60–69 | 22.6(53) | 18.3(55) | 20.2(108) | ||
| 70–79 | 7.3(17) | 11(33) | 9.3(50) | ||
| ≥80 | 6(14) | 9.3(28) | 7.9(42) | ||
| Diabetes | |||||
| Yes | 35.3(77) | 26.1(74) | 30.1(151) | 0.02 | Chi-Square |
| No | 64.7(141) | 73.9(210) | 69.9(351) | ||
| Hypertension(HTN) | |||||
| Yes | 42.2(92) | 26.8(76) | 33.5(168) | 0.001 | Chi-Square |
| No | 57.8(126) | 73.2(208) | 66.5(334) | ||
| Asthma or chronic obstructive pulmonary disease(COPD) | |||||
| Yes | 17.4(38) | 19.4(55) | 18.5(93) | 0.58 | Chi-Square |
| No | 82.6(180) | 80.6(229) | 81.5(409) | ||
| Malignancy | |||||
| Yes | 4.6(10) | 4.6(13) | 4.6(23) | 0.99 | Chi-Square |
| No | 95.4(208) | 95.4(271) | 95.4(479) | ||
| Organ transplant | |||||
| Yes | 0(0) | 0.4(1) | 0.4(1) | 0.37 | Chi-Square |
| No | 100(219) | 99.6(282) | 99.6(501) | ||
| Other chronic conditions | |||||
| Yes | 74.3(162) | 60.9(173) | 66.7(335) | 0.002 | Chi-Square |
| No | 25.7(56) | 39.1(111) | 33.3(167) | ||
| Smoker | |||||
| Yes | 0.6(1) | 4.6(11) | 3(12) | 0.02 | Chi-Square |
| No | 99.4(163) | 95.4(228) | 97(391) | ||
| Addictions | |||||
| Yes | 2.4(4) | 4.2(10) | 3.5(14) | 0.34 | Chi-Square |
| No | 97.6(160) | 95.8(229) | 96.5(389) | ||
| Initial Lung CT result | |||||
| Positive | 6.9(15) | 6.7(19) | 6.8(34) | 0.93 | Chi-Square |
| Negative | 93.1(203) | 93.3(265) | 93.2(468) | ||
| CT scan results during discharge | |||||
| Positive | 73.9(17) | 89.7(26) | 82.7(43) | 0.13 | Chi-Square |
| Negative | 26.1(6) | 10.3(3) | 17.3(9) | ||
| Patient admission | |||||
| Inpatient | 95.3(222) | 97.4(296) | 96.5(518) | 0.19 | Chi-Square |
| Outpatient | 4.7(11) | 2.6(8) | 3.5(19) | ||
| ICU admission | |||||
| Yes | 11.7(26) | 13.2(39) | 12.5(65) | 0.61 | Chi-Square |
| No | 88.3(196) | 86.8(257) | 87.5(453) | ||
| Outcomes | |||||
| Partial recovery | 3.9(9) | 7(21) | 5.7(30) | 0.08 | Chi-Square |
| Recovery | 82(187) | 76.5(231) | 78.9(418) | ||
| Death | 7(16) | 9.3(28) | 8.3(44) | ||
| Unknown | 7.1(16) | 7.2(22) | 7.1(38) | ||
| Initial symptomsFever chill | |||||
| Yes | 55.4(128) | 61.5(184) | 58.9(312) | 0.15 | Chi-Square |
| No | 44.6(103) | 38.5(115) | 41.1(218) | ||
| Cough | |||||
| Yes | 76.2(176) | 72.2(216) | 74(392) | 0.30 | Chi-Square |
| No | 23.8(55) | 27.8(83) | 26(138) | ||
| Cough (sputum) | |||||
| Yes | 17.7(41) | 17.4(52) | 17.5(93) | 0.091 | Chi-Square |
| No | 82.3(190) | 82.6(247) | 82.5(437) | ||
| Shortness of breath | |||||
| Yes | 65.8(152) | 67.6(202) | 66.8(354) | 0.67 | Chi-Square |
| No | 34.2(79) | 32.4(97) | 33.2(176) | ||
| Myalgia | |||||
| Yes | 8.7(20) | 7(21) | 7.7(41) | 0.48 | Chi-Square |
| No | 91.3(211) | 93(278) | 92.3(489) | ||
| Headache | |||||
| Yes | 14.3(33) | 11(33) | 12.5(66) | 0.26 | Chi-Square |
| No | 85.7(198) | 89(266) | 87.5(464) | ||
| Fatigue | |||||
| Yes | 16.5(38) | 16.1(48) | 16.2(86) | 0.90 | Chi-Square |
| No | 83.5(193) | 83.9(251) | 83.8(444) | ||
| Chest pain | |||||
| Yes | 5.2(12) | 6.4(19) | 5.8(31) | 0.57 | Chi-Square |
| No | 94.8(219) | 93.6(280) | 94.2(499) | ||
| Runny nose | |||||
| No | 100(231) | 100(229) | 100(530) | ||
| Sore throat | |||||
| Yes | 3(7) | 3.7(11) | 3.4(18) | 0.68 | Chi-Square |
| No | 97(224) | 96.3(288) | 96.6(512) | ||
| Nausea/vomiting | |||||
| Yes | 13.9(32) | 10.4(31) | 11.9(63) | 0.22 | Chi-Square |
| No | 86.1(199) | 89.6(267) | 88.1(466) | ||
| RT-PCR | |||||
| Not taken | 94.9(224) | 92.5(284) | 93.6(508) | 0.5 | Chi-Square |
| Positive | 3.8(9) | 5.2(16) | 4.6(25) | ||
| Negative | 1.3(3) | 2.3(7) | 1.8(10) | ||
Laboratory data of COVID-19 patients with and without oseltamivir medication regimen. Values are expressed as median rank (mean rank).
| Laboratory data | Medication regimen containing Oseltamivir | Medication regimen without Oseltamivir | All | sig | ||
|---|---|---|---|---|---|---|
| Leukocyte count (×10^3/μL) | Initial | 61.8 (269) | 38.2(166) | 435 | 0.53 | Mann–Whitney |
| discharge | 64.5(53) | 35.5(29) | 82 | 0.85 | ||
| Lymphocyte count (×10^3/μL) | Initial | 61.8(269) | 38.2(166) | 428 | 0.04 | |
| discharge | 64.5(53) | 35.5(29) | 82 | 0.78 | ||
| Neutrophil count (×10^3/μL) | Initial | 61.4(263) | 38.6(165) | 428 | 0.83 | |
| discharge | 64(52) | 36(29) | 81 | 0.98 | ||
| Platelet count (×10^3/μL) | Initial | 62(263) | 38(163) | 426 | 0.11 | |
| discharge | 64(52) | 36(29) | 81 | 0.37 | ||
| ESR(mm/h) | Initial | 61(188) | 39(120) | 308 | 0.55 | |
| discharge | 56.7(21) | 43.3(16) | 37 | 0.74 | ||
| CRP | Initial | 63.5(241) | 36.5(138) | 379 | 0.03 | |
| discharge | 71.1(37) | 28.9(15) | 52 | 0.59 | ||
| LDH(U/L) | Initial | 60.7(193) | 39.3(125) | 318 | 0.01 | |
| discharge | 66.7(20) | 33.3(10) | 30 | 0.11 | ||
| Cr(mg/dL) | Initial | 63(263) | 37(155) | 418 | 0.74 | |
| discharge | 55.5(65) | 44.5(52) | 117 | 0.05 | ||
| AST(U/L) | Initial | 64.5(201) | 35.5(111) | 312 | 0.20 | |
| discharge | 57.4(27) | 42.6(20) | 47 | 0.49 | ||
| ALT(U/L) | Initial | 64.3(200) | 35.7(111) | 311 | 0.26 | |
| discharge | 57.4(27) | 42.6(20) | 47 | 0.48 | ||
| PTT(Sec) | Initial | 63.3(50) | 36.7(29) | 79 | 0.41 | |
| discharge | 44.1(15) | 55.9(19) | 34 | 0.25 | ||
| PT(Sec) | Initial | 62.8(49) | 37.2(29) | 78 | 0.13 | |
| discharge | 44.1(15) | 55.9(19) | 34 | 0.30 | ||
| INR | Initial | 62.5(47) | 37.5(28) | 75 | 0.10 | |
| discharge | 45.1(14) | 54.9(17) | 31 | 0.25 | ||
| BillT(mg/dL) | Initial | 63(149) | 37(89) | 238 | 0.16 | |
| discharge | 72.7(16) | 27.3(6) | 22 | 0.73 | ||
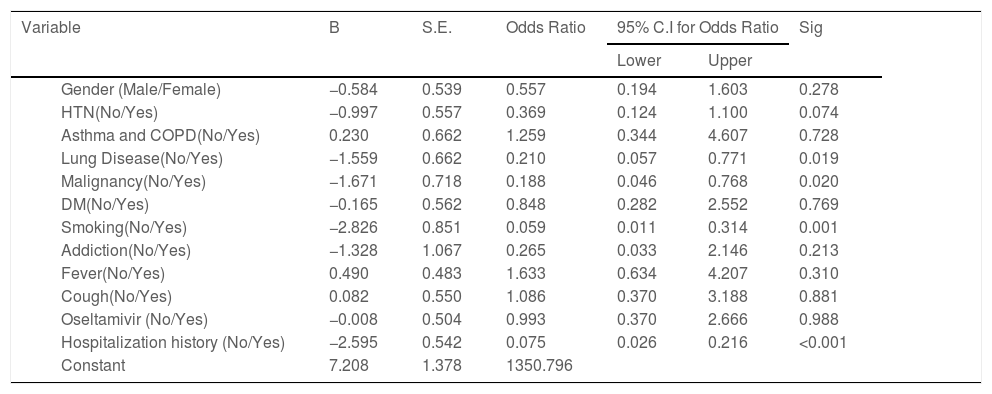
Finally, to show the effects of risk factors and demographic characteristics on the outcome (survival or death) of treatment, univariate logistic regression was used. The results are shown in the Table 3 and Fig. 2. This step of the analysis was performed to identify effective variables that have significant potential for entering multivariate logistic regression. Variables with a significance level of less than 0.3 were selected for the final analysis. According to the Table 3, the effect of oseltamivir was not significant with respect to the p-value (p = 0.99). Therefore, it did not enter the multivariate model. Smoking, lung disease, malignancy, hypertension, and patient gender were included in the multivariate model and the model was implemented as follows.
Determining effect of oseltamivir and other factors on the treatment of COVID-19 patients using univariate logistic regression.
| Variable | B | S.E. | Odds Ratio | 95% C.I for Odds Ratio | Sig | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gender (Male/Female) | −0.584 | 0.539 | 0.557 | 0.194 | 1.603 | 0.278 | |
| HTN(No/Yes) | −0.997 | 0.557 | 0.369 | 0.124 | 1.100 | 0.074 | |
| Asthma and COPD(No/Yes) | 0.230 | 0.662 | 1.259 | 0.344 | 4.607 | 0.728 | |
| Lung Disease(No/Yes) | −1.559 | 0.662 | 0.210 | 0.057 | 0.771 | 0.019 | |
| Malignancy(No/Yes) | −1.671 | 0.718 | 0.188 | 0.046 | 0.768 | 0.020 | |
| DM(No/Yes) | −0.165 | 0.562 | 0.848 | 0.282 | 2.552 | 0.769 | |
| Smoking(No/Yes) | −2.826 | 0.851 | 0.059 | 0.011 | 0.314 | 0.001 | |
| Addiction(No/Yes) | −1.328 | 1.067 | 0.265 | 0.033 | 2.146 | 0.213 | |
| Fever(No/Yes) | 0.490 | 0.483 | 1.633 | 0.634 | 4.207 | 0.310 | |
| Cough(No/Yes) | 0.082 | 0.550 | 1.086 | 0.370 | 3.188 | 0.881 | |
| Oseltamivir (No/Yes) | −0.008 | 0.504 | 0.993 | 0.370 | 2.666 | 0.988 | |
| Hospitalization history (No/Yes) | −2.595 | 0.542 | 0.075 | 0.026 | 0.216 | <0.001 | |
| Constant | 7.208 | 1.378 | 1350.796 | ||||
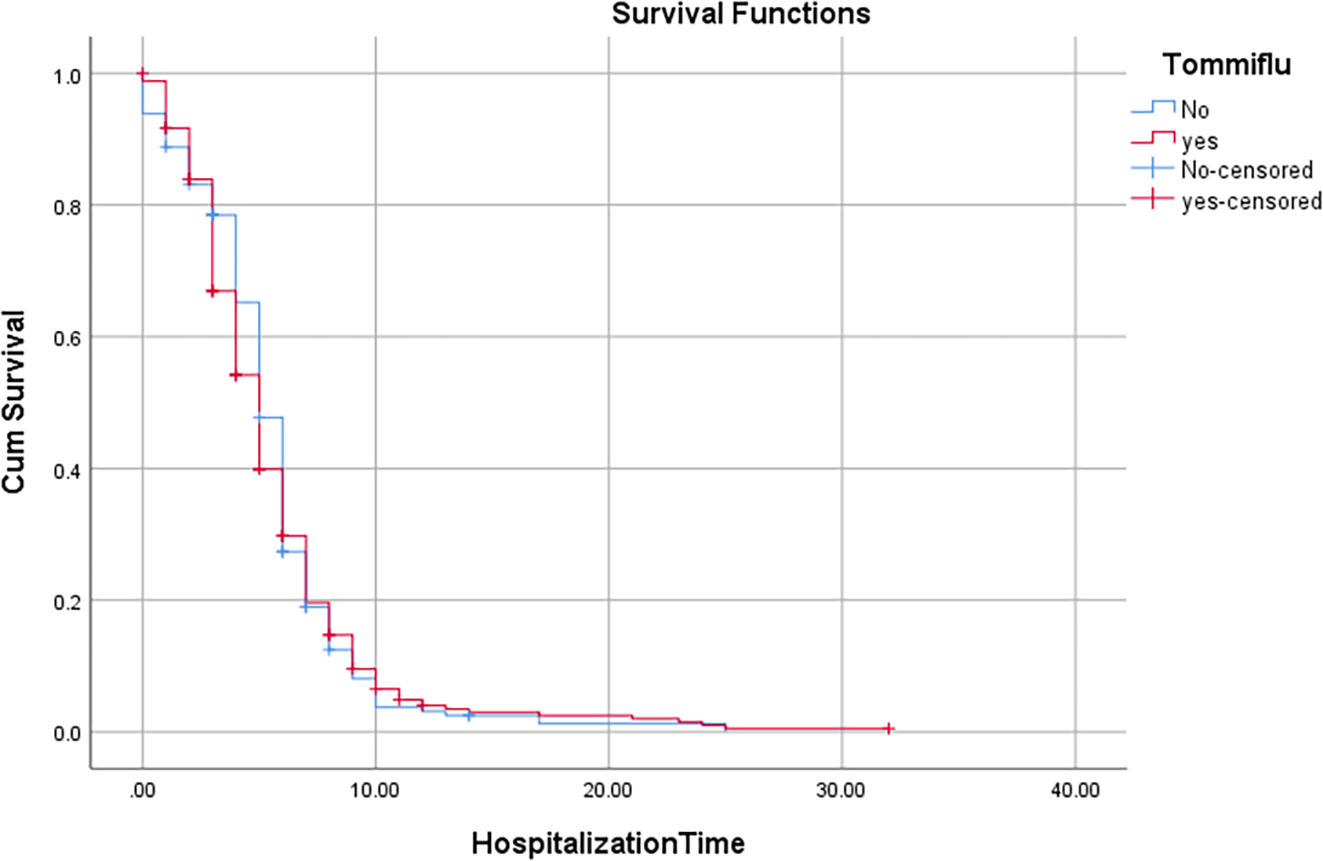
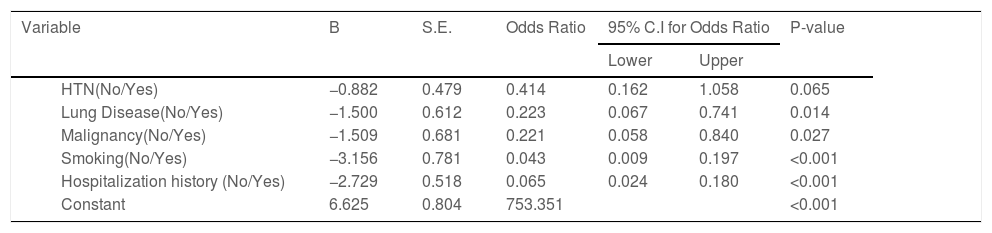
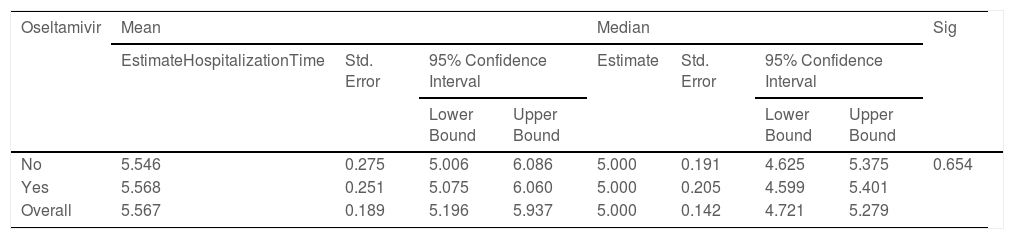
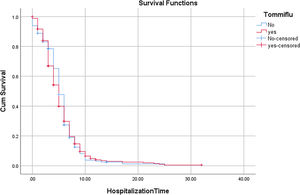
According to the variables of Table 4, the patients with lung disease have a 4.48 (1.35, 14.94) higher rate of death than those without the disease (P = 0.014). Moreover, the death rate in patients with malignancy is higher than patients without malignancy (P = 0.027). Patients with cigarette history in the hospital were 23.26 (5.07, 111.11) more likely to die than those without a history of smoking (P < 0.001). Patients with a history of hospitalization for any reason have a 15.38 (5.56, 41.67) higher rate of death than those without a history of hospitalization (P < 0.001). Kaplan-Meyer method was used to compare the duration of treatment in patients receiving oseltamivir versus those who did not receive it, and the mean hospital stay in patients receiving oseltamivir was 5.57 (5.20,5.94) days. The group that did not receive was 5.55 (5.01, 6.09) days, which was compared using the logrank test and no significant difference was observed (P = 0.654) (Table 5).
Determining effect of different factors on the treatment of COVID-19 patients using multivariate logistic regression.
| Variable | B | S.E. | Odds Ratio | 95% C.I for Odds Ratio | P-value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| HTN(No/Yes) | −0.882 | 0.479 | 0.414 | 0.162 | 1.058 | 0.065 | |
| Lung Disease(No/Yes) | −1.500 | 0.612 | 0.223 | 0.067 | 0.741 | 0.014 | |
| Malignancy(No/Yes) | −1.509 | 0.681 | 0.221 | 0.058 | 0.840 | 0.027 | |
| Smoking(No/Yes) | −3.156 | 0.781 | 0.043 | 0.009 | 0.197 | <0.001 | |
| Hospitalization history (No/Yes) | −2.729 | 0.518 | 0.065 | 0.024 | 0.180 | <0.001 | |
| Constant | 6.625 | 0.804 | 753.351 | <0.001 | |||
Comparison of hospitalization time in the COVID-19 patients with and without oseltamivir medication regimen using logrank test.
| Oseltamivir | Mean | Median | Sig | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EstimateHospitalizationTime | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | ||||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||||
| No | 5.546 | 0.275 | 5.006 | 6.086 | 5.000 | 0.191 | 4.625 | 5.375 | 0.654 |
| Yes | 5.568 | 0.251 | 5.075 | 6.060 | 5.000 | 0.205 | 4.599 | 5.401 | |
| Overall | 5.567 | 0.189 | 5.196 | 5.937 | 5.000 | 0.142 | 4.721 | 5.279 | |
This study was one of the few retrospective cohort studies that investigated the effect of oseltamivir prescription for patients with COVID-19. To date, a large numbers of antiviral drugs has been tested to find some positive results against COVID-19 infection and also its consequences such as pneumonitis.34–38 A computational study suggested that oseltamivir may be useful for fighting against SARS-CoV-2.39 Furthermore, a case study suggested that oseltamivir may be safe for patients with COVID-19. However, hydroxychloroquine another famous drug during COVID-19 may lead to serious side effects.40–42 Experimental and clinical studies are ongoing to determine the possible beneficial effects of various types of drugs such as antiviral, anti-inflammatory and also natural drugs to reduce time of hospitalization, alleviate side effects and improve survival rate.43,44
In the current clinical study, we aimed to evaluate possible positive effects of oseltamivir for COVID-19 patients. Patients with different sex and age included within study. Other possible interventions such as underlying medical conditions, smoking, lung diseases and malignancy considered and some endpoints such as CT scan of lung, blood test, fever and other patient's conditions were checked. Our investigations showed that patients with malignancy or respiratory diseases have higher risk for death. Furthermore, smoking led to more morality in COVID-19 patients than those without a history of smoking. A history of hospitalization has also caused a similar result. For evaluating the effect of administration of oseltamivir on survival rate and hospitalization time duration we used Kaplan-Meyer analyses test. Results indicated that treatment with oseltamivir can't reduce the duration time of hospitalization. Furthermore, no increase in survival observed following treatment with oseltamivir. Multivariable logistic regression analyses confirmed that oseltamivir had no improvement in recovery or survival. Multivariable logistic regression analyses also indicated that hospitalization, smoking, malignancy and hypertension are associated with more mortality in patients with COVID-19.
Blood analyses test showed that oseltamivir can't change most of parameters. However, CRP level reduced at the first stage of oseltamivir administration and lymphocytes count increased. CRP is a critical parameter for infection and inflammatory disorders and it has used as a marker for early detection of infection with SARS-CoV-2. It has reported that the level of CRP has a direct correlation with severity of COVID-19 symptoms.45 CRP level can reflect the severity of pneumonitis in patients with COVID-19.46 Logistic regression analyses showed that treatment with oseltamivir can be effective for patients with lung disease. This result may indicate that oseltamivir may be useful for alleviation of lung pneumonitis. One of the limitations of this study was the non-confirmation of COVID-19 in all patients because at the beginning of the pandemic, molecular testing was not possible for all patients and the diagnóstico of COVID-19 in most cases was based on CT scan findings and laboratory data.
To date, some other studies have evaluated the efficiency and the safety of oseltamivir for COVID-19 patients. A study suggested that oseltamivir isn't suitable for patients with COVID-19. This study compared the structure of proteins in COVID-19 and influenza. Furthermore, they analyzed molecular docking for binding of oseltamivir with key proteins in these viruses. The results suggested that oseltamivir can't bind to active site of COVID-19. The results also suggested that oseltamivir isn't effective against COVID-19 and can't improve the patients' symptoms.47 Results of this study were agree with our results in the current study. By contrast, another study suggested that oseltamivir can be recommended to improve symptoms and survival rate following infection with COVID-19. This study compared the efficacy of two different regimens including Azithromycin and Hydroxychloroquine or oseltamivir for patients with COVID-19. Results of this study suggested that administration of oseltamivir is more effective for the treatment of COVID-19. Patients that received oseltamivir showed a shorter hospitalization time and faster recovery compared to patients that received Azithromycin and Hydroxychloroquine. Furthermore, patients that received oseltamivir showed a lower mortality.48
ConclusionOseltamivir administration for COVID-19 patients didn't cause any increase in survival rate during 1 month following starting treatment. Furthermore, no improvement in treatment duration and hospitalization time observed following treatment with oseltamivir. However, oseltamivir may be useful for reduction of pneumonitis via reduction of CRP, and it may boost immune system via an increase in lymphocytes count.
This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran.