The aim of this study was to evaluate the morphological changes of rat lacrimal glands at the third month following radioiodine (RAI) treatment and the radioprotective effect of montelukast (ML) sodium against RAI-related lacrimal gland damage.
MethodsFifty female Wistar Albino rats were divided into three groups. The control group (n=10) consisted of rats with no intervention. RAI group (n=20) consisted of rats treated with oral 131I (111 MBq). The ML group (n=20) consisted of rats treated with intraperitoneal 10mg/kg/day ML sodium, starting three days before and continuing for one week after oral RAI administration. Intraorbital (IG), extraorbital (EG) and Harderian glands (HG) were removed bilaterally after three months.
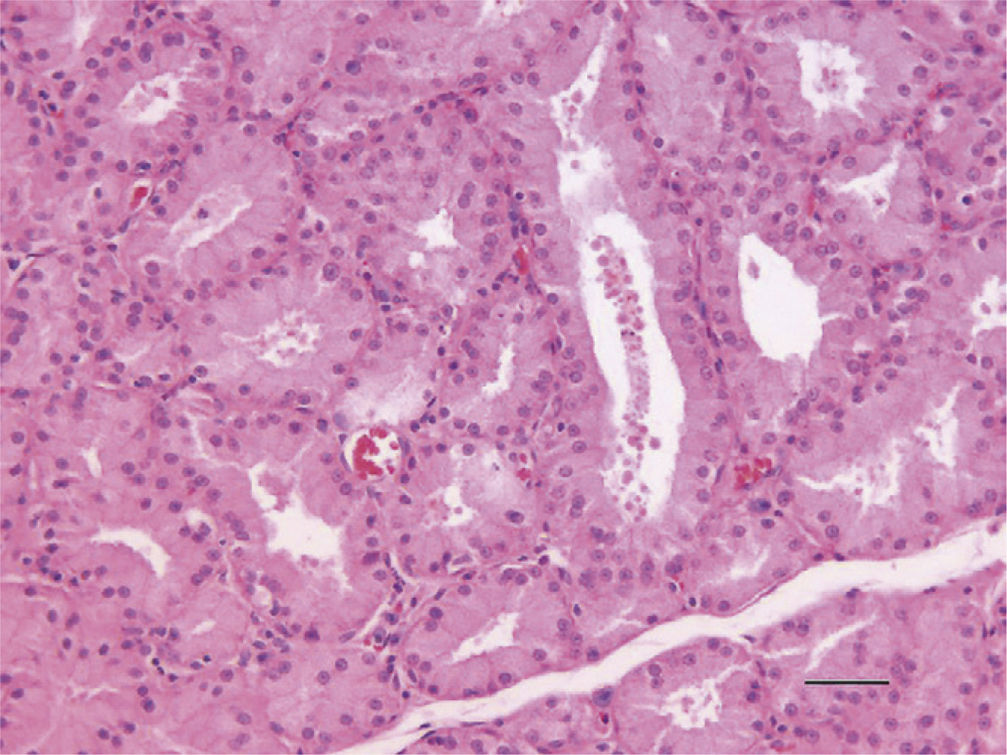
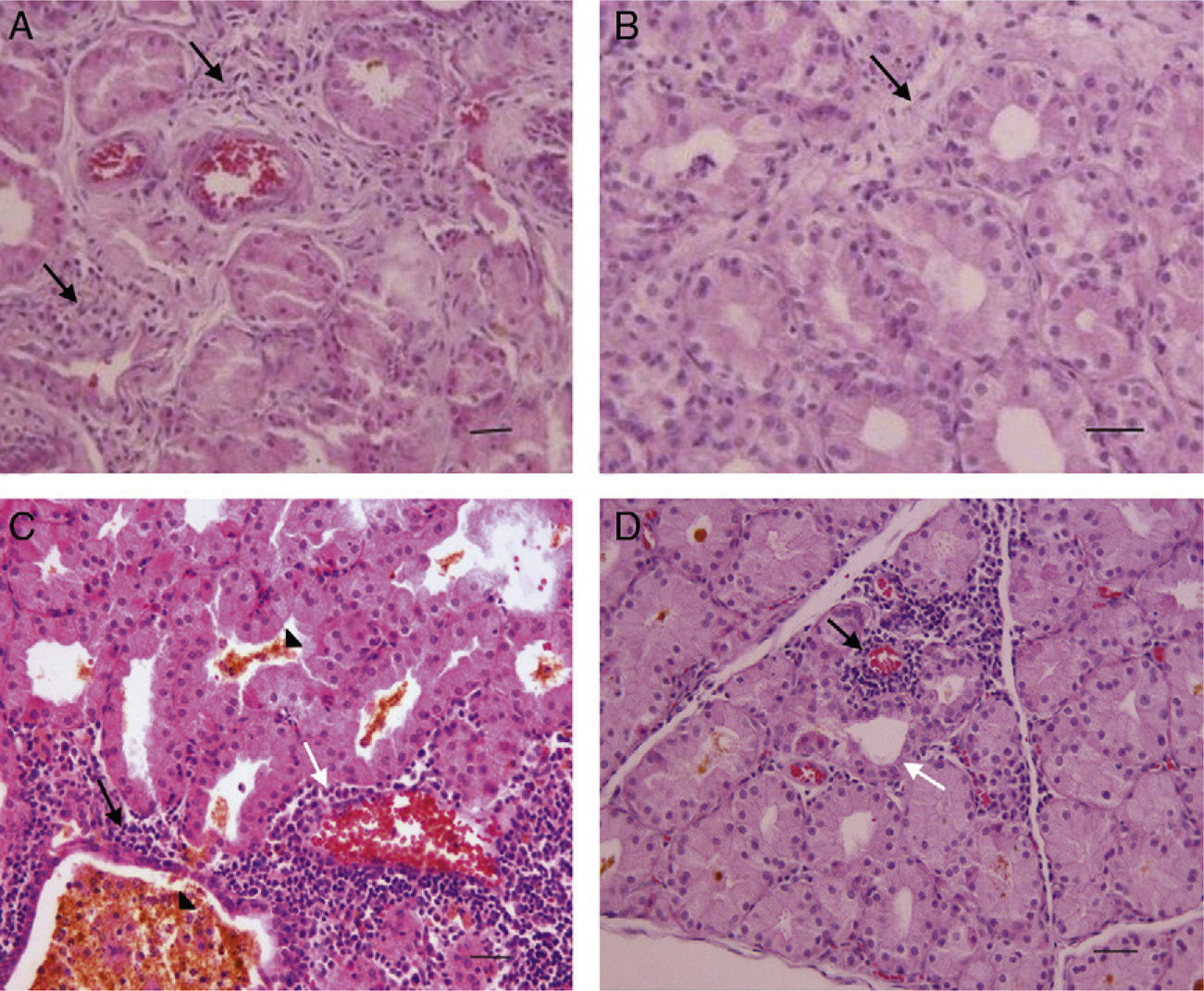
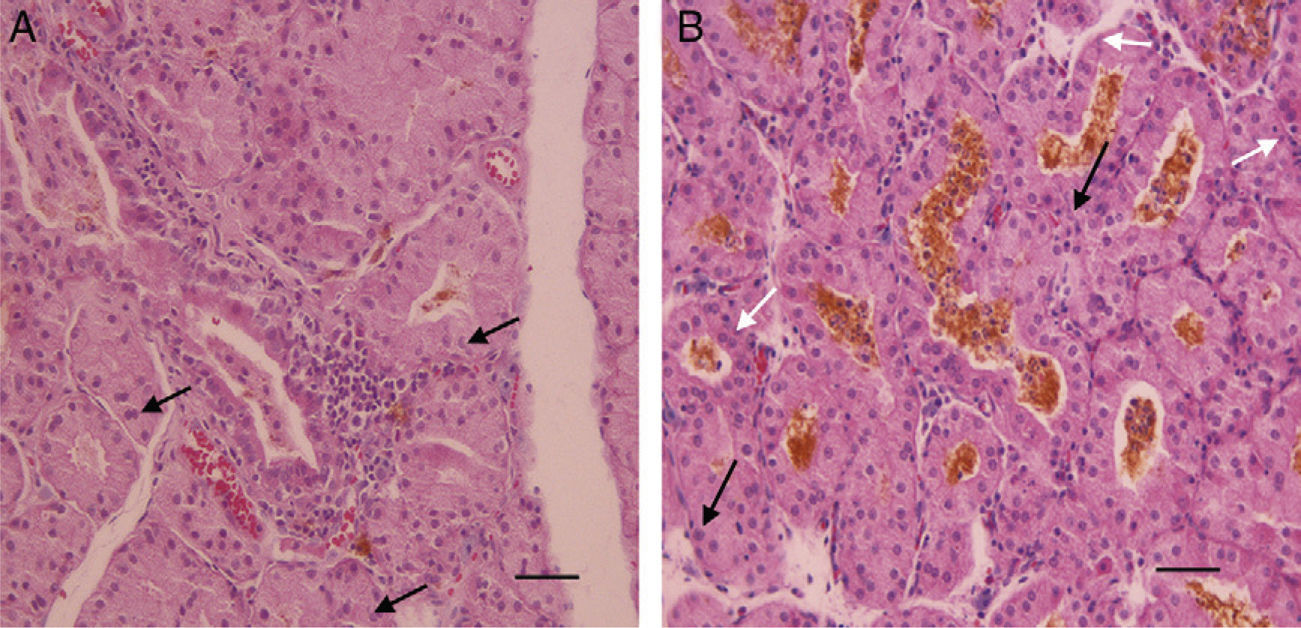
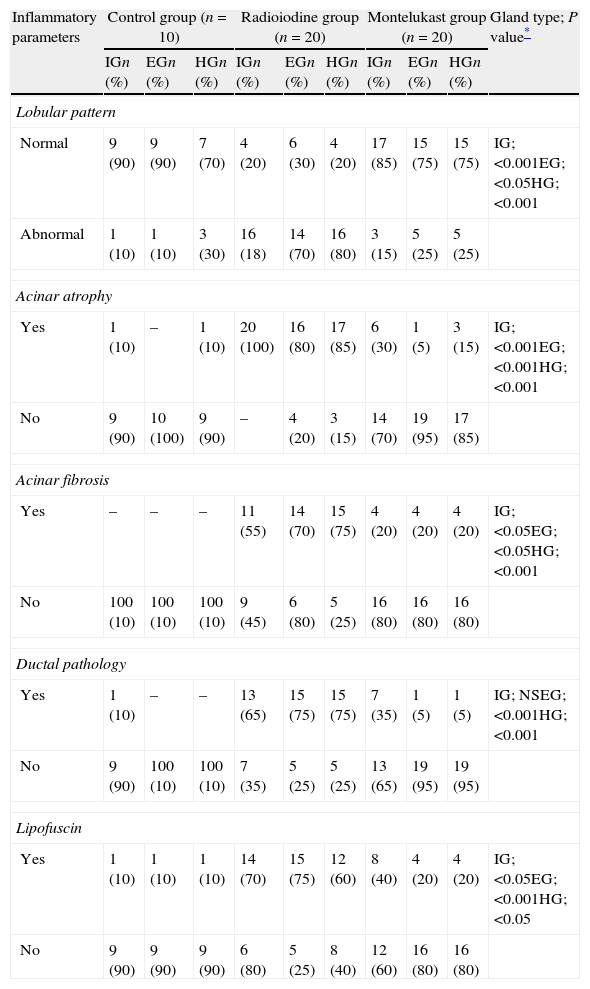
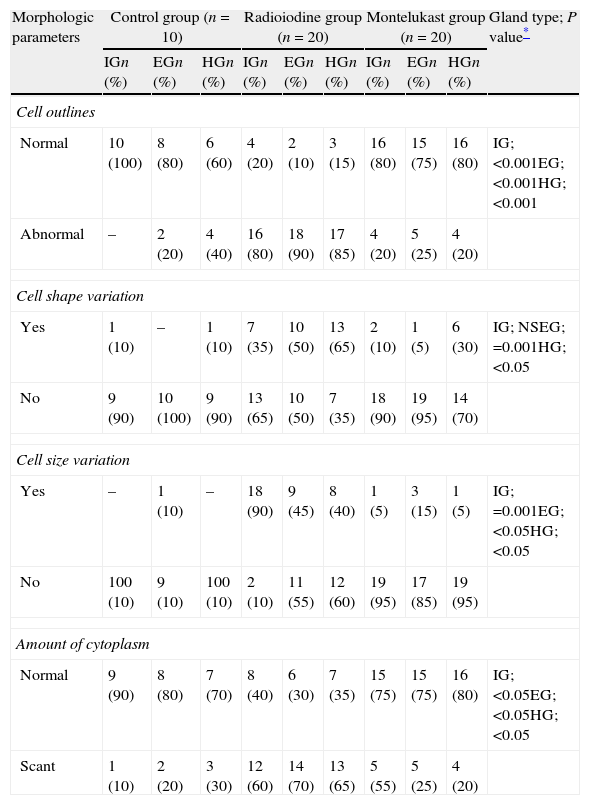
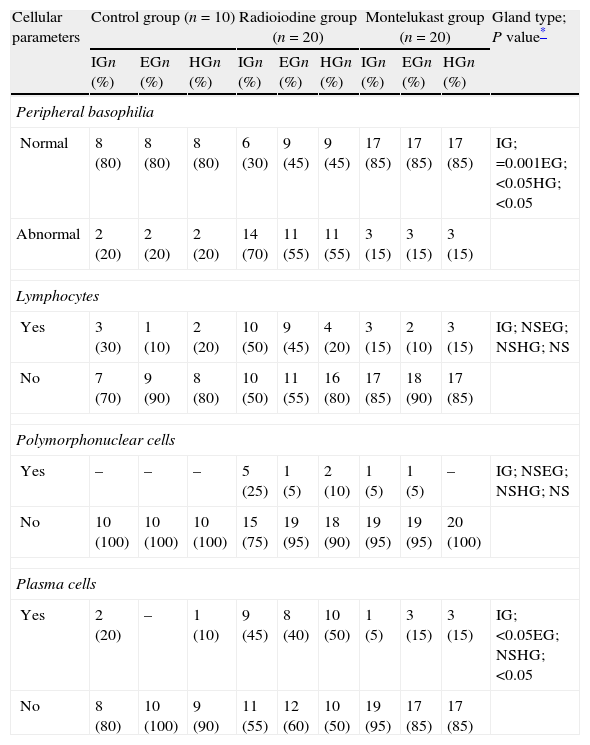
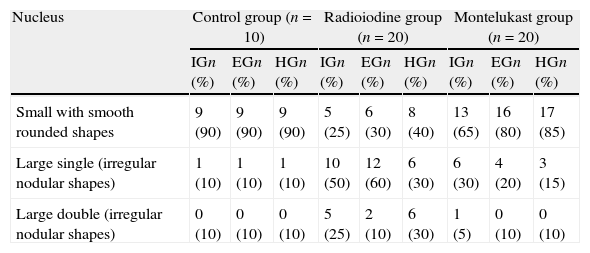
ResultsThe existence of acinar atrophy, acinar fibrosis, abnormal cell lines, peripheral basophilia, cell size variation and decrease in amount of cytoplasm was significantly more common in the RAI-rat treated group than in the ML group, in each of the glands. The ML-treated group had less-frequent cell shape variation in EG (P=0.001) and HG (P=0.027), cell size variation in IG (P<0.001) and HG (P=0.01), ductal pathology in EG (P<0.001) and HG (P<0.001) and lipofuscin accumulation in EG (P=0.001) and in HG (P=0.01) than the RAI-treated group.
ConclusionsRAI treatment seems to cause morphological damage to rat lacrimal glands, and ML sodium effectively protects against damage to lacrimal glands.
El objetivo de este estudio fue evaluar los cambios morfológicos de las glándulas lagrimales de las ratas en el tercer mes después del tratamiento con iodo radiactivo (RAI) y el efecto radioprotector de montelukast (ML) sódico contra los daños causados por el RAI en la glándula lagrimal.
MétodosCincuenta ratas hembra albinas de raza Wistar fueron divididas en 3 grupos. El grupo de control (n=10) estaba formado por ratas no intervenidas. El grupo de RAI (n=20) estaba formado por ratas tratadas con 131I orales (111MBq). El grupo ML estaba formado por ratas tratadas con 10mg/kg/d de ML sódico intraperitoneal comenzando 3d antes y continuando durante una semana después de la administración oral del RAI. Las glándulas intraorbitarias (IG), las extraorbitarias (EG) y las de Harder (HG) se eliminaron bilateralmente en 3 meses.
ResultadosLa existencia de atrofia acinar, fibrosis acinar, líneas celulares anormales, basofilia periférica, la variación de tamaño de las células y la disminución de la cantidad de citoplasma eran significativamente más común en todas las glándulas separadas en el grupo de ratas tratadas con RAI que en el grupo de ratas tratadas con ML. El grupo tratado con ML presentaba menor variación frecuente de la forma celular en las EG (p=0,001) y las HG (p=0,027), variación de tamaño de las células en las IG (p<0,001) y las HG (p=0,01), enfermedad ductal en las EG (p<0,001) y las HG (p<0,001) y menor acumulación de lipofuscina en las EG (p=0,001) y en las HG (p=0,01) que el grupo tratado con RAI.
ConclusionesEl tratamiento con RAI parece causar daño morfológico en las glándulas lagrimales de las ratas y el ML sódico protege eficazmente a las glándulas lagrimales de ese daño.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)