Hematological parameters are considered to be implicated in the pathogenesis of acute ischemic stroke. Otherwise, to our knowledge, it is still not known whether there are any differences between small and large vessel strokes, in terms of these parameters.
MethodsProspectively included in the study, a hundred small-vessel stroke (n = 50) and large-vessel stroke patients (n = 50) were managed in Ibn-sena teaching hospital, Mosul between Oct. 2023 and Aug. 2024. Complete history, neurological examination was done at admission, and blood samples for CBC were collected within the first 48 h of admission. White blood cells (WBCs), neutrophils, lymphocytes, neutrophil/lymphocyte ratio (N/L ratio), monocytes, eosinophils, basophil, hemoglobin (HB), RBC count, packed cell volume (PCV), red cell distribution width (RDW), red cell distribution width index (RDWI), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, platelet distribution width (PDW) and mean platelet volume (MPV) were obtained, and comparison between the two groups in terms of these parameters was done and with 50 control patients.
ResultsNeutrophil to lymphocyte ratio was highest in large vessel disease group and lowest in control group (small versus large, small versus control, large versus control, P = 0.003, <0.001, <0.001 respectively), and the opposite was the case with lymphocyte count (small versus large, small versus control, large versus control P = 0.003, <0.001, <0.001, respectively). We have found expressively higher total white blood cell (P = 0.004, <0.001), and lower monocyte count (P = 0.001, <0.001) in patients with small and large vessel diseases respectively compared to controls. Platelet count and mean platelet volume were significantly higher in small vessel disease compared to controls (P = 0.04, 0.02, respectively). Neutrophil count and RDW were inferentially higher in the large vessel group compared to controls (P = 0.005, 0.03, respectively), and no other significant differences were found among groups.
ConclusionsHematological parameters are invaluable tools to confirm the diagnosis of ischemic stroke and to predict stroke subtypes with modest sensitivity.
Se considera que los parámetros hematológicos están implicados en la patogénesis del accidente cerebrovascular isquémico agudo. Sin embargo, hasta donde sabemos, aún no se ha establecido si existen diferencias entre los accidentes cerebrovasculares de pequeños y grandes vasos en términos de estos parámetros.
MétodosDe manera prospectiva, se incluyeron en el estudio cien pacientes con accidente cerebrovascular de pequeños vasos (n = 50) y de grandes vasos (n = 50), atendidos en el Hospital Docente Ibn-Sina, Mosul, entre octubre de 2023 y agosto de 2024. Se realizó historia clínica completa y examen neurológico al ingreso, y se recolectaron muestras de sangre para hemograma en las primeras 48 horas de admisión. Se midieron los niveles de leucocitos (WBC), neutrófilos, linfocitos, relación neutrófilo/linfocito (N/L), monocitos, eosinófilos, basófilos, hemoglobina (HB), recuento de glóbulos rojos (RBC), volumen corpuscular medio (MCV), hemoglobina corpuscular media (MCH), concentración de hemoglobina corpuscular media (MCHC), volumen plaquetario medio (MPV), ancho de distribución de plaquetas (PDW), volumen plaquetario medio (PCT), ancho de distribución de glóbulos rojos (RDW), índice de distribución de glóbulos rojos (RDWI), y se compararon estos parámetros entre los dos grupos y con 50 pacientes controles.
ResultadosLa relación neutrófilo/linfocito fue mayor en el grupo de enfermedad de grandes vasos y menor en el grupo control (pequeños vs grandes, pequeños vs control, grandes vs control, p = 0.003, <0.001, <0.001 respectivamente), y lo opuesto ocurrió con el conteo de linfocitos (pequeños vs grandes, pequeños vs control, grandes vs control, p = 0.003, <0.001, <0.001 respectivamente). Se encontró un aumento significativo en el recuento total de leucocitos (p = 0.004, <0.001), y una disminución en el conteo de monocitos (p = 0.001, <0.001) en pacientes con enfermedad de pequeños y grandes vasos, respectivamente, en comparación con los controles. El recuento de plaquetas y el volumen plaquetario medio fueron significativamente mayores en pacientes con enfermedad de pequeños vasos en comparación con controles (p = 0.04, 0.02 respectivamente). El recuento de neutrófilos y RDW fueron inferencialmente mayores en el grupo de grandes vasos en comparación con los controles (p = 0.005, 0.03 respectivamente), sin que se encontraran otras diferencias significativas entre los grupos.
ConclusionesLos parámetros hematológicos son herramientas valiosas para confirmar el diagnóstico de accidente cerebrovascular isquémico y para predecir los subtipos de ictus con una sensibilidad modesta.
Of the distinguished ischemic stroke subtypes are large territorial infarction which is the most common type caused by atherothrombosis of large vessels or thromboembolization from the heart or extracranial vessels. Lacunar infarction which represents roughly for 15% of ischemic stroke is due to occlusion of deep penetrating branches of large cerebral blood vessels mainly caused by thrombosis in situ.1 A limit of 15–20 mm in diameter size distinguishes between the two subtypes radiologically.2 Changes in vessel wall, blood flow or in circulating blood elements are the major causes of stroke according to Virchow's theory.3 A substance of researches has emerged in recent years to elucidate the role of blood elements in diagnosis and management of ischemic stroke patients.4–6 The changes in these elements advocate their potential role as inflammatory markers in ischemic stroke pathophysiology.7 While most researches focused on the relation between these parameters and length of hospital stay or prognosis in general, little differentiated between small and large-vessel strokes in terms of these parameters.8–11 We are coming to see whether these elements are changing in all infarction types and whether they can predict stroke subtypes.
Material and methodsStudy design and populationIt is a prospective case-control study that included ischemic stroke patients who were admitted to Ibn-sena teaching hospital, Mosul, Iraq from Oct. 2023 to Aug. 2024. Randomization of the sample was done by selecting patients who were admitted during the authors' call duty; a total number of 100 patients applied the inclusion criteria in the study. After admission, complete history and physical examination were done and a diagnosis of ischemic stroke was confirmed using CT scan and/or MRI. Patients were divided into 2 groups according to the size of infarction. Fifty small vessel stroke (lacunar infarction); those patients who presented with first-ever acute onset irreversible focal neurological deficit and imaging study showed an ischemic area of less than 15 mm in diameter, and fifty large vessel stroke patients who presented with first-ever acute onset focal deficit and imaging study showed territorial infarction larger than 15 mm in diameter. Patients with severe anemia, fever on the day of admission, patients with end-stage kidney, liver disease or a history of underlying neoplasm were excluded from our study. A hundred cases were matched with 50 control cases. Control patients were those who were seen in out-patient clinic complaining from other than vessel, heart, kidney, liver or neoplastic diseases, e.g. cases of uncomplicated diabetes or hypertension. Blood was drawn from all participants, and a complete blood count was done within the first 48 h of admission.
Data collectionPatient data, including age, sex, underlying diseases (hypertension, diabetes, hyperlipidemia, and ischemic heart disease), complete blood count and parameters including white blood cells (WBC), neutrophils, lymphocytes, neutrophil/lymphocyte ratio (N/L ratio), monocytes, eosinophils, basophil, hemoglobin (HB), RBC count, packed cell volume (PCV), red cell distribution width (RDW), red cell distribution width index (RDWI), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count, platelet distribution width(PDW) and mean platelet volume (MPV) were gathered using hematology analyzer (NIHON KOHDEN, cell tac α, Japan, 2023). Comparison among small, large vessel stroke and control groups was done using these parameters.
Data analysisThe collected data were introduced into Microsoft Excel 2010 and analyzed by SPSS statistical analysis software version 23 (SPSS Inc., Chicago, IL, USA). First, the Smirnov–Kolmogorov test was used to measure the normal distribution of variances. Demographic variables were analyzed by descriptive statistical methods, and presented with percentage, frequency, mean and standard deviation (SD). Analysis of quantitative variables was performed using one-way ANOVA or Kruskal-Wallis test depending on the distribution of variables. Chi-square test was used for the analysis of categorical data. The P-values less than 0.05 were considered statistically significant.
ResultsThere were 50 small vessel stroke patients, where the size of infarction was less than 15 mm with a mean age (58.15 years ± SD 13.2), and 50 large territorial infarction with mean age (65.2 years ± SD 13.3), and 50 control cases with mean age (56.2 years ± SD 11.4). Sided weakness, numbness and dysarthria were the main presentations of patients with lacunar infarctions (32, 7, 4 cases, respectively), whereas sided weakness and disturbed level of consciousness ranked first in large vessel occlusion cases (27, 11 cases, respectively). The latter was mainly due to a large area of ischemia, perifocal edema or hemorrhagic transformation. Feeling of spinning environment was the complaint of 3 patients having small pontine or medullary infarctions and a patient with a large cerebellar infarction. Left cerebellopontine angle small infarction caused left-sided incoordination in one patient, whereas large cerebellar infarctions produced hemi ataxia in two patients. Aphasia was the presentation of two patients with large vessel occlusion but one with small-sized left thalamic ischemia. There was a small vessel disease patient who presented with difficulty to swallow and a large vessel disease patient presented with a fall attack. Complaining from abnormal body movement, a patient with caudate ischemia and two with cortical infarctions ended the list (Table 1).
Patients’ presentations at emergency room and definite diagnoses made by head CT or brain MRI scan.
| Presentation | Small Vessel | Head CT or brain MRI | Large Vessel | Head CT or brain MRI |
|---|---|---|---|---|
| Sided weakness | 32 | Infraction at different areas | 27 | Superior branch of middle cerebral artery infarction |
| Sided numbness | 7 | Infarction at different areas | 3 | Inferior branch of middle cerebral artery infraction |
| Hemi ataxia | 1 | Lacunar infarction at Cerebellopontine Angle | 2 | Cerebellar infarctions |
| Aphasia | 1 | Left thalamic infraction | 2 | Broca area infarction |
| Abnormal movement | 1 | Caudate infarction | 2 | Cortical infraction |
| Disturbed level of consciousness | 0 | 11 | Large middle cerebral artery infarction | |
| Vertigo & dizziness | 3 | Pontine or lateral medullary infraction | 1 | Cerebellar infarction |
| Dysarthria | 4 | Infarction at different areas | 1 | Large territorial infarction |
| Fall | 0 | 1 | Occipital lobe infarction | |
| Dysphagia | 1 | Pontine Infarction | 0 |
No significant differences were found between small and large vessel stroke patients in terms of sex, hypertension, diabetes, or smoking status (P = 0.32, 0.22, 0.1, 1, respectively). Atrial fibrillation was significantly higher in the large vessel group (P = 0.04). Chi-square test was used to compare between categorical variables, and p value of <0.05 was considered significant (Table 2).
Clinical features of patients with small and large vessel infarction.
| Categorical Variable | Small vessel | Large vessel | Odd ratio | Confidence interval (95%) | P-value |
|---|---|---|---|---|---|
| Sex M/F⁎ | 30/20 | 25/25 | 1.5 | 0.68–3.31 | 0.32 |
| Hypertension P/A | 22/28 | 16/34 | 0.6 | 0.27–1.40 | 0.22 |
| Diabetes P/A | 34/16 | 26/24 | 0.51 | 0.23–1.10 | 0.10 |
| Smoking Status P/A | 16/34 | 16/34 | 1.00 | 0.43–2.3 | 1.00 |
| Atrial fibrillation (ECG) P/A | 3/47 | 10/40 | 3.9 | 1.01–15.22 | 0.04 |
In Table 3, one-way ANOVA was used for comparison among normally distributed variances. Tukey's HSD post-hoc test was used for multiple group comparisons after performing one-way ANOVA. Elements that were compared by this method were as follows: neutrophil, lymphocyte, RBC count, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), and platelet count. The key differences among small, large vessel occlusion and control groups were in lymphocyte count that was highest in the control group and lowest in the large vessel disease group (small vs large, small vs control, large vs control P = 0.003, <0.001, <0.001, respectively). Neutrophil and red cell distribution width were inferentially higher in the large vessel occlusion group in comparison to the control group (P = 0.03, 0.005, respectively). A significantly higher mean platelet count was recorded in the small vessel occlusion group compared to the control group (P = 0.04).
Differences among the small, large vessel stroke and control groups in equally distributed variances.
| Variables | Subgroups | Mean ± SD (S) | Mean ± SD (L) | Mean ± SD (C) | P-value |
|---|---|---|---|---|---|
| Neutrophil count % | small–largesmall–controllarge–control | 70.67 ± 10.2 | 92.7 ± 108.2 | 59.8 ± 10.4 | 0.190.670.03 |
| Lymphocyte count % | small–largesmall–controllarge–control | 23.58 ± 9.58 | 17.37 ± 8.32 | 32.72 ± 9.79 | 0.003<0.001<0.001 |
| RBC count ×1012/L | small–largesmall–controllarge–control | 4.97 ± 0.97 | 4.81 ± 0.62 | 4.78 ± 0.63 | 0.550.420.98 |
| PCV%⁎ | small–largesmall–controllarge–control | 40.68 ± 6.68 | 47.2 ± 51.89 | 39.73 ± 5.00 | 0.530.990.44 |
| MCV (fL) | small–largesmall–controllarge–control | 80.61 ± 11.4 | 83.09 ± 8.41 | 83.51 ± 7.35 | 0.370.260.97 |
| MCH (pg) | small–largesmall–controllarge–control | 27.58 ± 3.14 | 27.5 ± 3.48 | 27.69 ± 2.7 | 0.990.980.94 |
| MCHC (g/dL) | small–largesmall–controllarge–control | 33.5 ± 1.15 | 33.04 ± 1.34 | 33.22 ± 1.41 | 0.180.530.77 |
| RDW (fL) | small–largesmall–controllarge–control | 41.54 ± 3.08 | 42.76 ± 4.37 | 40.07 ± 4.66 | 0.310.210.005 |
| Platelet count ×109/L | small–largesmall–controllarge–control | 268.32 ± 77.87 | 238.54 ± 77.80 | 230.76 ± 69.89 | 0.120.040.86 |
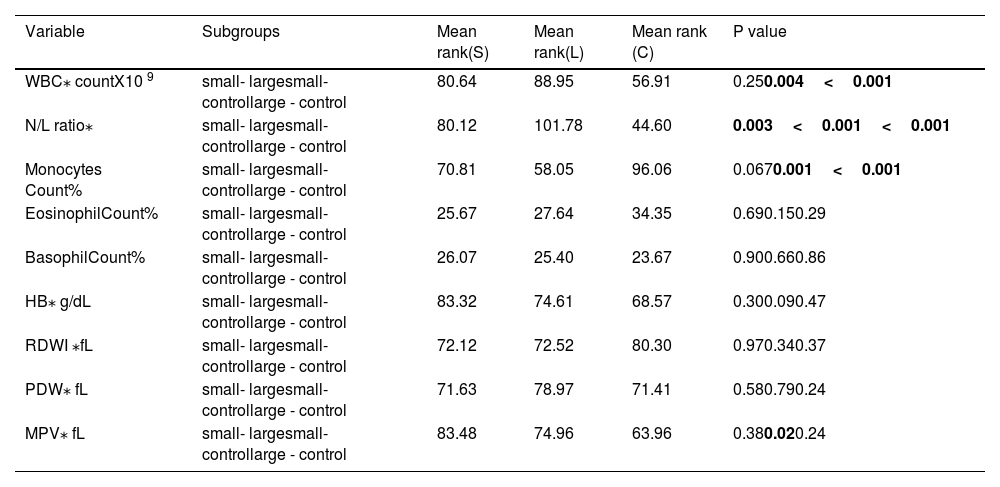
Using non-parametric tests, namely Kruskal-Wallis and Wilcoxon rank–sum tests, we compared three independent samples that were not following normal distribution curve. Parameters that were compared by this method were as follows: WBC count, neutrophil to lymphocyte ratio (N/L ratio), monocytes, eosinophils, basophil count, hemoglobin (HB), red cell distribution width index (RDWI), platelet distribution width (PDW) and mean platelet volume(MPV). Remarkable differences were found between small and large vessel disease groups on one side and control group on the other side in terms of WBC count (P = 0.004, <0.001, respectively), neutrophil/lymphocyte ratio(P < 0.001,<0.001, respectively), monocyte count (P = 0.001, <0.001, respectively) and mean platelet volume (small vessel disease group vs control group P = 0.02). See Table 4. N/L ratio ranked highest for large in comparison to the small vessel disease group (P = 0.003). For other parameters, no significant differences were found among the three groups.
Differences among small, large vessel stroke and control groups in unequally distributed variances.
| Variable | Subgroups | Mean rank(S) | Mean rank(L) | Mean rank (C) | P value |
|---|---|---|---|---|---|
| WBC⁎ countX10 9 | small- largesmall- controllarge - control | 80.64 | 88.95 | 56.91 | 0.250.004<0.001 |
| N/L ratio⁎ | small- largesmall- controllarge - control | 80.12 | 101.78 | 44.60 | 0.003<0.001<0.001 |
| Monocytes Count% | small- largesmall- controllarge - control | 70.81 | 58.05 | 96.06 | 0.0670.001<0.001 |
| EosinophilCount% | small- largesmall- controllarge - control | 25.67 | 27.64 | 34.35 | 0.690.150.29 |
| BasophilCount% | small- largesmall- controllarge - control | 26.07 | 25.40 | 23.67 | 0.900.660.86 |
| HB⁎ g/dL | small- largesmall- controllarge - control | 83.32 | 74.61 | 68.57 | 0.300.090.47 |
| RDWI ⁎fL | small- largesmall- controllarge - control | 72.12 | 72.52 | 80.30 | 0.970.340.37 |
| PDW⁎ fL | small- largesmall- controllarge - control | 71.63 | 78.97 | 71.41 | 0.580.790.24 |
| MPV⁎ fL | small- largesmall- controllarge - control | 83.48 | 74.96 | 63.96 | 0.380.020.24 |
Some sorts of confusion happen when a patient is coming to the emergency room with sided weakness or numbness or other neurological manifestations, and the physician is asking himself whether the stroke is large enough to admit him to the neurological care unit.
Research on risk factors for various ischemic stroke subtypes has produced mixed results. While some studies found no significant differences in hypertension, diabetes, or smoking status between lacunar and non-lacunar infarctions,12 others reported inconsistent findings. Jackson et al. observed similar prevalence of hypertension and diabetes in small and large vessel occlusion diseases, but found lower rates of cardioembolic sources and carotid stenosis in lacunar strokes.13 Though Schulz & Rothwell found only a weak association between hypertension and lacunar infarctions, no association with diabetes was found.14 Besides, both Shi et al. and Ntaios et al. noted smoking as a risk factor for large vessel occlusion.12,15 Our findings were consistent with Jackson and colleagues' report. Small sample size, single-center experience and ethnic variances are the main limitations of the latter studies. These discrepancies highlight the need for further research to clarify risk factor profiles across stroke subtypes.
We have found notably higher total white blood cell (WBC), neutrophil count, and lower monocyte count in patients with large and small vessel diseases compared to those without. Regarding WBC and neutrophil count, both Wang et al. and Takayani et al. addressed the same issue and reached similar findings.16,17 Investigation on monocyte counts in stroke patients has yielded inconsistent results. While some papers found increased monocyte count in stroke patients compared to controls18,19; others reported significantly lower monocyte count in stroke patients consistent with our findings.20 Moreover, Cortina et al. reported that monocyte count is an underlying marker of the lacunar subtype of small vessel disease.21 Further studies are needed to elucidate the potential role of monocyte count in various stroke subtypes. The neutrophil-to-lymphocyte ratio (NLR) has arisen as an important predictor of stroke subtypes in emergency settings. Significant differences among groups were found with this ratio in our study. Desai et al. predicted large vessel stroke with a higher ratio compared to those without vessel occlusion.22 Though in their study, they had not addressed the small vessel occlusion issue. In accord with our findings, Zeng et al. found that the lymphocyte count was highest in the control group and lowest in the large vessel disease group.23 These markers showed moderate ability to predict stroke subtypes, with neutrophil-to-lymphocyte ratios (NLRs) and lymphocyte count representing the strongest relationship in our study. Generally, these findings highlight the potential role of inflammatory markers in distinguishing stroke subtypes that could have effects for early diagnosis and management decisions.
This study has shown that red cell distribution width (RDW) is dramatically higher in large vessel stroke patients compared to controls and can be a valuable marker of stroke severity. Kara et al. and zhao et al. demonstrated that higher RDW levels correlated with poorer functional outcomes and increased mortality in stroke patients.24,25 Principally, these findings were highly reliable and advocate that RDW could work as a cost-effective indicator for stroke risk and prognosis.
Multiple studies had found that platelet count and mean platelet volume (MPV) were profoundly higher in patients with ischemic stroke compared to healthy controls.26,27 We have found that platelet count and MPV were statistically higher in the small vessel group in comparison to the control group (P = 0.04, 0.02, respectively). Contrary to ours' in their paper, Butterworth & Bath found that the elevation in MPV is more obvious in large vessel strokes.28 However, this finding was not constant across all studies.26 Moreover, their study did not find differences in platelet count between stroke groups, opposite to the authors' assumption.
Small sample size, single-center involvement and covert confounders that might affect results are the main limitations of our findings. However, this should be considered as a pilot study to easily perform a more extensive, multinational study to more elucidate the role of these blood elements in the pathogenesis of stroke from one perspective and to differentiate between different stroke subtypes in term of these markers from another perspective.
Sharing similar risk factors and clinical characteristics when are coming in emergency settings, conclusively, small and large vessel stroke groups may be different in pathophysiology, hematology and inflammatory markers. This difference might serve as an aid for diagnosis and management of different stroke subtypes.
Ethics approval and consent to participateInformed consent was obtained from all participants in the study. The study was approved by the Mosul medical college ethics committee (Ref. No. UOM/COM/MREC/23–24/SP13) on 19/9/2023.
FundingNo funding was received.
Patient consent for publicationNot applicable.
CRediT authorship contribution statementO.A. Mahmood: Conceptualization, Data curation, Formal analysis, Investigation, Methodlogy, Progect admistration, Software, Supervision. M.G. Aliraqi: Writing – original draft, Writing – review & editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Not applicable.