To assess the radiopharmacist’s role in a multidisciplinary team focused on the contraindications of regadenoson in order to ensure the safe use of pharmacologic vasodilator stress agents in patients undergoing SPECT-MPI.
MethodsWe ambispectively studied its safe use in 1905 patients (54.1% female, mean age: 66.6±11.7 years, range: 20–95 years). Sex, age, medical history, medications, drug allergies, and contraindications for stress testing were registered together with recommendations for the nuclear physician in charge.
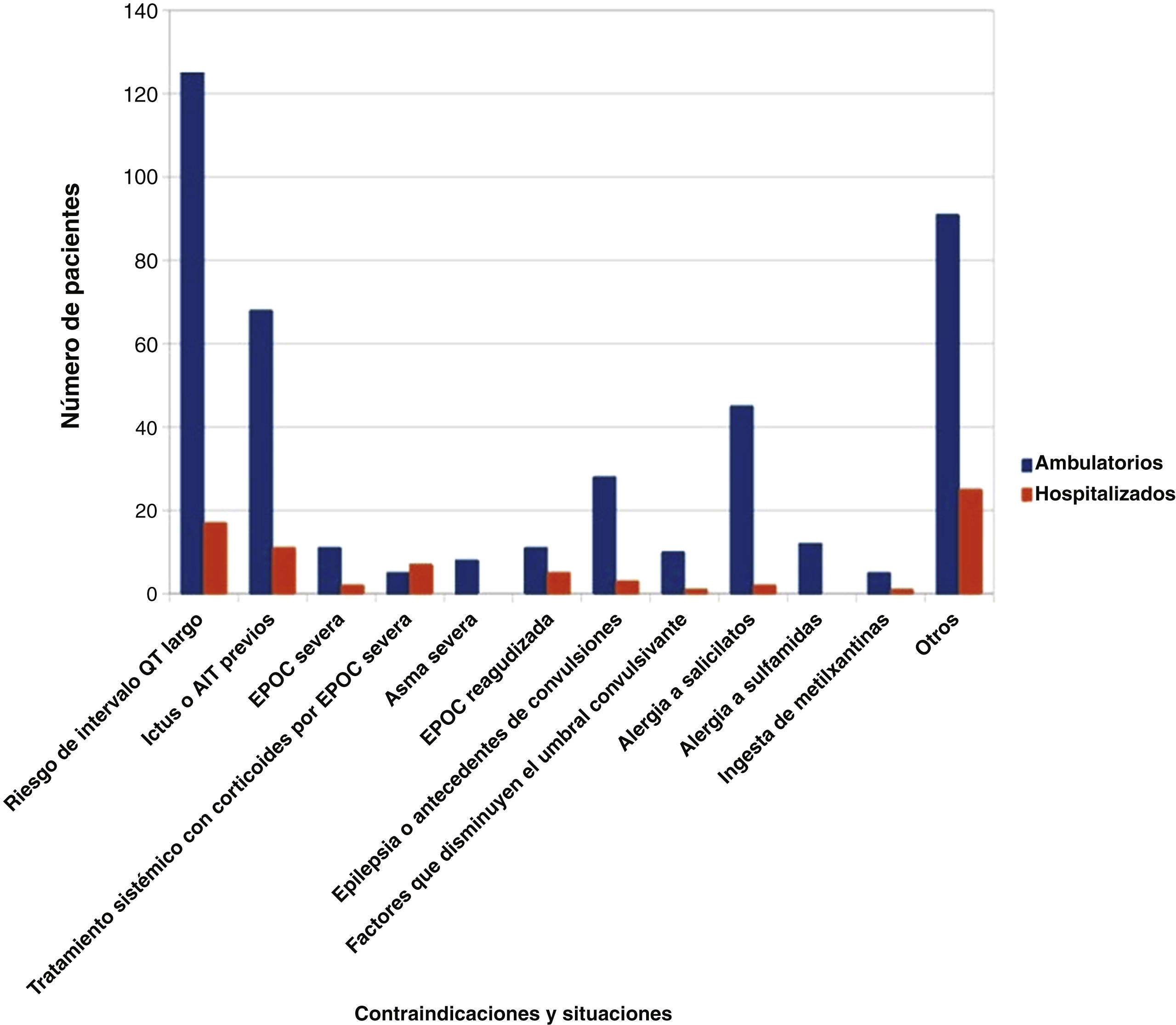
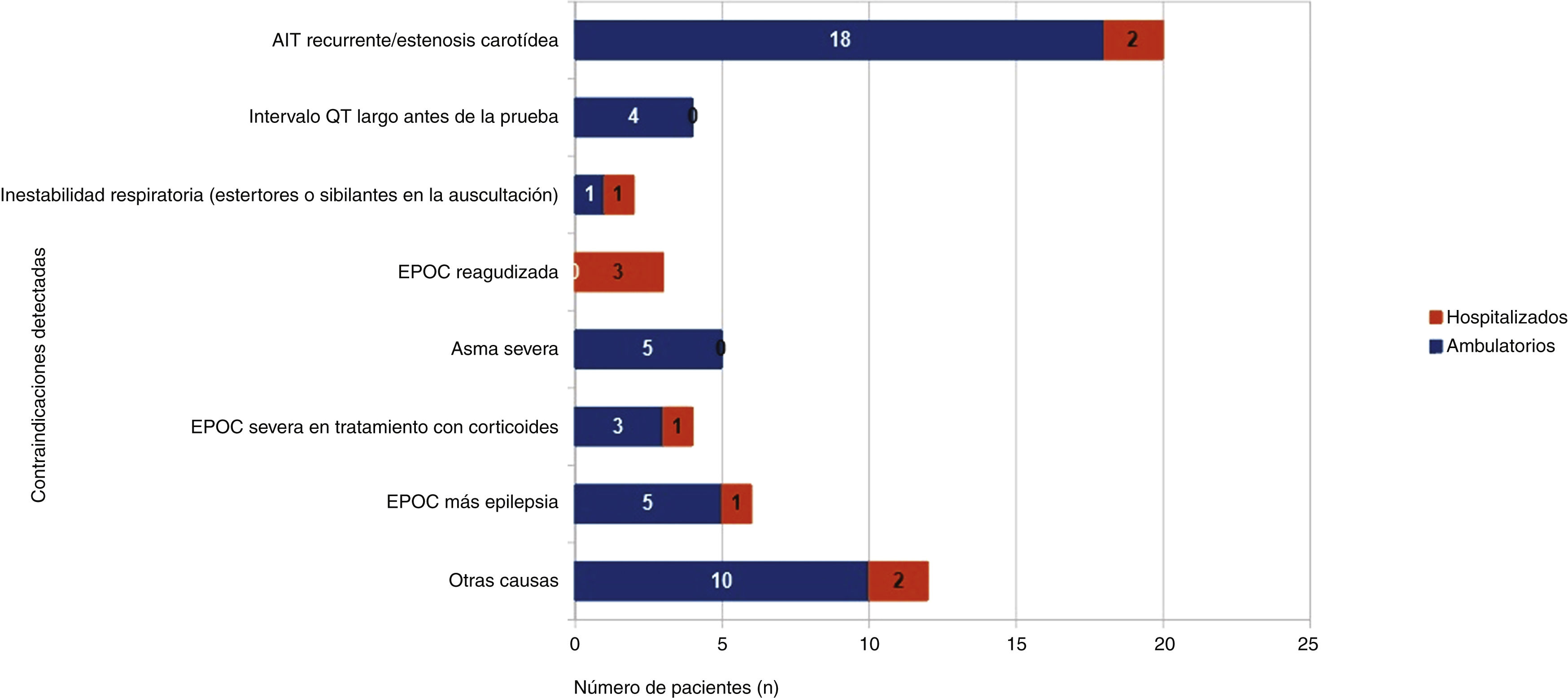
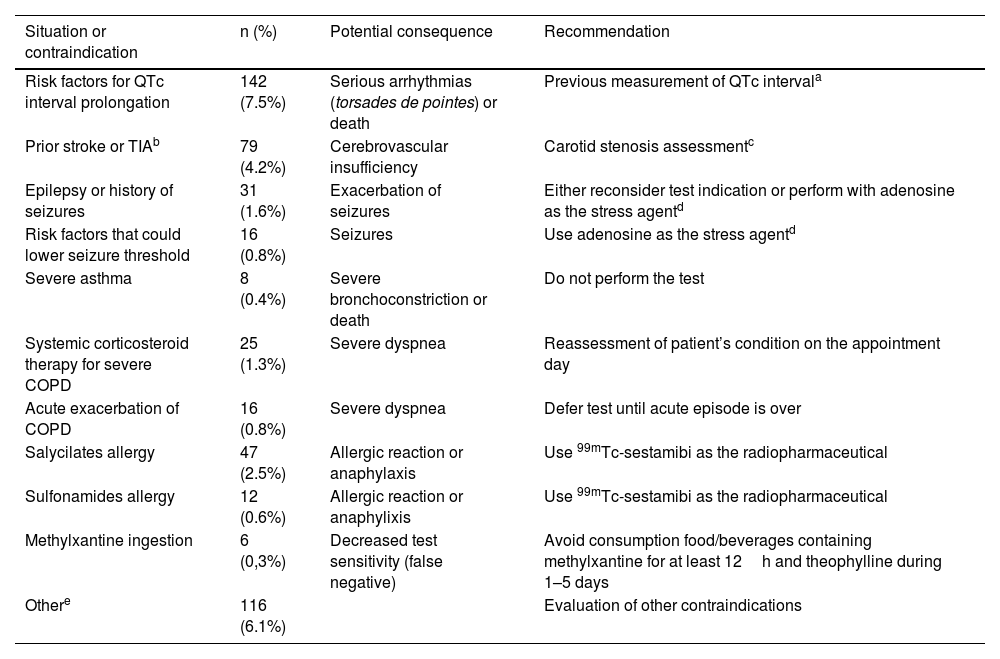
ResultsDetected contraindications and corresponding recommendations were as follows: risk factors for QTc interval prolongation 7.5% – measurement of QTc interval previously to test and monitor ECG; prior stroke or TIA 4.2% – consider carotid stenosis assessment; salicylates/sulfonamides allergy 3.1% – use 99mTc-sestamibi; epilepsy or risk factors for seizures 2.4% – use of adenosine or reconsider test indication; systemic corticosteroid therapy for severe COPD 1.3% – reassessment of patient’s condition; acute exacerbation of COPD 0.8% – defer test until acute episode is over; severe asthma 0.4% – do not perform test; methylxanthine ingestion 0.3% – avoid consumption previously; other 6.1% – evaluation of other contraindications. No contraindications were detected in 73.6% of patients. The test was canceled due to absolute contraindications in 2.9% of the requests.
ConclusionsWorking in a systematic way, the radiopharmacist was able to detect a high number of issues related to regadenoson, with one out of four patients presenting some clinical contraindication. The recommendations given by the radiopharmacist were well accepted by the nuclear physicians who changed their approach contributing to increase the safety of patients referred for MPI.
Evaluar el papel del radiofarmacéutico en un equipo multidisciplinar en la detección de contraindicaciones del regadenosón para su uso seguro en pacientes a los que se les solicitó una Single Photon Emission Computed Tomography (SPECT) de perfusión miocárdica.
MétodosSe estudió ambispectivamente su uso seguro en 1.905 pacientes (54,1% mujeres, edad media: 66,6±11,7, rango: 20–95). Se registraron datos de género, edad, historial médico, medicación, alergias medicamentosas y contraindicaciones para el estrés farmacológico, así como las recomendaciones realizadas al médico nuclear responsable.
ResultadosLas contraindicaciones detectadas y las correspondientes recomendaciones fueron las siguientes: riesgo de prolongación del intervalo QT corregido por la frecuencia cardiaca (QTc) (7,5%) – comprobación previa del intervalo QTc y monitorización del electrocardiograma (ECG), ictus o accidente isquémico transitorio (AIT) previo (4,2%) – evaluación de estenosis carotídea, alergia a salicilatos y/o sulfamidas (3,1%) – empleo de 99mTc-sestamibi, epilepsia o riesgo de convulsiones (2,4%) – uso de adenosina o reconsiderar indicación, tratamiento con corticoesteroides sistémicos en enfermedad pulmonar obstructiva crónica (EPOC) severa (1,3%) – reevaluar condiciones del paciente; exacerbación de EPOC (0,8%) – posponer hasta resolución del episodio agudo; asma grave (0,4%) – no realizar la prueba; toma de metilxantinas (0,3%) – evitar su consumo previo; otras (6,1%) – evaluación de cada restricción. No se observó rechazo en 73,6% de los usuarios. Se anularon 2,9% de las peticiones debido a contraindicaciones absolutas.
ConclusionesTrabajando de forma sistemática, el radiofarmacéutico detectó un elevado número de incidencias, presentando uno de cada cuatro pacientes alguna contraindicación clínica. Las recomendaciones dadas fueron aceptadas por los médicos nucleares, que modificaron su enfoque, incrementando así la seguridad de estos usuarios.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)