The culmination of the process of creating the Institutes of Legal Medicine (IML) with the commissioning of the IML of Madrid in 2020 homogenizes the competences of forensic medicine throughout the country. Recent legislative reforms in specialized medical training, expand their responsibilities to cover, in addition to the expert function, a stronger role in teaching and research. The design and implementation of quality systems must become a priority for IMLs in order to guarantee their effectiveness and efficiency by providing accurate, reliable and timely results. This article provides a detailed review of the procedure to be followed to design a quality strategy in Forensic Pathology Services.
La culminación del proceso de creación de los Institutos de Medicina Legal (IML) con la puesta en funcionamiento al IML de Madrid en 2020, homogeniza las funciones de la medicina forense en todo el territorio nacional. Recientes reformas legislativas en materia de formación médica especializada amplían sus competencias para abarcar, además de la función pericial, responsabilidades en materia docente y de investigación. El diseño e implementación de sistemas de calidad, debe convertirse en una prioridad de los IML, con el objetivo de garantizar su eficacia y eficiencia ofreciendo resultados exactos, fiables y en los plazos apropiados. El presente artículo ofrece una revisión detallada del procedimiento a seguir para diseñar una estrategia de calidad en los Servicios de Patología Forense
Forensic medicine in general, and forensic pathology in particular, have a core purpose within the framework of judicial proceedings: to supply medical knowledge applied to the specific case in question so that the judge can base his decisions on them. Any error in any of the processes involved in forensic pathology, which run from the death of an individual until the issue of a report, as well as the collection of clues or samples, or the chain of custody, may lead to a sentence that is based on facts which do not fit with the scientific evidence, which would be enormously serious.1 Guaranteeing optimum levels of quality will lead to better working of the Administration of Justice and therefore an unquestionable benefit for society as a whole.
Accreditation was defined in the ISO/IEC guide 2:1996 norms, revised by the ISO/IEC GUIDE 2:2004, as well as by a “procedure by which a service, institution or person is formally recognised to be competent for carrying out specific tasks”. Developing an appropriate framework of quality standards within the field of forensic pathology is still a question that is pending, and this requires a solution without delay. This element is indispensable to cover the needs of the justice system and offer the population the trust that the forensic science used in judicial investigations is robust and fit for purpose.2
However, the benefits of guaranteeing the quality of forensic medicine working within the context of the Institutes of Legal Medicine (IML) goes beyond the purely juridical. As its areas of competence are extended to cover the world of training and investigation, its responsibilities also increase, with the potential to affect health and the advance of science. Now that the process of setting up the IMLs at national level terminated when the Madrid IML started working in February 2020, urgent progress has to be made in both areas, for which purpose quality management is an indisputably valuable instrument.
The gradual fall in the number of clinical autopsies performed in hospitals means that cadaveric studies have been restricted almost exclusively to those carried out in forensic pathology departments. To the degree that quality guarantee systems exist which enable them to combine their function of working for the Administration of Justice with the other functions described in the regulations for IML, they may become referral centres for the initial and on-going training of other specialists, as well as for the advancement of knowledge.
The previous point is especially important in those IML which perform a high number of autopsies and a broad range of studies. In the Madrid IML, for example, which is now located in a new facility with state-of-the-art installations, more than 2,000 autopsies per year are carried out, of which more than 60% are found to be natural deaths without penal relevance.
The natural deaths which are eventually involved in judicial proceedings are usually sudden or unexpected, so that they are classified as “with the suspicion of criminality”. When a death is due to a disease that was unknown during life, and which is also hereditary, it is enormously important for the family members to be able to request early diagnosis and thereby prevent other similar outcomes. Forensic medicine therefore ceases to have a role that is restricted to deaths that can be attributed to third parties, and now plays a clear supporting role for medical care, making it possible to diagnose diseases which although not legally relevant are vitally important for those who suffer them. The performance of this function makes it necessary to design quality systems which guarantee appropriate ethical standards as well as the functioning and traceability of all of the processes involved.
Respecting the teaching function, in 2020 RD 704/2020 of 28 July was published. This established internship as the system giving access to the qualification of Forensic and Legal Medicine doctor/specialist.3 Article 4 of this Royal Decree offers IML the possibility of requesting accreditation as a teaching unit for specialized training. Responding to this challenge involves fulfilling the quality requisites that Ministry of Health demands to grant the necessary recognition through the Dirección General de Ordenación Profesional of the Ministry of Health.
When implementing the strategic plans of the IML no effort should be spared when designing and implementing a quality plan that makes it possible to work with guarantees that cover the responsibilities arising from modern forensic medicine, with the Administration of Justice as well as with research and teaching, and eventually with society as a whole.
Objectives and methodologyThis work consists of a bibliographic review of the methodology used to implement a quality management system in the Forensic Pathology Departments of the IML.
Theoretical frameworkGeneral quality aspectsAlthough from a historical point of view the concern about the quality of medical care is not recent, it started to develop after the industrial revolution in the 19th century. Above all it was in the 20th century when it developed the most, thanks to the effect of work by authors such as Nightingale (1855), Donabedian (1966) or Williamson (1978).4
The growing interest in the implementation of quality systems in organizations has occurred more or less simultaneously at an international level. This has made it possible to develop the concept of quality and analyse the elements or dimensions within it. Thus the concept of quality has evolved and become richer until our days, making it possible to adapt it to the evolution of society itself on a path which aims at excellence.
Donabedian defined quality based on the structures, process and results of an organization, establishing the importance of measuring quality using indicators. Since his works, which were used by the Joint Commission on Accreditation of Hospitals (JCAH), the concept of quality has gradually been completed, progressively adding new dimension to be taken into account when drawing up a quality plan.
There is now no doubt that if quality involves structural elements of an organization, the processes carried out within it and the results obtained, it is also certain that other elements are highly important, too. These elements (or dimensions) include leadership, customer (or user) perceptions, or the total effect that the organization and its products or services have on its environment.
Several models are used to guide organizations during the implementation and management of quality systems, offering international recognition of a quality guarantee when they meet certain appropriate standards. The following models stand out among these:
- -
The JCAH model: this is structured on the basis of the “structure, process and results” dimensions, for which quality criteria are established, together with optimum standards and standards that can be attained.
- -
The European Foundation for Quality Management (EFQM) model - the most recent version of this model in 2020 aspires to be a model for excellence and transformation. This requires the commitment of the organization management and the leadership and involvement of all of its members, together with the management of processes (total quality systems and continuous improvement). It includes 7 criteria for excellence that are structured in 3 blocks: management, execution and results. It proposed evaluating each criterion by applying the modified REDER logical pattern, Deming’s variant of the circle of continuous improvement or the PDCA cycle (plan–do–check–act).4–6,10
- -
The ISO model - which was last updated in 2015. This offers a series of standards which function as guidelines as to how a service or organization should act to achieve its goal. The ISO 9000 series is dedicated to quality management, specifying quality system requisites based on processes and continuous improvement systems (ISO 9001).
Organizations are able to select the system which best fits their needs within the context of their strategic plan. However, it has to be pointed out that the quality systems described above are mutually compatible in many respects. They share the idea of searching for excellence by creating values for all of the interest groups involved, designing a quality plan and quantifying quality using indicators as an instrument to discover the starting point, the goal and possible deviations that arise in the path.
Implementing quality management systems. Mention of the forensic fieldQuality management in the field of medical care in Spain has grown in importance since the passing of “General Public Health Law 14/1986”.7 Article 69.2 of this law states that the “evaluation of the quality of care given must be a continuous process that covers all of the activities of medical personnel and the personnel of the health services within the National Health System”. Since then different accreditation systems have gradually been introduced, with certification or evaluation of quality while seeking to achieve excellence in the service offered to users of the health care system.4,8
Anatomical Pathology Departments are similar in some ways to Forensic Pathology Departments. They have not remained on the side-lines and have worked to meet relevant quality standards in terms of laboratory functioning, the techniques used or the issue of reports, as well as in computer systems and digitalization.9
In 2013 the Anatomical Pathology Quality Management and Accreditation Workgroup of the Spanish Society of Anatomical Pathology published the manual “Reglas y Consejos sobre Buenas Prácticas Profesionales en Anatomía Patológica”. This manual covers structural and organizational questions in Anatomical Pathology Units, although it also recognises the importance of pre- and post-diagnostic process for the quality of the final result of laboratory working. It defines good practices as the “set of norms, organizational structures and knowledge management which ensure the issue of the best possible final report for each patient”.10
The manual includes a self-evaluation formula based on the EFQM model. It states that the first step in adopting quality guarantee policies is to know the department in depth, with the aim of identifying the areas where work is required to achieve the desired level of quality. It also encourages acceptance of the challenge to subject departments to external audits through accreditation processes. For this purpose it considers the UNE-ENISO 15189 Standard, which is the most suitable route because of its high level of technical exigency. It should be pointed out that the accreditation process involves an authorized external body recognizing that an organization is competent to the activity it undertakes, after subjecting it to evaluation in terms of compliance with quality standards. Although implementing quality standards may be a requisite when requesting accreditation, these concepts are not the same as it is possible to progress in quality management, with the resulting benefits for the organization, without requesting formal accreditation, even though this would be desirable.
The manual therefore sets out the minimum quality standards that can be demanded for the good practice of Anatomical Pathology in terms of structure, information systems and processes.
During many years in forensic medicine no specific quality standards were defined or formally regulated at an international level, and in general public investment to do so was very limited. Nevertheless, the accreditation bodies in different countries have been gradually working towards establishing international standards.11
For a long time Spanish forensic laboratories have been aware of the need to comply with applicable quality standards, especially concerning DNA analysis. This is not only so for the analytical methods used, to ensure the reliability of results, or the traceability of process and the guarantee of the chain of custody. It is also necessary to minimize the risks of sample contamination, so that risk mapping and the preparation of security protocols are indispensable.12,13 The National Institute of Toxicology and Forensic Science, which has been a member for more than 20 years of the European Network of Forensic Science Institutes (ENFSI), is therefore accredited according to ISO/IEC 17025 for different methods of analysis as well as the Biology Services of its different departments and delegations. This guarantees compliance with the standards stipulated by “Royal Decree 1977/2008 of 28 November, regulating the composition and functions of the National Commission for the forensic use of DNA.”14
The achievements in the field of quality by bodies such as the Guardia Civil should also be underlined. Through their Department of Quality Management, which was created in 2003, the laboratory of the Guardia Civil was the first to be accredited by ENAC (the National Body for Accreditation) according to the requisites of the UNE-EN-ISO 17025 standard. In 2019, the Servicio de Criminalística obtained accreditations in a study of digital evidence and ocular technical inspection in open and enclosed spaces. The latter was awarded the accreditation file 314/EI520 as it complied with the UNE-EN ISO/IEC 17020: 2012 standard.15,16
Looking solely at the Forensic Pathology Departments in our country, the development of quality control and guarantee systems has not been uniform. Some IML, such as the one in Valencia, which is a pioneer in this field, have clearly invested in the implementation of quality management systems. This is shown by the recent ISO 9001:2015 accreditation obtained by the Anatomical Pathology Department and Toxicology Section of the Valencia IML. However, it is not possible to talk of the existence of a widespread culture of quality, and much work remains to be done in the majority of Forensic Pathology Departments. Without denying the need for this work, the experience acquired in areas close to forensic pathology offers the possibility of including this accumulated knowledge within our own sector.
It is well-known that the maximum quality in a department is achieved when all of the processes involved in reaching a result are considered as a whole and subjected to continuous evaluation and improvement. These include structural, administrative and management aspects, together with professional practice and qualification, procedures and the issue of reports. This approach, known as total quality, is expressed in the ISO 9000 standards of the International Organization for Standardization (ISO).
In the field of forensic pathology, in 1999 the WHO published a “Manual for the implementation and monitoring of quality systems in anatomical and forensic pathology laboratories”, with the aim of offering a practical guide to aid compliance with ISO standards.17 The eight chapters of this manual cover general questions to be included in the quality plan (describing aspects of health and safety at work, the identification of risks at work and preventive measures, procedures for what to do in case of accident, etc.), as well as the minimum elements which a “Quality Manual” should contain respecting personnel and continuous training, management and administrative, financial and economic management, the management of personnel, aspects in connection with samples, cadaveric investigations, quality monitoring, laboratory techniques, infrastructures and agreements, etc. Its appendices also contain checklists for the self-evaluation of services.
Thus through the quality manual the quality management system sets out a series of measures that, once implemented, guarantee the effectiveness and efficiency of the service, offering results that are exact, reliable, reproducible and within appropriate time-spans.
Along the same line as the said manuals, the work undertaken by the European Council of Legal Medicine (ECLM) also stands out, with the 2014 validation of a checklist for use in Forensic Pathology Departments.18 The ECLM is an official body at European level, and its objectives include the harmonization of legal medicine practices in the member states, while guaranteeing the quality of forensic pathology work. This checklist was drawn up by a specially convened workgroup, with the participation of international experts. The aim was to offer an instrument that would be useful for organizations as well as external assessors, to enable a successful process of ECLM accreditation. The checklist contains a series of items classified in different domains:
A) General. This includes sub-domains on infrastructure, security, administrative area, protection against risks, maintenance, action plans in case of catastrophe, quality guarantee and statistical reports.
B) Investigations: this includes sub-domains on the removal of a cadaver and identification procedures.
C) Action in the Forensic Pathology Department: with subdomains on handling and moving cadavers, interventions in the autopsy room, radiological installations, post-mortem examinations, taking samples and the chain of custody.
D) Histology: its subdomains include installations and investigations.
E) Toxicology: the subdomains of this heading include the toxicology laboratory, processes, department personnel and samples for toxicological analysis.
F) Reports and the custody of documentation: with subdomains on report registering and custody, documentation delivery procedures, the preparation of reports on cadaver removal and autopsy, and photographic reporting.
G) Staff: this includes the following as subdomains: qualified forensic pathologists, technical and auxiliary personnel and non-technical personnel.
H) Support services and consultants: with the subdomains of support services and consultants.
The WHO manual and the SEAP Rules and Recommendation for Good Practice in Anatomical Pathology or the ECLM checklist reflect and approach to quality that involves breaking it down into its different dimensions, offering formulas for self-assessment.19
According to the recommendation contained in the said guides, the departmental quality report should cover a very wide range of matters in a structured and complete way. These include infrastructures, laboratory accreditation, personnel qualifications, continuous training, questions regarding health at work, the preparation of protocols and guides for action, etc. All of this has the aim of guaranteeing it is possible to check and monitor forensic working, so that it is possible to know the degree to which the interventions implemented offer a result which fulfils the objectives of the organization and, if applicable, to develop corrective measures.20
In connection with the indicators, although any quality management system has to cover the design of processes according to the target standards, the design alone is insufficient as it must be possible to quantify the degree to which the said standards have been met. It is therefore indispensable to design and define the key performance indicators and the outcome-based indicators.
Different indicators are suggested within the forensic science bibliography. Some emphasise the economic aspect21 and others productivity,22 while others emphasise utility or results.23 Although the majority of the proposed indicators are not expressly designed for a Forensic Pathology Department, they could be adapted for use in this field. This is the case, for example, with productivity indicators (reports issued /reports requested × 100), time in the issue of reports, percentage of reports issued after delay, or the efficiency of the service in terms of average cost per report (the sum of installations and personnel costs/total number of reports issued).
The College of American Pathologists (CAP) recommends relating processes and the design of quality indicators for them in its Laboratory Accreditation Programme, to implement a quality guarantee programme for clinical autopsies. This monitors the pre-analytical phase, analysis and post-analysis, as well as the time taken to issue reports and client satisfaction. It proposes monitoring a series of key indicators for this purpose.24,25
The CAP includes identification of the cadaver within the pre-analytical phase, together with collecting the necessary documentation (consent, medical history and demographic data, etc.) and communication with the family and other professionals (undertakers and investigators, etc.). The analytical phase refers to practicing the autopsy and includes safety measures (protective equipment, etc.) as well as macro- and microscopic studies (applying general good practice protocols as well as ones for specific cases). The post-analytical phase covers the preparation of the cadaver after the autopsy, the issue of reports and the degree of satisfaction with the results.
All of the different phases are monitored by questionnaires of the Yes/No type that should be completed for each cadaver. Table 1 shows some questions for each phase, as an example:
Quality indicators and monitoring.
| Pre-analytical phase |
| a) Permits and communication - with the objective of complying in 100% of cases for items 1-6 |
| Has the patient been correctly identified? |
| Has the informed consent form been signed by the authorized family member? |
| Has the request for post-mortem study been signed by the doctor in charge? |
| b) Transport of the cadaver - with the objective of complying in 100% of cases for items 1-3 |
| Is the body properly identified according to centre policy? |
| During transport: is the body correctly protected? |
| Was the body properly registered in the morgue according to centre regulations? |
| Was the documentation in connection with the cadaver delivered to the pathologist in a timely manner? |
| Analytical phase |
| a) Macroscopic examination - 100% compliance target |
| Was appropriate protective equipment used during the autopsy? |
| Were examinations appropriate to demonstrate the findings? |
| Were weights and measures taken properly? |
| b) Microscopic examination and diagnosis - 100% compliance target |
| Did the microscopic description correspond to histological findings? |
| Did the definitive diagnosis include macro- and microscopic findings? |
| Post-analytical phase |
| a) Care after the autopsy - 100% compliance target |
| Was the body cleaned and appropriately prepared? |
| Was it placed appropriately in the shroud? |
| Was the body placed at the disposal of the family in a reasonable time? |
| b) Autopsy report - 100% compliance target |
| Did this include the final diagnosis? |
| Did it include a proper description of the external and internal examination? |
| Does the information it contains make it possible to infer the main pathological process and the cause of death? |
| c) Clinical and autopsy audit |
| d) Time the report was issued |
| e) Satisfaction questionnaires |
Translation adapted from the Royal College of Pathologists.27
Within the post-analytical phase and more specifically in the section on the quality of results, it is both fitting and of interest when designing quality indicators to take into account the Daubert criteria, as shown in Table 2, as a guarantee of the scientific nature of the test.26
Criteria for the scientific nature of the test.
| Daubert criteria |
| It must be possible to empirically corroborate or falsify the scientific theory on which production is based |
| The possibility of determining the percentage of error in connection with the technique used |
| The existence of control applied by other experts; peer review of the discipline in question |
| The existence of a general consensus in the scientific community about the validity of the foundations of the scientific theory |
| The need for a direct connection between the test and the facts within a specific case |
Within the context of forensic pathology, the guide to good practice in forensic pathology “Code of practice and performance standards for forensic pathology in England, Wales and Northern Ireland” also stands out. This has been in force since 2018 in most of the United Kingdom (except for Scotland). It was prepared and published with the backing of the Justice Department and the Royal College of Pathologists.27 This manual runs through — and lists criteria for good practice— all of the processes involved in the investigation of suspicious or violent deaths, from a cadaver found at the scene to the issue of a report after the autopsy and the taking of samples.
Finally, although they refer to clinical autopsies, the guides published in this field by the NHS Scotland Quality Improvement Unit in 2003 and 2016 may be useful when setting quality standards and objectives and monitoring procedures.28,29 The way these describe the standards and objectives of good practices is highly illustrative, so that an adapted translation is included in Table 3 of standard number 4 of the 2016 Scottish guide.
Translated and adapted from the "Management of Hospital Post-mortem Examinations Standards – June 2016" (24).
| Standard 4: | Post-mortem examination and the issue of a report |
|---|---|
| Description of the standard | |
| The post-mortem examination in the hospital and the issue of the corresponding report are carried out according to national law and clinical practice guides. | |
| Justification | |
| The requisites for the post-mortem examination in the hospital, the issue of reports and the formal requisites are clearly shown in the legislation and in the clinical practice guides at national level. Autopsy reports are issued without delay to guarantee that the information for family members, primary care doctors and the other medical professionals reaches them as soon as possible.Sometimes the autopsy and issue of a report will require more time due to the characteristics of the investigation, for example, neuropathology, cytogenetics or when there is the risk of the transmission of diseases to staff, such as hepatitis B or Creutzfeldt Jakob’s disease. Family members must be informed of any possible delays while maintaining the confidentiality of the deceased individual. | |
| Criteria | |
| 4.1 | The post-mortem examination in the hospital, the issue of reports and the audits are undertaken according to the Human Tissue (Scotland) Act 2006 and professional guidance, for example, of the Royal College of Pathologists. |
| 4.2 | Protocols and procedures exist to guarantee the correct identified of the deceased. |
| 4.3 | The requesting doctor has exactly completed the authorization document. |
| 4.4 | The pathologist in charge of the post-mortem examination in the hospital has access to:
|
| 4.5 | The post-mortem examination in hospital is undertaken or supervised by a pathologist who is registered as a specialist by the General Medical Council (GMC). Paediatric and perinatal autopsies and neuropathological post-mortem examinations are carried out or supervised by pathologists with training in these fields. |
| 4.6 | Hospital autopsies are performed in the responsible department in the 3 working days following reception of the complete authorization document. |
| 4.7 | In the process of performing a hospital autopsy and the corresponding complementary tests, the pathologist is to supply the requesting doctor and other personnel with:
|
| 4.8 | When neuropathological or paediatric studies or specific tests are required, the time limits for issuing reports will be longer. |
| 4.9 | When a delay is required in the autopsy or the issue of reports, this fact will be documented in the clinical history and the rest of the personnel will be informed, as well as family members and those close to the deceased. |
| 4.10 | The histological, cytological descriptions and that corresponding to any other sample taken and the results will be included in the final autopsy report. |
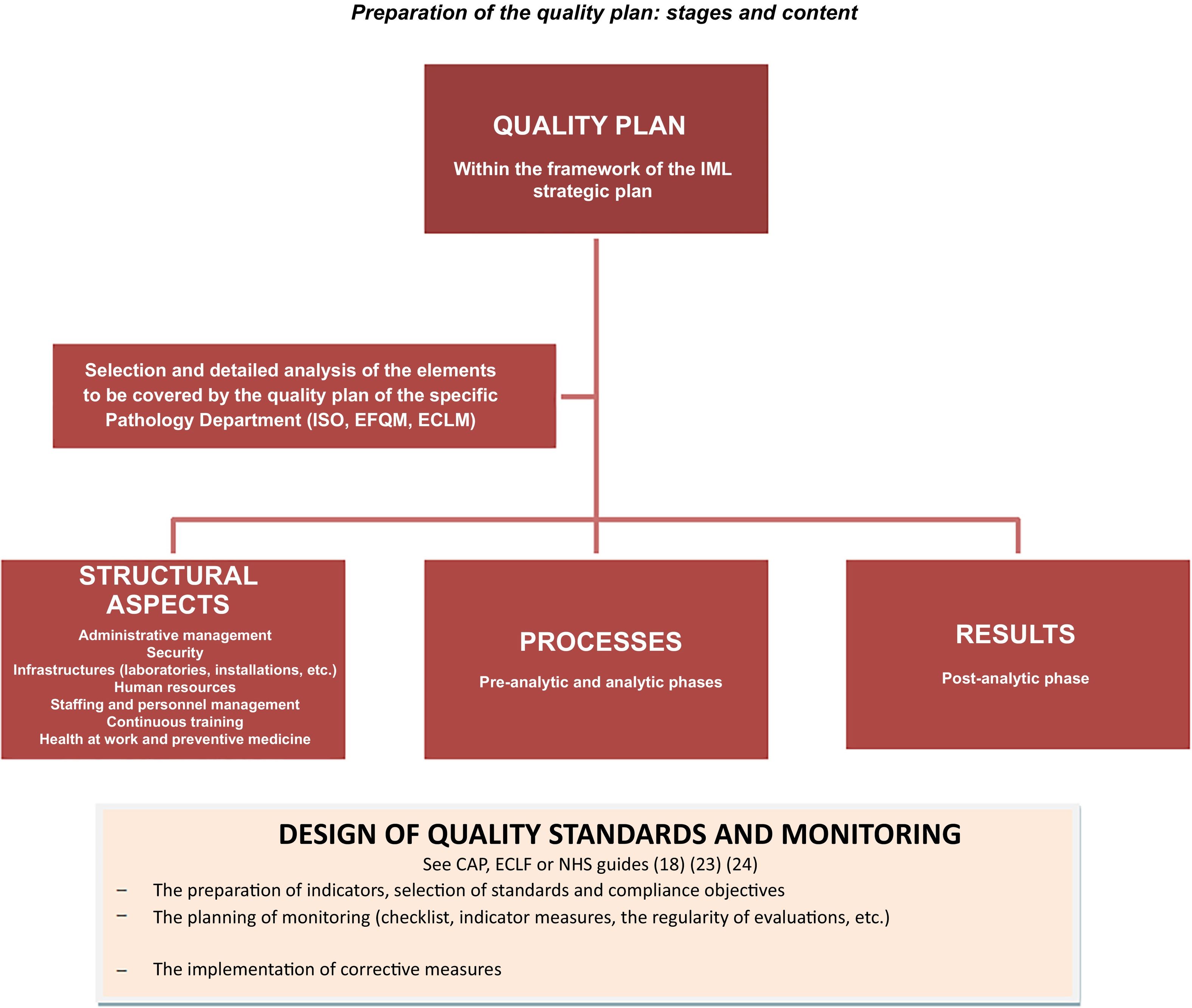
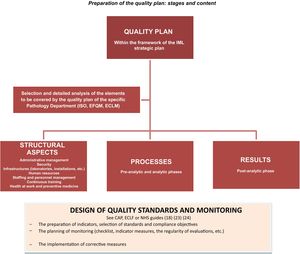
Fig. 1 shows a graphic summary of the stages of preparing and designing the content of the quality plan in Forensic Pathology Departments.
ConclusionsAthough preparing and implementing quality plans in Forensic Pathology Departments may seem laborious, there can be no doubt that it is worthwhile. This is because it is an indispensable means of transparently guaranteeing that the results obtained fulfil the objectives of the organization and respond to the commitment to the administration of justice.
The documents described establish, although they do so indirectly, the dimensions of which quality is composed in the context of forensic and/or anatomical pathology. That is, the aspects which the organization should centre on describing and planning when designing its quality plan.
The specific plan should be adapted to the specific characteristics of the IML in question, as well as its objectives and resources. All of the professionals who will take part in its subsequent implementation should be involved in its design, under the leadership of the managers.
FinancingThis research has not received specific support from public sector funding agencies from public sector funding agencies, the commercial sector or non-profit commercial sector or non-profit organisations.
Please cite this article as: Miguel Alhambra L, Zarco Rodríguez J, Andreu Tena E. Revisión sobre la metodología para el diseño de la estrategia de calidad en los servicios de patología forense. Revista Española de Medicina Legal. 2022. https://doi.org/10.1016/j.reml.2022.03.003