This paper reviews the association of clinical symptoms and their clinical and forensic implications in a patient with Down's syndrome and congenital cervical synostosis who experienced a traumatic spinal cord injury. To date, no prevalent association between Down's syndrome and cervical synostosis has been reported in the literature. Given that both entities are prone to cause degenerative or traumatic cervical myelopathy, the combination of both conditions must be seen as a risk factor for spontaneous, and particularly traumatic, spinal cord damage. In these cases, radiological examination must be exhaustive, including MRI, given the possibility of spinal cord injury even after minimal trauma.
In the event of death, spinal cord autopsy is mandatory as it may reveal subclinical lesions, clarify the nature and extension of the spinal cord and skeletal injuries and help to establish an improved anatomo-clinical correlation.
Con ocasión de un caso de asociación entre síndrome de Down y sinostosis vertebral congénita que sufrió lesión medular traumática, se revisa la asociación de estos cuadros y sus implicaciones clínicas y forenses. La literatura no ha comunicado hasta ahora una asociación prevalente entre síndrome de Down y sinostosis cervicales. Ambos cuadros por separado propenden a la mielopatía cervical, bien de manera degenerativa, bien traumática. En consecuencia, la asociación de ambos debe verse como un factor de riesgo de daño medular o de manera espontánea o, sobre todo, después de traumatismos. En estos casos, la exploración radiológica debe ser exhaustiva incluyendo RMN ya que pueden existir lesiones medulares incluso ante traumatismos mínimos.
En caso de fallecimiento, la autopsia medular es obligada ya que puede revelar lesiones subclínicas, clarificar la naturaleza y extensión de las lesiones medulares y esqueléticas, así como ayudar a establecer una mejor correlación anatomoclínica.
Central post-contusive myelopathy or Schneider syndrome was first described in 1954.1 The typical presentation is that of a greater neurologic deficit in the upper limbs compared to the lower ones. In the original description, it was attributed to an anterior medullary contusion due to a herniated disc or other causes after a trauma.
Klippel–Feil syndrome (KFS) was described by these two authors in 1912.2 Initially, the diagnostic criteria were clinical (short, stiff neck, and low posterior hairline). Later on, radiological requirements were added, with the main characteristic being the presence of congenital block vertebrae in the cervical spine.3 It is known that the existence of vertebral synostosis between 2 or more spinal mobile segments promotes spinal cord injury over the course of a hyperextension, this greater propensity being attributed to adjacent hypermobile block segments.4–6 This spinal cord injury may, in the cervical region, constitute Schneider syndrome.
Down's syndrome (DS) may predispose the development of degenerative cervical myelopathy,7–9 with the changes increasing with age.
Being 2 conditions that independently tend towards degenerative or traumatic myelopathy, the association between cervical synostosis and DS may involve a high risk of neurologic injury, even in the face of minor trauma. This may present as primary or secondary injury, and is not always identified by routine tests.10
In this paper, we present a case of associated vertebral synostosis, DS and traumatic cervical myelopathy (Schneider syndrome). Clinical, radiological and autopsy data have been analysed and related literature reviewed, with the objective of considering the clinical and medico-legal issues posed by the possible coexistence of these 3 diseases.
As far as we know, no association between KFS and DS has been described in the literature.
Case reportOn arrival at the hospital emergency department, the patient was conscious and presented with a lacerated contused wound to the scalp of approximately 4cm without skull depression. Normal neck. Lacerated contused wound in superior maxillary region that communicates with oral cavity with loss of teeth.
The general examination revealed blood pressure of 56/33mmHg, afebrile, conscious, reactive, agitated, nauseous, O2 saturation of 94%. Nasal bone crepitus, periorbital haematoma in right eye and right forehead. Infranasal and upper lip lacerated contused wounds with loss of teeth in upper jaw.
Heart auscultation: sinus rhythm 90bpm. No murmur. No jugular ingurgitation.
Lung auscultation: good ventilation in all lung regions. Pain on palpation in right inferior costal cartilage.
Erosion in left breast.
Abdominal auscultation: soft abdomen, depressible, erosion in abdominal wall at right flank level with haematoma in said area of approximately 7×9cm in diameter. Erosion in pubic area.
Limbs: all 4 limbs moving. No focal neurologic deficit.
Additional emergency department tests:
- -
Blood and urine analysis and coagulation study: no significant abnormalities.
- -
Cranial CT scan with emergency intravenous contrast medium administration: no evidence of foci of intracranial haemorrhage or midline shift. Cortical and subcortical atrophy. No skull fracture lines observed. Paranasal sinuses with no warning signs. Deviated nasal septum. Nasal bone fracture.
- -
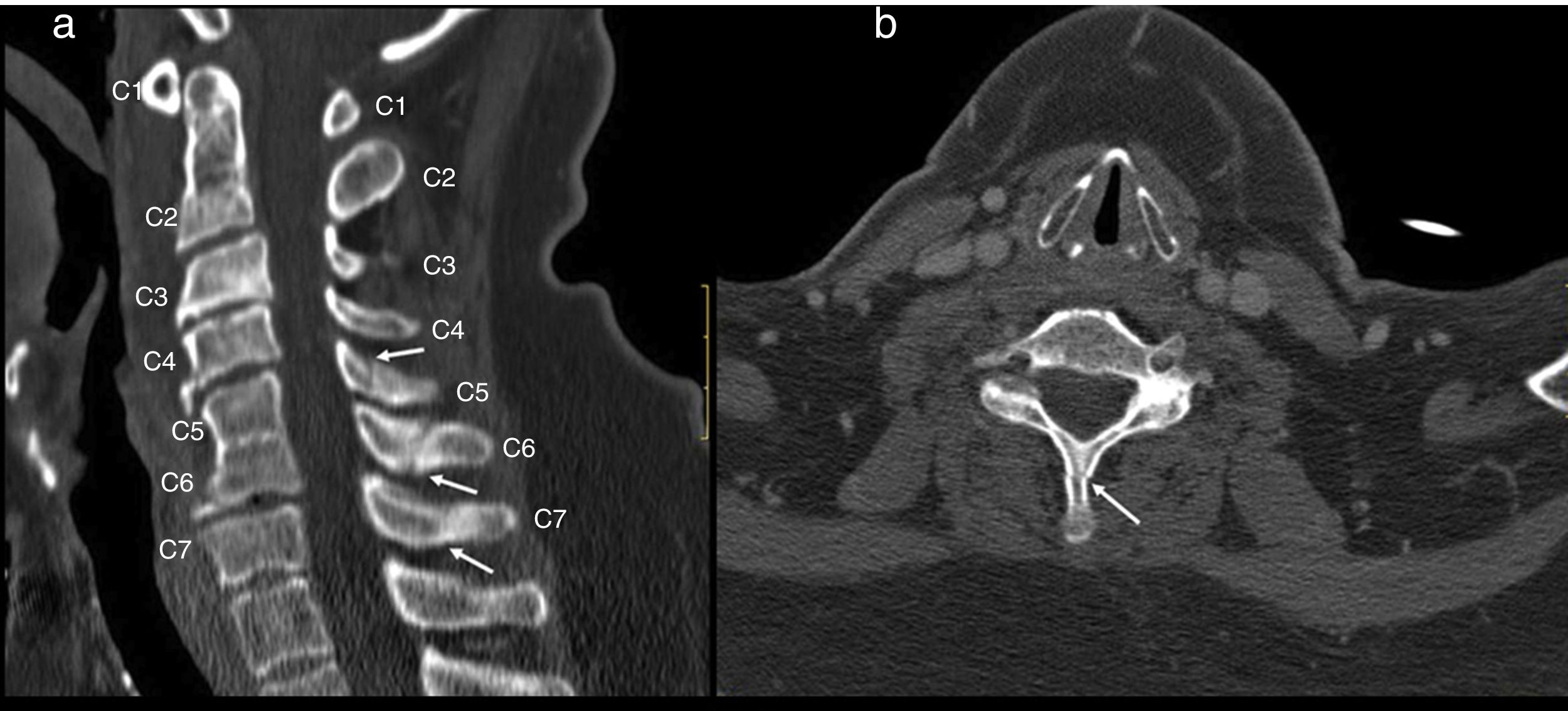
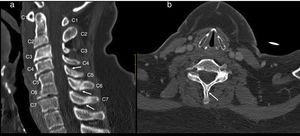
Cervical CT scan: no vertebral alignment disruption. Fusion of C5–C6 vertebral bodies. Spinous process fractures of C6 and C7. Cervical arthritis. No soft tissue masses (Fig. 1).
Figure 1.Cervical CT study on sagittal (a) and axial (b) planes. Hypoplasia of the vertebral bodies C5 and C6 with “wasp waist” typical of congenital fusion, with fusion of the facet joints, without fusion of the spinous processes is shown. Anterior osteophytes identified, mainly in the bodies adjacent to the vertebral fusion and rectification of the lordosis, conditioning a canal stenosis in C3 and C4. A discrete subluxation of C2 on C3 is observed, without associated atlantoaxial or atlanto-occipital subluxation, or hypoplasia of the atlas or odontoid hypoplasia. Undisplaced fractures in the C5, C6 and C7 spinous processes (arrows).
(0.22MB). - -
Thoracoabdominal CT scan with intravenous contrast medium: lung parenchyma with areas of increased attenuation of predominance in both lung bases with respiratory movement. Mediastinum with no pathological images. Heart, pericardium and thoracic aorta without significant changes. Fracture of costal arches 10 and 11 of the right hemithorax. Liver, pancreas, kidneys and adrenal glands normal. Hypodense image seen in the inferior pole in the spleen interpreted as respiratory motion artefact. No dilated intestinal loops were observed. Uncomplicated umbilical hernia. Haematoma of anterior abdominal wall in right hemiabdomen was observed. No free intraperitoneal fluid or presence of ectopic air was observed.
After suturing the head and face injuries, the patient was moved to the observation area of the emergency department to receive analgesic treatment and assess progress. The patient was admitted here with a blood pressure of 68/40mmHg, O2 saturation of 100% and heart rate of 89bpm. The patient was complaining, with multiple haematomas in the facial region. Normal breath sounds anteriorly, no crepitations. In the abdomen, haematoma and excoriation on the right flank wall was observed. The patient's limbs were moving and lab tests, chest X-ray and abdominal ultrasound were pending.
The day after admission, blood analysis was requested with significant haemoglobin values of 10.6 and leukocytes of 13,800, chest X-ray without appreciable changes at the bilateral pleural parenchymal level and a normal abdominal ultrasound.
In the ICU, the patient was agitated, pale and progressive neurological deterioration began, with progressive hypotonia in the 4 limbs, except the right leg, which moved to pain. Preserved sensitivity to pain.
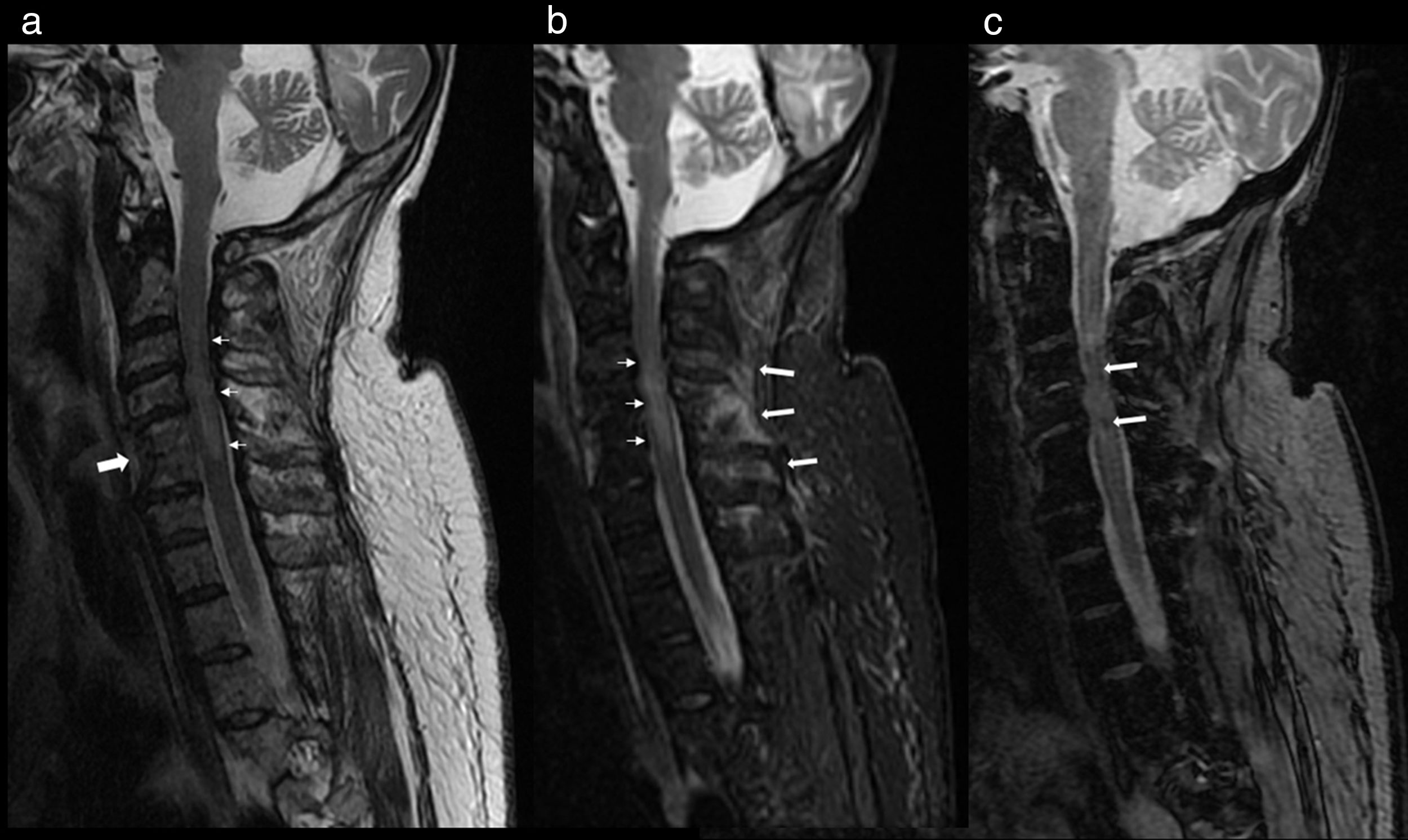
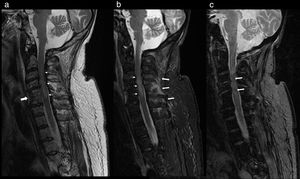
At 2 days after the trauma, magnetic resonance imaging of the cervical region was carried out, where cervical canal stenosis was observed. Fusion of C4–C5 vertebrae. Signs of spinal cord contusion from C3 to fused vertebrae (C4–C5). No evidence of intracanalicular bleeding or other processes that compromise space. Oedema in posterior interspinous ligament (Fig. 2).
Cervical MRI study on sagittal planes, sequences FSE T2 (a), STIR (b) and GRE T2* (c). In the FSE T2 and STIR sequences, significant canal stenosis in C3–C4 is shown, with hyperintense spinal cord signal (thin arrows), in relation to oedema secondary to spinal cord compression. Congenital vertebral fusion of C5–C6 is also identified, with a very rudimentary disc (thick arrow in “a”). In the STIR sequence, oedema between the spinous processes C4–C7 and adjacent paraspinal muscles is identified, in relation to partial injury to the interspinous ligaments (thick arrows in “b”). The gradient echo sequence T2* shows an intramedullary hypointense linear image at C3 and another punctiform at C5 in relation to haemorrhagic content (arrows in “c”) a finding for a poor prognosis.
The patient was assessed by the Department of Neurosurgery. The patient was sedated with morphine hydrochloride. She is a carrier of oxygen therapy and enteral nutrition through a nasogastric tube. Tachypnoea and dyspnoea at rest, flaccid tetraparesis with areflexia of upper limbs. It was explained to the family that the therapeutic options consist of cervical decompression surgery and possible sequelae with very difficult rehabilitation, to which the family responded by preferring to wait out the natural evolution, accepting the limit to therapeutic measures.
Neurologically, the patient remained stable with flaccid tetraparesis with areflexia of the upper limbs. Treatment was continued with NASCIS III (initially a 30mg/kg bolus of methylprednisolone was administered to pass in one hour, continuing with an infusion of 5.4mg/kg/h, for 48h).
The patient was assessed by the Spinal Cord Injury Unit, but, due to a lack of collaboration, it was not possible to perform a formal exploration of the ASIA scale. Spontaneous and voluntary movements were observed in the upper and lower right limbs. Deep tendon reflexes were present in the lower right limb, which could not be assessed in the upper right or left limbs.
No signs that led to suspicion of pain. The patient was terminally ill, so it was decided to limit therapeutic actions.
Six days after the trauma, at 5.00 p.m., the patient died. Causes of death were established as:
Immediate cause: cardiorespiratory arrest.
Intermediate cause: respiratory failure.
Underlying cause: traumatic cervical spinal cord injury.
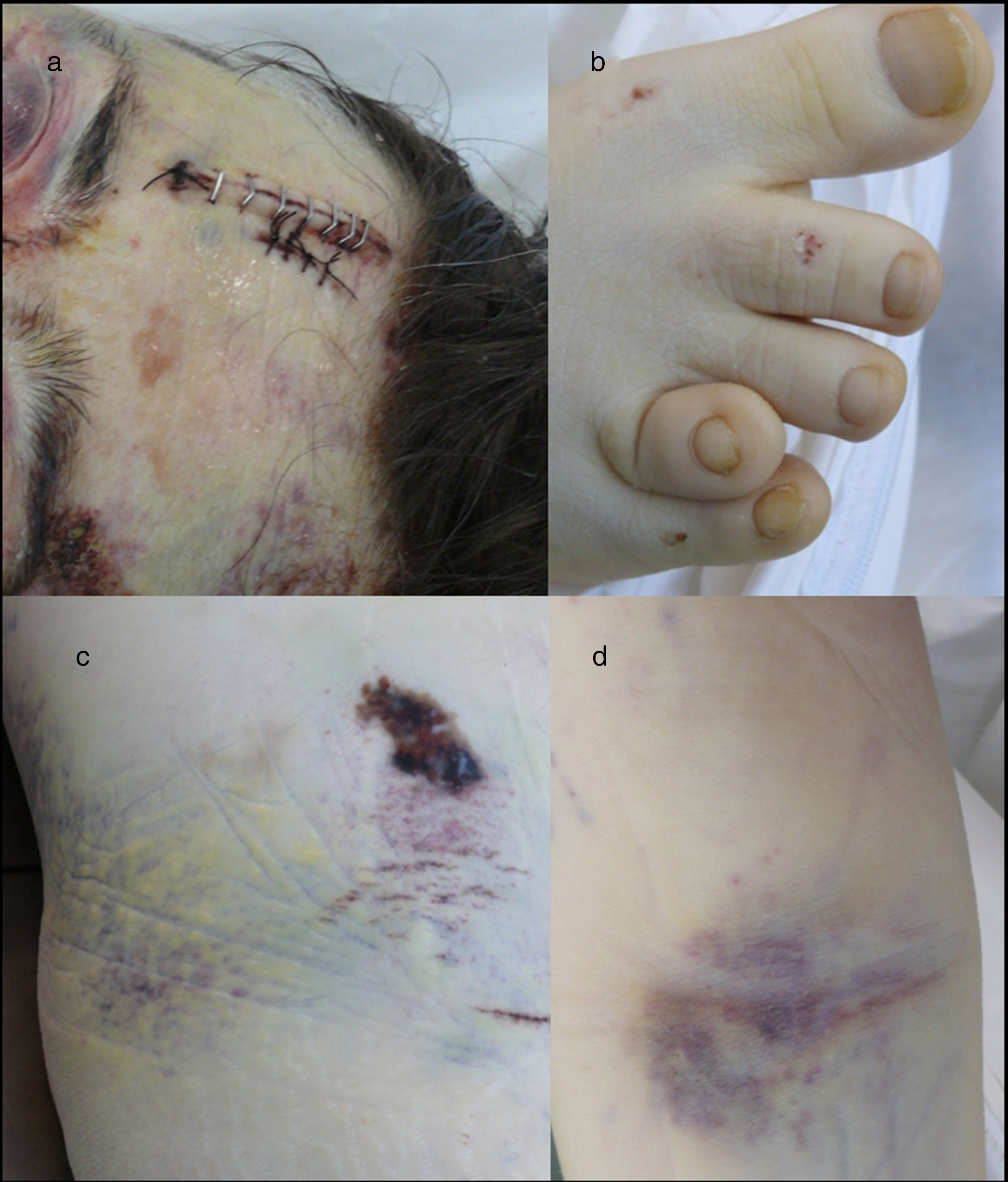
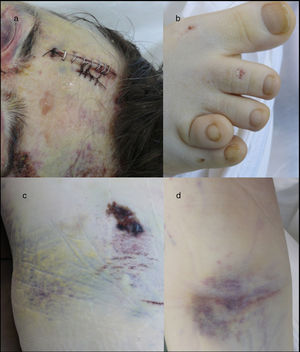
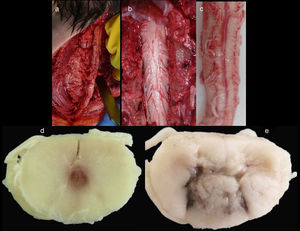
Medico-legal autopsyExternal examinationThe main findings included: DS phenotype. Periorbital haematomas in both eyes, lacerated contused wound sutured with staples in the right forehead and occipital nasal regions (Fig. 3a), nasal oedema, several haematomas in flexure of both arms as a result of punctures to place intravenous lines (Fig. 3d), haematoma resolution in both hips with extension to the thighs and ecchymosis in right vacuum and umbilical region on the right side (Fig. 3c). Shortening of the fourth toe on both feet (Fig. 3b), as well as cadaveric lividity on back.
External examination. Note the forehead impact injuries (sutured contusion and bilateral orbital haematoma (a). In (b), partial hypoplasia-syndactyly of the fourth toe can be seen. The images in “c” are located in the right hemiabdomen, responding to a friction mechanism. The injuries visible in (d) occur on the front side of the right forearm.
A mento-pubic incision was carried out in which the haematoma of the right abdominal wall could be seen in the dissection. Fractures of the right 10th and 11th ribs, with haemorrhagic infiltration that compromised the right hemi-diaphragm, and pericardial adhesions without signs of pericarditis were observed. Rest of thoracoabdominal viscera with no pathological significance.
Cranial autopsyMata's technique [lateral opening of the cranial cavity]. After the skull was opened up, the brain was extracted with cerebellum. Only slight cortical atrophy in parietal lobes of greater extension on the left side were observed, and once the dura mater of the bone was removed, a tegmen tympani could be observed on the right side, possible dehiscence and only covered by periosteum.
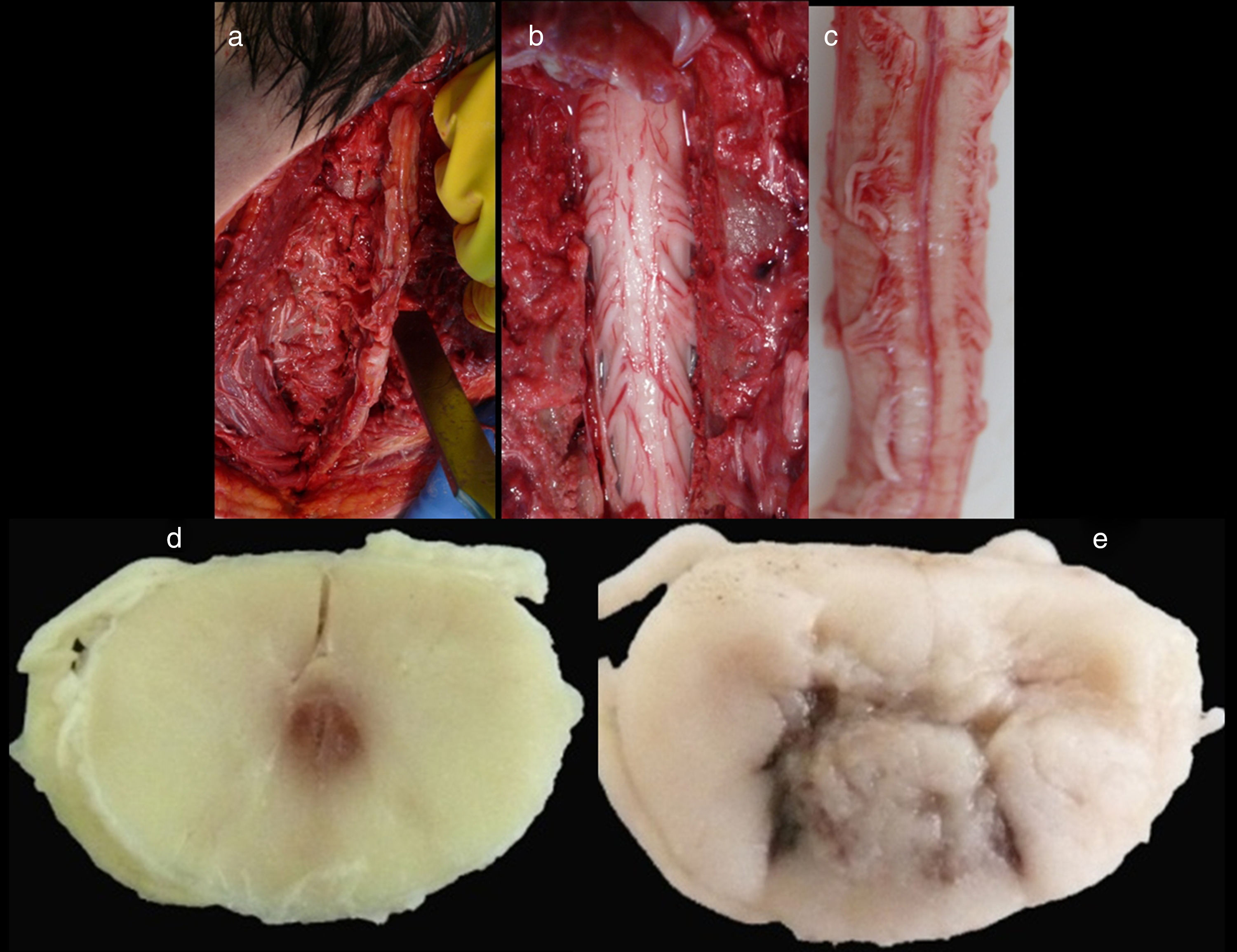
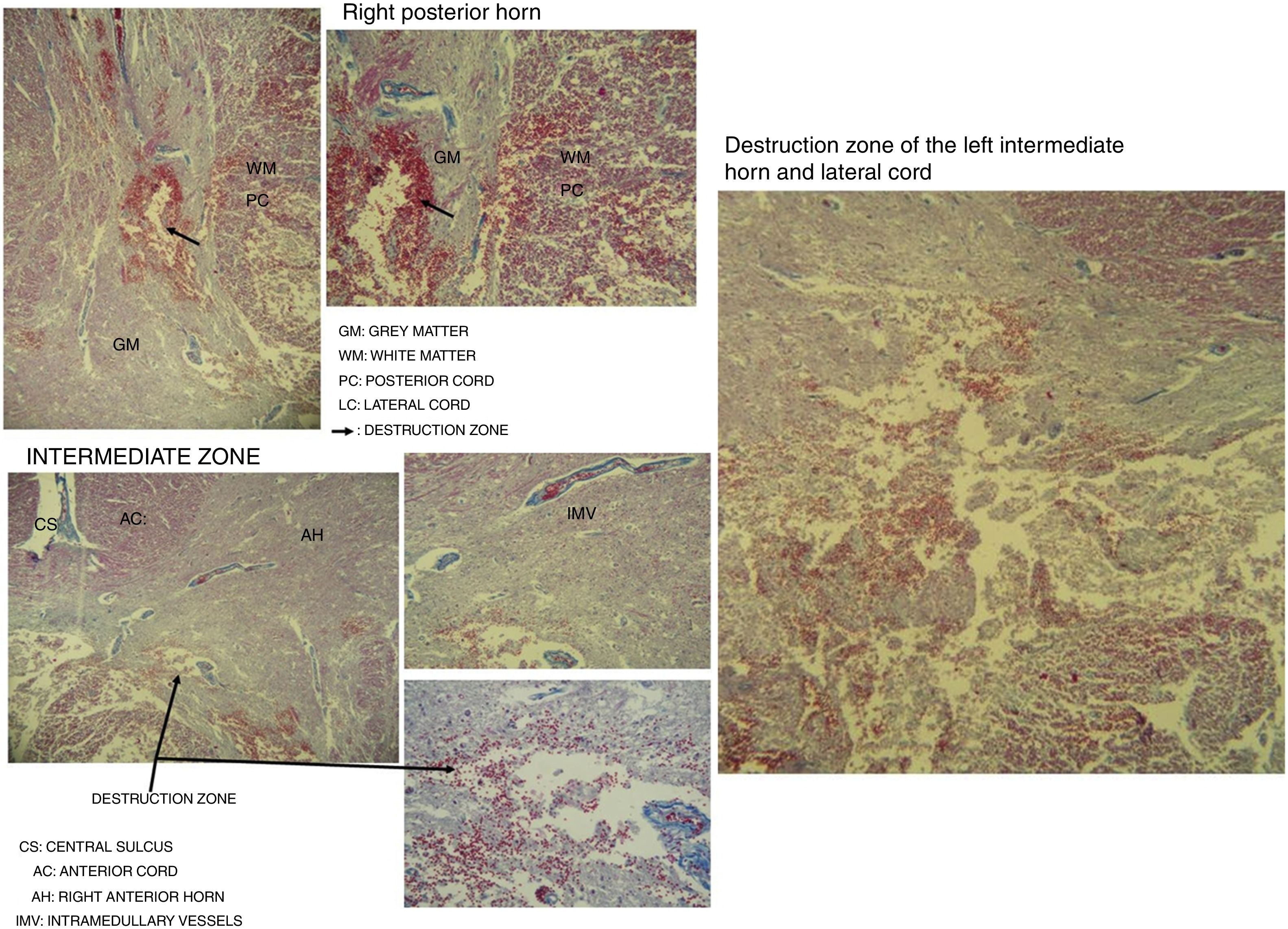
Spinal cordWith the corpse in the decubitus prone position with the cervical region in slight flexion and once the spinous processes had been marked, a midline incision was made. After separating the paravertebral muscles, the spinous processes and the cervical vertebrae laminae were accessed where the fractures of the spinous processes of C5, C6 and C7 were observed. After sectioning the vertebral laminae, the marrow was removed between C3 and D2 (Fig. 4a–c).
Images of the spinal cord trauma macroscopic study. In “a”, part of the spinal autopsy at the time of elevating the laminar webbing is shown. In “b” the webbing has been removed and the dorsal dura mater opened up to extract the complete cervical spinal cord, from the foramen magnum, along with the encephalon. No external injuries are identified on the spinal cord or spine additional to the fractures to the spinous processes. In “d” and “e”, corresponding to the specimen fixed in formalin, an intramedullary haemorrhagic focus C2–C3 (“d”) and C5–C6 (“e”) is identified, this last one more extensive and affecting anterior and posterior horns. In “e”, a softened intramedullary region is also identified, probably due to secondary injury.
The specimen was fixed in 10% formalin and carved 2 days later. Macro- and microscopic studies were performed.
Macroscopic studyAn intramedullary haemorrhagic infiltrate was observed at C3 level that in successive cuts extended up to C5 (Fig. 4d, e). The spinal cord looked normal below this level.
Microscopic studyThe anatomical specimen, after formalin fixation, was cut after the macroscopic study. The fragments required for microscopic study were embedded in paraffin, previously dehydrated (alcohol) and embedded in a miscible liquid (xylene) with the paraffin.
A Martin's trichrome stain was performed on the preparations duly fixed on the slides.
Once the preparations had been stained using the aforementioned technique, they were studied under an optical microscope.
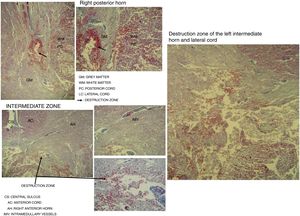
Destruction of the right posterior horn at the level of the neck, affecting the Rexed III and IV laminae, extending to the right posterior cord, was observed. Another area of destruction was observed in the left intermediate zone of grey matter, which compromised the Rexed VIII and IX laminae. Destruction of the posterior horn of the base and neck with involvement of the Rexed V and VI laminae, along with a large area of destruction of the left lateral cord, was identified (Fig. 5).
Microscopic study of spinal cord cut at level C3 with Martin's trichrome stain, where we can observe in the right posterior horn the destruction of the Rexed III and IV laminae and part of the right posterior cord. In the intermediate zone, the destruction of the Rexed VIII and IX laminae of the left side and zone of destruction of the left intermediate horn and lateral cord.
DS can be considered a risk factor for cervical myelopathy. Neurologic injury has mainly been associated with atlanto-occipital and atlantoaxial instability, that may occur in 13% of cases and cause neurologic deficit in 1.5%.11–13 This means that, in the face of cervical trauma in these patients, performing not only X-rays but, at the very least, a CT scan is required.14,15
Also, frequent association between subaxial cervical myelopathy (under the axis) and DS has been reported, confirming a higher prevalence of spondylosis and cervical myelopathy.8 Bosma et al. reported that spondyloarthritic cervical myelopathy seems to be a common condition in DS, as well as manifesting itself at a relatively young age.16 Frost et al.17 reported a case of an autopsy study in which triplegia, respiratory deterioration and death had occurred, which was attributed to severe osteoarticular degenerative changes and canal stenosis. It appears that age is a factor to take into account since atlantoaxial instability predominates in the paediatric or youth population, and is rare in adults with Down's syndrome, in whom subaxial osteoarticular changes are common.17
Other studies have reported that the existence of inflammatory processes of the cervical spine could also be linked to the development of myelopathy in DS.18
Cervical synostosisSpontaneous cervical fusion is a rare malformation. On many occasions it is assimilated with KFS. However, it has been argued that in many cases the typical associations with KFS are missing, such as the short neck.19 Therefore, it would be better to speak of vertebral synostosis in cases where the cardinal signs of the syndrome are not present. Abnormalities associated with synostosis, such as scoliosis, Sprengel's deformity,20 and heart and eye problems,21 have also been described.
Regarding the pathogenesis of synostosis, the lack of sclerotome segmentation would be behind fusions for some, while others suggest different mechanisms, such as disc tissue proliferation or lack of zygapophyseal joint development.22,23
KFS is classified according to 3 types24–26: Type I is “massive” congenital fusion of cervical vertebrae into one block and can also include thoracic vertebrae. Type II is congenital fusion at one or two non-contiguous intervertebral spaces. Type III is congenital fusion of multiple contiguous segments of cervical, thoracic or even lumbar vertebrae. Clinically, Type I is associated with cervicalgia and cervical mobility restriction, while Types II and III have a greater incidence of radiculopathy or myelopathy.
The frequency of neurological complications of KFS have been reported.27 The main ones are related to occipitocervical anomalies, medullary malformations, canal stenosis and instability.27,28 The existence of block vertebrae causes greater biomechanical stress on adjacent segments, causing premature degenerative changes on adjacent mobile segments.29,30 It may also cause disc, transverse ligament and odontoid fractures and spondylosis.31 Neurologic deficits have been described after minor trauma.32,33
People with blocks or KFS are more prone to myelopathy.34 The reasons for greater prevalence of myelopathy in this population is yet to be precisely confirmed. On the one hand, biomechanical reasons are adduced, which would affect the adjacent segments causing degeneration and discoarthritic bars capable of causing canal stenosis or damaging the spinal cord in the face of minor or heavy trauma. It has been reported that the greatest cause of instability in fused segments is the translation of the superior vertebra to the synostosic level, which would tend to then move around in hyperextension, this being the key factor in spinal cord compression.19
There is also a dose-dependent criterion. The more fused segments there are, the more predisposed the patient is to excess mobility and overload on adjacent mobile segments, which leads to a faster degeneration of discs and protrusions or hernias at these levels. This in turn increases the risk of traumatic spinal injury.35,36 On the other hand, factors deriving from neurologic or genetic anatomical aberrations are adduced to justify neurologic damage.37
To date, no significant association between DS and vertebral synostosis has been found in the literature. During the extensive review of spinal abnormalities in DS carried out by Frost,17 no cases of synostosis were reported.
Acute traumatic myelopathyAcute spinal cord injury is a dynamic, evolutionary and multi-phase process. Primary injury resulting from mechanical destruction of nerve structures, direct vascular injury and haemorrhage are present.38,39 But there is also secondary injury derived from inflammatory, vascular and neurochemical changes, which mainly and initially involve the central grey matter, extending dorsally and caudally. It has been determined that the optimal interval for trying to stop and reverse this torrent of events responsible for secondary injury is 4h (ideally 2), as the inhibition of axoplasmic transport begins in this period. It is prominent at 4h and complete at 6h after trauma.38,40
The secondary lesion is determined by inflammatory phenomena with release of mediators and lysosomal enzymes, changes to the vascular endothelium with microthrombi and microhaemorrhages, and neurochemical imbalances with associated vasospasm, oedema and haemorrhagic necrosis.38
In patients who suffer from hyperextension of the neck and have cervical stenosis, this can result in intramedullary injury (Schneider syndrome). A cervical hyperextension with posterior translation can cause foraminal narrowing and narrowing of the diameter and volume of the canal, which can exacerbate clinical manifestations in patients with stenosis.41 It has been reported that minor trauma can cause neurologic symptoms in the presence of a narrow canal.42,43
The immediate consequences of a spinal cord injury are translated into different degrees and combinations of motor, sensory and/or autonomic neurological deficit produced, depending on its severity, location (in the transverse plane) and the affected level.38,44–46
The spinal cord injuries that we have observed in the spinal cord microscopic study in this case settle on the Rexed right III and IV laminae. These regions are responsible for the processing of afferent somatosensory information on the right side, and the nucleus itself is located in this region, which receives exteroceptive stimuli.47 The additional impact on the right posterior cord gives rise to impaired touch and epicritic sensation.
The destruction of the Rexed left VIII and IX laminae affects the group of central nuclei where at levels C3 and C4 (area of the cut studied) the nucleus of the phrenic nerve on the left side is located. If this is injured, it can cause paralysis of the diaphragm on the same side.48
The destruction of the base and neck of the grey matter of the left horn would also affect the processing of afferent somatosensory information,49 and damage to the most medial part of the lateral cord would compromise the crossed pyramidal tract of that same side, affecting mainly the upper extremities. This injury would have allowed the execution of spontaneous and voluntary movements in the upper and lower right limbs, and the presence of a deep tendon reflex in the right lower limb as the right lateral cord was less affected.
Judging from all these findings, we can say that the patient suffered from a central spinal cord injury at the cervical level (Schneider syndrome), with diaphragmatic paralysis and involvement of ascending and descending pathways, which would explain the clinical manifestations the patient presented with before death.
This could have been due to spinal shock causing hypotension due to loss of sympathetic tone, bradycardia or due to parasympathetic stimulation. Loss of muscle tone due to paralysis causes venous congestion with consequent hypovolaemia.
Given the characteristics of the case, possible association with atlantoaxial instability was not excluded by dynamic studies. However, despite several cases of severe autonomic changes having been reported for this reason,50 the absence of damage at this level in both MRI and histology does not allow us to ascribe the deadly mechanism to potential lesions in this region.
A matter of interest is that, from the outset, clinical neurologic manifestation was not accredited in this case. It is possible that the direct injury existed subclinically or, due to its nature or the characteristics of the case, went unnoticed. But most likely is the development of secondary injury at the expense of a focus of previous intramedullary contusion, which evolved progressively.
In our case, the autopsy showed signs of severe frontal impact. Spinal cord compression, fractures in the spinous processes, as well as the lesion of the interspinous ligaments, may be present with a mechanism of forced hyperflexion or hyperextension. However, since no injuries were identified by compression of the vertebral body, discs or longitudinal ligaments, it appears that the predominant axial loading occurred in the posterior elements, secondary to a dominant component of forced hyperextension.51
The radiological diagnosis in our case required a particular analysis. The tests carried out on admission (X-ray and CT) did not reveal the existence of neurologic injury, nor was suspicion of this raised.
There is a clinical picture, which has been called SCIWORA (acronym of “Spinal Cord Injury Without Radiographic Abnormality”), and defines a patient who has objective signs of myelopathy as a result of trauma without radiographic or CT evidence of a column fracture or ligament instability.10 It is thought that this picture is mainly due to a hyperextension of the rachis, and, accordingly, it is normally seen in rear-end collisions or direct craniofacial trauma.52 Another special characteristic of this picture is the concurrence of pre-existing cervical spondylosis, which predisposes the patient to spinal injury even with trivial trauma. In such cases, the presence of posterior discoarthritic bars, as well as the redundancy of ligaments owing to telescoping, may give rise to intramedullary injury, even after a slight hyperextension.53 Spinal cord concussion is also characterised by biochemical changes in the spinal cord. It most likely constitutes SCIWORA in the strictest sense.
The case we present would fit the concept of SCIWORA.
However, MRI is the main examination for ruling out spinal cord injury.54 In this sense, the most widespread classification of spinal cord injuries is that of Kulkarni et al.55 Even techniques capable of demonstrating non-visible lesions in conventional MRI have been reported, which may be useful in the assessment of spinal cord concussions.56–60
The analysis of all the above information emphasises the need to have both synostotic block vertebrae and DS present, which are the elements that promote the development of degenerative or traumatic cervical myelopathy (Schneider syndrome). As a consequence, the association of both entities must be seen as a clue for the physician to carry out in-depth studies (including magnetic resonance imaging) in the face of trauma, even if it is minor.
Equally, from the medico-legal point of view, the study of the previous state must be viewed as pathological analysis, emphasising the need for an autopsy study of the cervical spinal cord in these types of cases, to correlate with the results of radiological examinations carried out.
In short, our paper serves to reinforce the important role that, both clinically and medico-legally, the knowledge and study of the previous state in cervical trauma plays. Cases like those presented stimulate the physician to collect this history and to consider it as a risk factor in the causation of injuries or complications.
Equally, from the causal point of view, these abnormalities or changes constitute pre-existing causes to seriously consider during the assessment of corporal injuries, since their presence means that the criterion of proportionality loses strength in the face of previous indemnity, and, in other words, justifies injuries that would otherwise be difficult to explain causally due to the low scale of trauma.
ConclusionsThe association between DS and congenital vertebral synostosis has rarely been described in literature. Both pictures independently tend towards degenerative or traumatic cervical myelopathy. Consequently, the association between them must be seen as a risk factor for spinal cord injury, either spontaneously or, above all, after trauma. In these cases, radiological examination must be exhaustive, including magnetic resonance imaging, since spinal cord injury may be present even after minor trauma.
In the case of death, spinal cord autopsy is required since it can reveal subclinical injuries, clarify the nature and extent of spinal cord and skeletal injuries, and help to establish a better clinico-anatomical correlation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Arredondo Diaz JM, Aso Escario J, Obon J, Sebastián Sebastián C, Aso Vizan A, Martínez Quiñones J-V. Sinostosis cervical asociada a síndromes de Down y de Schneider. Implicaciones clínicas y médico-legales a propósito de un caso autópsico. Rev Esp Med Legal. 2018;44:73–82.