To determine the results of radioembolization transarterial (TARE), in the treatment of liver tumors, a retrospective evaluation was performed after 112 TARE with 90Y-microspheres administered in 82 patients in a single hospital, analyzing efficacy and safety, after a follow-up greater than or equal to 1 year post-TARE in all patients, and evaluating the possible relationship between treatment response and patient survival.
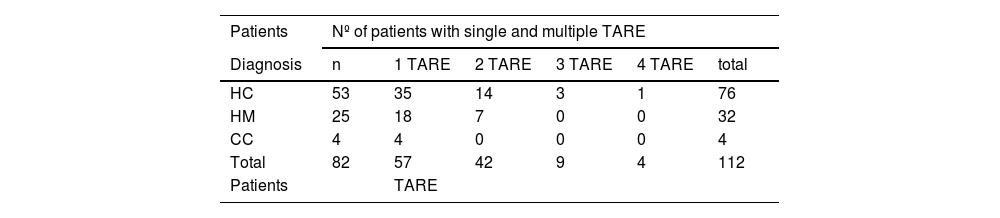
Material and methodsWe have administered 57 single TARE and 55 multiple TARE in patients with hepatocellular carcinoma (53), liver metastases (25) and cholangiocarcinoma (4), with prior multidisciplinary evaluation, clinical, angiographic and gammagraphic (planar/SPECT/SPECT-CT with 99mTc-MAA), multicompartment model (MIRD equations), post-TARE screening (planar/SPECT/SPECT-CT), clinical and radiological follow-up, tumor response evaluation (mRECIST criteria) and Kaplan–Meier analysis to determine progression-free survival (PFS) and overall survival (OS).
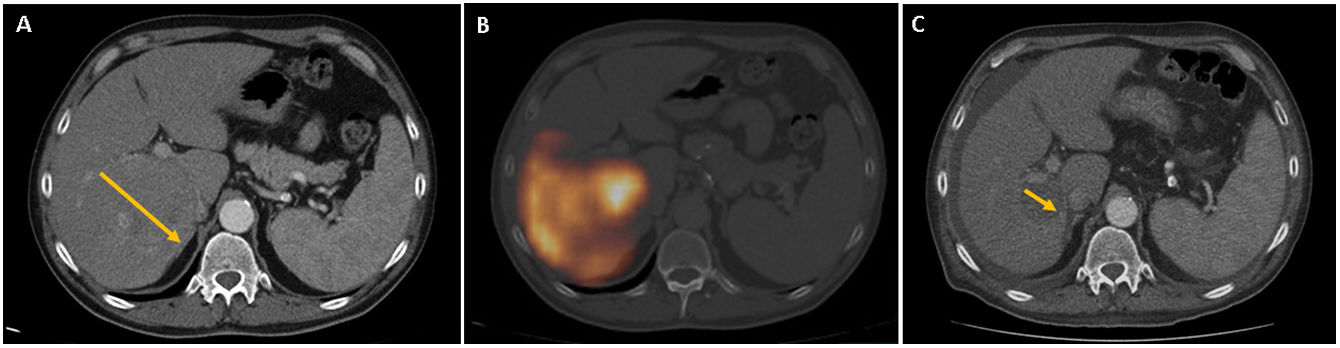
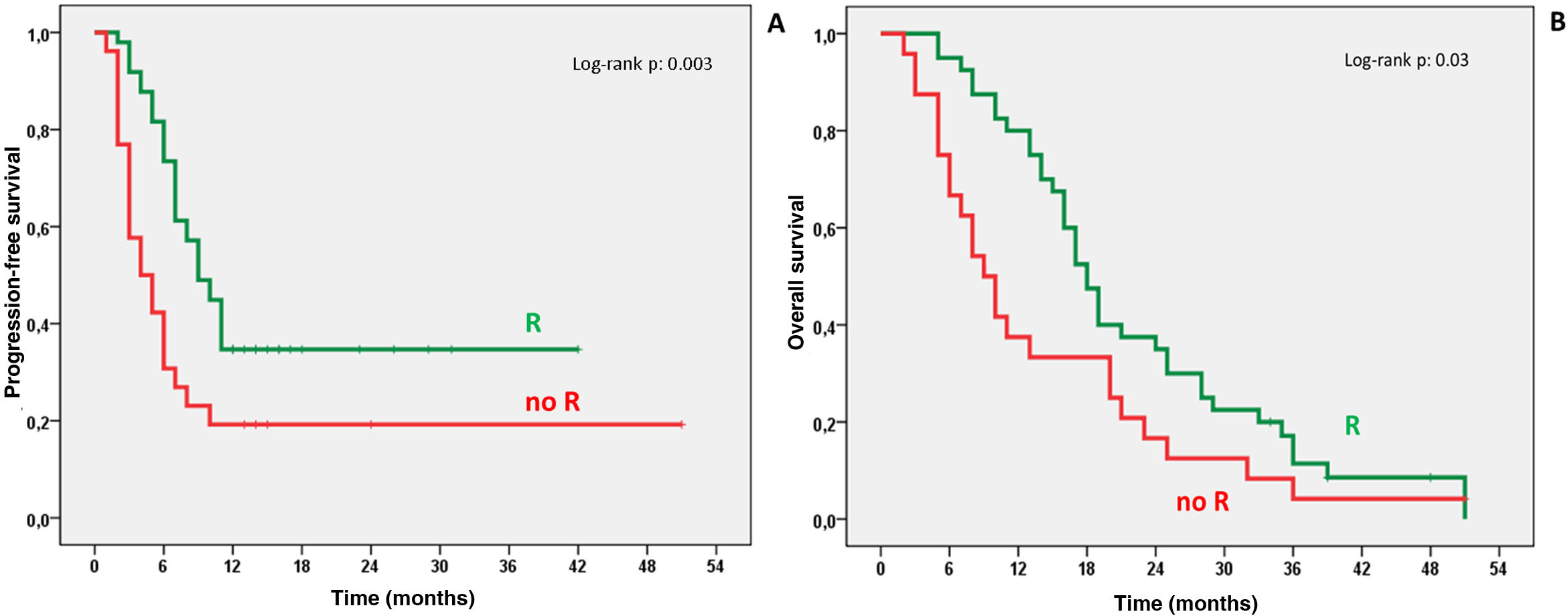
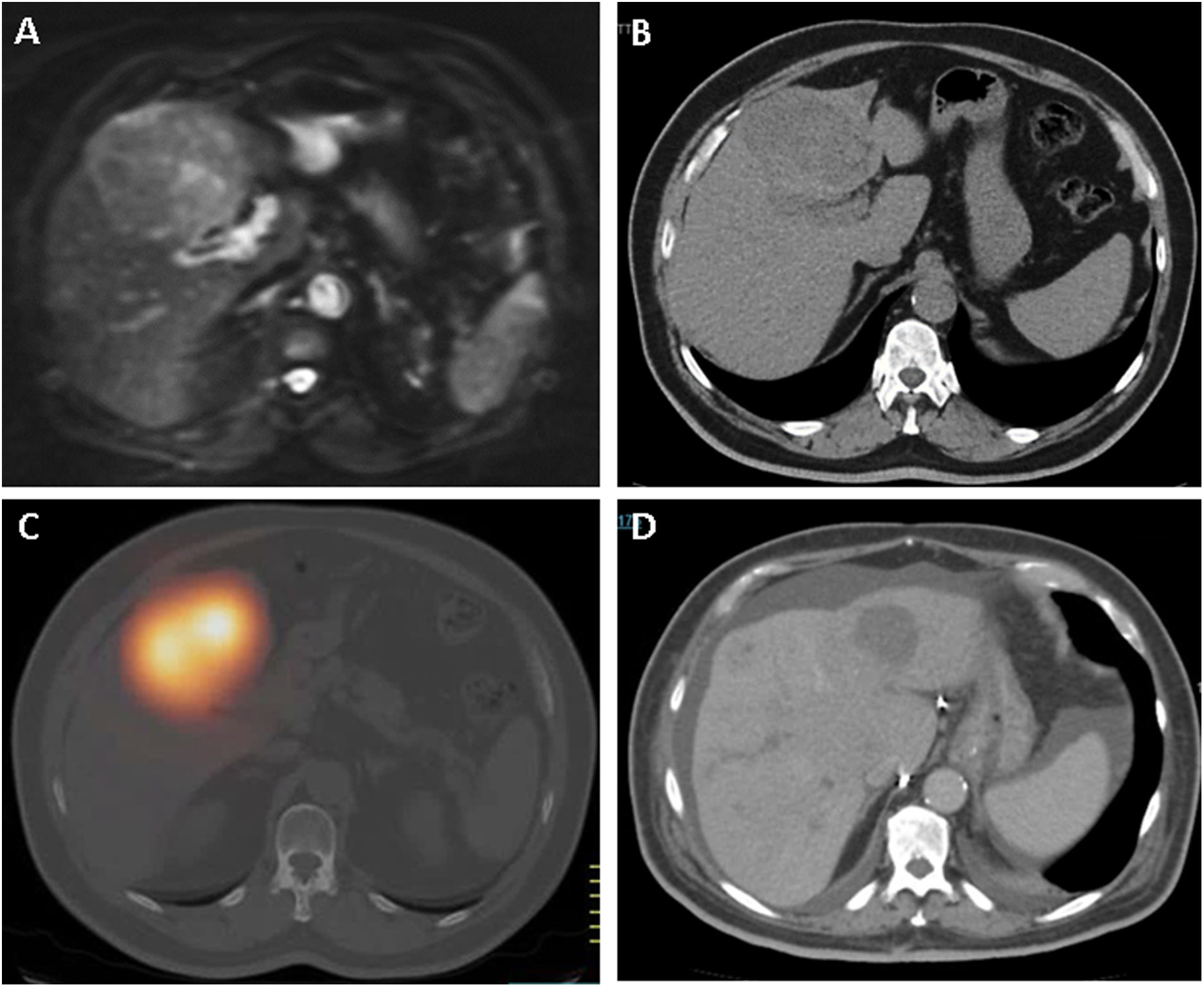
ResultsTherapeutic intention was palliative (82%) and as bridge to liver transplantation/surgical resection (17%). We obtained response (R), complete or partial, in 65.9% of cases. One year after TARE 34.7% of patients with R and 19.2% of non-R were progression-free (P: .003), with OS of 80% for R and 37.5% for non-R (P: .001). Survival analysis showed median OS of 18 months (95% CI 15.7–20.3) for R and 9 months (95% CI 6.1–11.8) for non-R (P: .03). We found mild (27.6%) and severe (5.3%) side effects, all of them resolved, without higher incidence after multiple TARE.
ConclusionTARE with 90Y-microspheres, in appropriately selected patients with liver tumors, provides therapeutic efficacy and low rate of toxicity, with higher PFS and OS in patients with TARE response compared to those who did not respond.
Para conocer los resultados de la radioembolización (transarterial radioembolization o TARE), en el tratamiento de tumores hepáticos, se realizó una valoración retrospectiva tras 112 TARE con 90Y-microesferas administradas en 82 pacientes en un único hospital, analizando la eficacia y la seguridad, tras un seguimiento mayor o igual a 1 año post-TARE en todos los pacientes, y evaluando la posible relación entre la respuesta al tratamiento y la supervivencia de los pacientes.
Material y métodosSe administraron 57 TARE únicas y 55 TARE múltiples en pacientes con hepatocarcinoma (53), metástasis hepáticas (25) y colangiocarcinoma (4), con evaluación previa multidisciplinar clínica, angiográfica y gammagráfica (planar/SPECT/SPECT-TC con 99mTc-MAA), modelo multicompartimental(ecuaciones MIRD), valoración gammagráfica post-TARE (planar/SPECT/SPECT-TC), seguimiento clínico-radiológico, evaluación de respuesta tumoral(criterios mRECIST) y análisis (Kaplan Meier) de supervivencia libre de progresión (SLP) y supervivencia global (SG).
ResultadosLa intención terapéutica fue paliativa (82%) y como puente a trasplante hepático/resección quirúrgica (17%). Se obtuvo respuesta(R), completa o parcial, en el 65.9% de los casos. Al año post-TARE estaban libres de progresión el 34.7% de pacientes con R y 19.2% de los no R (P: .003), con SG del 80% para los R y 37.5% para los no R (P: .001). Las curvas supervivencia mostraron mediana de SG de 18 meses (95% IC 15.7–20.3) para los R y 9 meses (95% IC: 6.1–11.8) para los no R (P: .03). Efectos secundarios leves (27.6%) y severos (5.3%) resueltos, sin mayor incidencia tras TARE múltiple.
ConclusionesLa TARE con 90Y-microesferas en pacientes adecuadamente seleccionados con tumores hepáticos, aporta eficacia terapéutica y bajo índice de toxicidad, con SLP y SG superiores en los pacientes con respuesta a la TARE respecto a los que no respondieron.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)