There is early evidence about Valproic acid (VPA) antiviral effect. Our aim was to investigate the incidence and severity of SARS-CoV-2 infection in VPA users as compared with the general population.
Material and methodsA case-control study nested within a cohort, carried out between March 1 and December 17, 2020. Retrospectively, we identified confirmed SARS-CoV-2 infection patients exposed to VPA in our health department (defined as case). We ascertained VPA regimen (all the time (AT) (292 days) or at least 20% of the study period (notAT) (≥58 days) and if VPA levels were in therapeutic range (ATR) (50–100mcg/mL) in the last 24 months. We calculated the cumulative incidence of SARS-CoV-2 infection and hospital admission in the cases, comparing it with the general unexposed VPA population (controls).
ResultsDuring the study period, 6183 PCR+ were detected among 281,035 inhabitants, of these, 746 were hospitalized. 691 patients were on VPA notAT and 628 (90.1%) AT. The indication for VPA use was epilepsy in 54.9%. The incidence of PCR+ was 1.736% (OR 0.785 (95%CI 0.443–1.390) and 1.910% (OR 0.865 (95%CI 0.488–1.533), on VPA notAT and VPA AT patients, respectively vs. 2.201% in people without VPA regimen. Those patients with VPA ATR had a lower risk of PCR + (OR 0.233 (95%CI 0.057–0.951) notAT; OR 0.218 (95%CI 0.053–0.890) AT). Hospital admission incidence was lower in patient on VPA (OR was 0.543 (95% CI 0.076–3.871).
ConclusionPatients with VPA within the therapeutic range had a reduction of SARS-Cov-2 infection incidence greater than 75%. There is a downward trend in the risk of COVID-19 admission by SARS-CoV-2 in patients on VPA therapy. These findings warrant further investigation.
Existe evidencia preliminar sobre el efecto antiviral del ácido valproico (VPA). Nuestro objetivo fue investigar la incidencia y la gravedad de la infección por SARS-CoV-2 en usuarios de VPA, en comparación con la población general.
Material y métodosEstudio de casos-controles anidado en una cohorte, realizado entre el 1 de marzo y el 17 de diciembre de 2020. De forma retrospectiva identificamos en nuestro departamento de salud a las personas con infección confirmada por SARS-CoV-2, usuarias de VPA (definido como caso). Comprobamos el régimen de VPA (todo el tiempo [TT], 292 días) o al menos el 20% del período de estudio (no-TT) (≥ 58 días) y si los niveles de VPA estaban en rango terapéutico (RT) (50-100 mcg/mL), en los últimos 24 meses. Calculamos la incidencia acumulada de infección por SARS-CoV-2 e ingreso en los casos, comparándola con la población general no expuesta a VPA (controles).
ResultadosDurante el período de estudio se detectaron 6.183 PCR + entre 281.035 habitantes, de estos, 746 fueron hospitalizados. Seiscientos noventa y un pacientes estaban en VPA no-TT y 628 (90,1%) TT. La indicación para el uso de VPA fue la epilepsia en el 54,9%. La incidencia de PCR + fue 1.736% (odds ratio [OR] 0,785; intervalo de confianza [IC] 95% 0,443-1.390) y 1.910% (OR 0,865; IC 95% 0,488-1.533), en pacientes con VPA no-TT y VPA TT, respectivamente, vs. 2.201% en personas sin indicación de VPA. Los pacientes con VPA en RT tenían un riesgo menor de PCR + (OR 0,233; IC 95%: 0,057-0,951) no-TT; OR 0,218 (IC 95%: 0,053-0,890 [TT]). La incidencia de ingreso hospitalario fue menor en pacientes con VPA (OR 0,543; IC 95%: 0,076 a 3,871).
ConclusiónLos pacientes con VPA dentro del rango terapéutico tuvieron una reducción de la incidencia de infección por SARS-CoV-2 superior al 75%. Existe una tendencia a la baja en el riesgo de admisión por COVID-19 por SARS-CoV-2 en pacientes en terapia con VPA. Estos hallazgos justifican una mayor investigación.
As the SARS-CoV-2 epidemic continues to expand, drug repurposing research is marked by failures (hydroxychloroquine, azithromycin, ivermectin, convalescent plasma), weak outcomes (remdesivir, baricitinib, tocilizumab), and small victories (dexamethasone).1
Epilepsy is a common neurological condition with a worldwide prevalence of around 1%.2,3 The association between epilepsy and COVID-19 outcomes remains unclear. Recently, a systematic review and meta-analysis suggested that patients with epilepsy are at risk of having poor COVID-19 outcomes, specifically in terms of disease severity and mortality rate.4 Therefore, patients with epilepsy need special attention and should be considered a population at risk during the COVID-19 pandemic.
The histone deacetylase inhibitor Valproic acid (VPA; 2-n-propylpentanoic acid or n-dipropylacetic acid) is a branched short-chain fatty acid widely used as an antiepileptic drug in the treatment of neurological disorders.5,6 Most people with epilepsy are treated with a single antiepileptic drug (monotherapy) and current guidelines from the National Institute for Health and Care Excellence (NICE) for adults (men and women who are not of childbearing potential) and children recommend sodium valproate (VPA) for generalized onset seizures (approximately 20–25% of epilepsy cases)3 and unclassified epilepsies (myoclonic seizures, tonic or atonic seizures, Dravet syndrome, Lennox–Gastaut syndrome and idiopathic generalized epilepsies in boys).7
VPA along with its amidic derivatives (valpromide and valnoctamide) has been shown as a potential broad-spectrum antiviral, since it inhibited in vitro infection by enveloped viruses from various viral families.8,9 Several studies have reported their antiviral activity against herpesviruses, cytomegalovirus, West Nile virus RNA, and the reactivation of Epstein–Barr virus.9 VPA and its amidic derivatives valpromide (VPD) and valnoctamide (VCD) acts at different molecular levels of the viral cycle, and may be part of the solution to the growing problem of viral resistance against traditional antivirals, that prominently affects the herpesvirus family.
Singh et al.10 have recently shown that VPA could also have an antiviral effect against SARS-CoV-2, as it reduces the amount of angiotensin-converting enzyme 2 (ACE-2) in endothelial cells, the main cellular receptor that this coronavirus uses to enter the cell. This new knowledge about “mechanism of action” of VPA provides a novel potential therapeutic drug target for prevention and treatment of COVID-19. The absence of published clinical data warrants immediate further investigation, while we await evidence from clinical trials.
Our aim was to investigate whether SARS-CoV2 infection was less common or less severe among VPA users.
Material and methodsWe performed a case–control study nested within a cohort in the General Hospital of Alicante health department, a tertiary hospital in the southeast of Spain. Between March 1 and December 17, all patients with suspected SARS-CoV-2 infection in the Health area (281,035 inhabitants) were included through RT-PCR determination. In those with diagnosis, it was verified if they were exposed to Valproic, defined as cases, and those who had not been exposed to Valproic, were defined as controls. VPA regimens were classified according to time of exposure: all the time (292 days) or at least 20% of the study period (≥58 days). Furthermore, the patients were classified according to the achievement of the VPA therapeutic range (50–100mcg/mL) in the last 24 months, following the usual clinical practice.
Data about VPA treatments (as main explanatory variable), reason for the prescription of VPA/prescribing specialist, demographics (age and gender), date of SARS-CoV-2 infection diagnosis by RT-PCR, and date of hospital admission were obtained from the electronic prescription records.
Outcome definitions- 1.
SARS-CoV-2 infection confirmed by RT-PCR in nasopharyngeal aspirate.
- 2.
Moderate-severe COVI19 disease, defined by the need for hospital admission.
For each patient, follow-up started on 1 March and ended on 17 December 2020. We therefore calculated the 292-day risk (cumulative incidence) and 95% CI for COVID-19 diagnosis, hospital admission, overall and by VPA regimen. The characteristics of the patients according to their exposure to Valproic acid were described, comparing the groups using the Chi square test or the Mann–Whitney U test. To study the association between SARS-CoV-2 infection and Valproic exposure, the Odds Ratio (OR) with its 95% confidence interval was calculated, both for diagnosis of Infection and for hospital admission. Analyses were performed with IBM SPSS Statistics V25 (Armonk, NY) and EPIDAT 3.1. The level of statistical significance is set at p<0.05.
This study was approved by the institutional review board at Alicante General University Hospital, Alicante, Spain (ref 2020-0388).
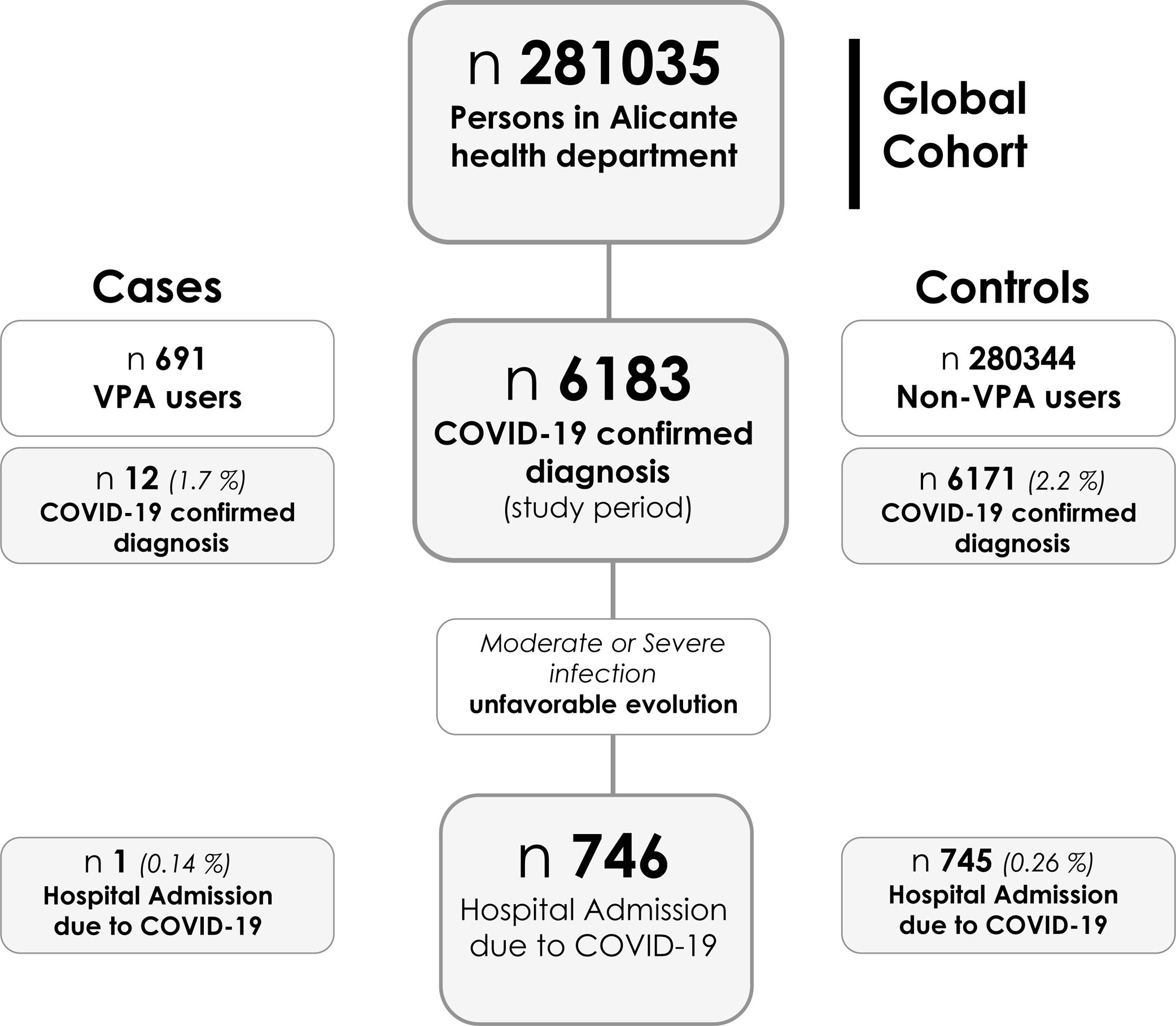
ResultsBetween 1 March and 17 December, 6183 PCR - confirmed diagnoses of SARS-CoV-2 infection were made among 281,035 persons in Alicante health department. Of these, 746 (0.265%) were hospitalized (see flowchart in Fig. 1). In the evaluated period, 697 patients received VPA treatment, 691 of them at least 20% of the time and 628 (90.1%) all the time. The indication for VPA use was epilepsy in more than half of the cases (54.9%), being neurologists (65.6%) and psychiatrists (25.3%) the main prescribers.
Age and sex distributions were similar between VPA infected patients and the rest of the population, median age 42.5 (IQR, 40.0–47.5) vs 40.0 (IQR 24.2–54) years old (p=0.24) and males 58.3% vs 48.3% (p=0.48), VPA use vs not, respectively.
Valproic acid regimen and diagnoses of SARS-CoV-2 infectionThe incidence of PCR – confirmed diagnoses of COVID-19 was 1.736% (12/691) in patients under VPA at least 20% of the evaluated period, and 2.201% (6171/280,344) in the general population, without VPA; estimated OR was 0.785 (95% CI 0.443–1.390) (p=0.405) (see Fig. 1). In those patients under VPA regimen all the evaluated period, the incidence of PCR – confirmed SARS-CoV-2 diagnoses was 1.910% (12/628) and the estimated OR 0.865 (95% CI 0.488–1.533) (p=0.620).
Valproic acid regimen and hospital admissionThe incidence of COVID-19 hospital admission was 0.144% (1/691) in patients under VPA at least 20% of the evaluated period, and 0.265% (745/280,344) in Alicante population without VPA; estimated OR was 0.543 (95% CI 0.076–3.871) (p=0.536) (see Fig. 1). In those patients under VPA regimen all the evaluated period, the incidence of COVID-19 hospital admission was 0.159% (1/628) and the estimated OR 0.598 (95% CI 0.084–4.262) (p=0.604).
The only VPA admitted patient was a middle age male with infantile cerebral palsy with mild COVID-19 and a favorable outcome.
Valproic acid therapeutic range and outcomesWe have information about VPA therapeutic range in 442 (63.9%) of the patients included in the study. Patients with VPA within the therapeutic range had a lower risk of COVID-19 diagnosis (PCR – confirmed SARS-CoV-2) during the study period, in the global cohort – patients under VPA at least 20% of the evaluated period (OR 0.233 (95% CI 0.057–0.951), p=0.042) and in the subpopulation on AVP regimen during the whole study period (OR 0.218 (95% CI 0.053–0.890), p=0.034). No patient with VPA within therapeutic range was admitted to the hospital.
DiscussionThe results of this epidemiological study on a large population, show half the risk of COVID-19 admission in patients on VPA therapy. This downward trend in risk is consistent across age and sex, although was not statistically significant. Globally, there is no clear reduction in the risk of PCR confirmed diagnoses of COVID-19 in patients with VPA use; however, in those patients with AVP within the therapeutic range the risk reduction of SARS-Cov-2 infection is greater than 75%.
Although there are currently no published clinical trials due to the novelty of SARS-CoV-2 infection, there is pathophysiologic rationale for exploring the use of VPA in this global pandemic, supported by early evidence about its broad-spectrum antiviral effect.11
Previous studies have positioned VPA as a potential broad-spectrum antiviral, since it inhibited in vitro infection by enveloped viruses from various viral families.9 VPA could act at different steps of enveloped virus infection; although West Nile virus RNA and protein synthesis were drastically abolished, Vesicular stomatitis virus RNA and protein synthesis remained unaltered.8 In herpes virus, the half maximal inhibitory concentration (IC50) for VPA inhibition of HSV-1 infection was 0.55mM,12 the regular dosage recommended for epilepsy which ranges between 0.3 and 0.6mM in the plasma. In a nested case-control study, patients on VPA therapy for more than 90 days showed a reduced risk of clinical infections by herpesviruses as compared with non-users (OR 0.84; CI 95% 0.7–1.0; p=0.057).5 Finally, some studies have reported antiviral activity of VPA, against cytomegalovirus, and the reactivation of Epstein–Barr virus.9
This VPA potential benefit may be explained by an interference with different cellular and viral processes.11 VPA may interfere with the initial steps of the viral cycle, specifically during virus entry13; it can affect lipid metabolism (including the synthesis of phosphatidylinositols) and formation of cell membranes, so it may damage the viral acquisition of lipid envelope, resulting in unstable virions8; it can affect the recognition of gB – interaction of its glycoprotein gB with cellular heparan sulfate proteoglycans in CMV.13,14
Singh S. et al.10 demonstrated in human umbilical vein endothelial cells (ECs) that VPA-treatment significantly reduced ACE-2 expression (a cell “entry door” for “SARS-CoV-2”) in endothelial cells.15 So, VPA in at risk individuals could reduce the risk of COVID-19 or reduce the viral load, both by an effect on ACE2 as well as Transmembrane protease, serine 2 reduced the expression.16 Singh S. et al.10 also reported that VPA-treatment significantly reduced the expression of inflammatory cytokines IL-6 along with the endothelial activation marker inter-cellular adhesion molecule-1 (ICAM-1). Finally, PCR array analysis for human endothelial-related genes in VPA-treated ECs showed a beneficial Plasminogen activator, tissue (T-PA) expression.17 This antithrombotic potential, along with its proven antiplatelet effects,18 would have potential benefits in coagulopathy a hallmark of severe COVID-19.17 These in vitro data would support our working hypothesis and data, so that, patients on VPA therapy could present a lower rate of infection by SARS-CoV-2, and in those who become infected, less viral replication, which was related to less severe disease and COVID-19 admission.19
Epilepsy is a neurological comorbidity that needs special attention in the outbreak of COVID-19.4 The effect of the pandemic in patients with epilepsy is controversial. Several reports have shown that most patients with epilepsy experience worsened seizures during this pandemic, which may lead to higher morbidity and mortality rates.20,21 Lallana et al.22 have reported an increase in depression rates after the first wave, that along with drug-resistant epilepsy and a reduction in family income were independent risk factors for an increased seizure frequency. Nonetheless, other studies have shown that seizure frequency and severity remained unchanged in most patients during the COVID-19 pandemic and the lockdown.23,24 Regarding the longitudinal effects of the COVID-19 pandemic, Gonzalez-Martinez et al.24 found lower QoL, particularly related to daily activities, and higher somnolence.
Regarding epilepsy and COVID-19 severity, a systematic review and meta-analysis of 13 studies with 67,131 patients with COVID-19 suggested that patients with epilepsy are at risk of having poor COVID-19 outcomes, specifically in terms of disease severity (OR, 1.69; 95%CI: 1.11–2.59) and mortality rate (OR, 1.71; 95%CI: 1.14–2.56).4 The results also showed that the association between epilepsy and increased risk of developing severe COVID-19 is influenced by sex and neurodegenerative disease.
Considering this evidence together, the use of VPA in patients with epilepsy as an antiepileptic and potential antiviral drug seems an extremely interesting strategy, with potential benefits in reducing SARS-CoV-2 infection and its unfavorable clinical evolution toward serious forms that require hospital admission.
Some important limitations need to be addressed. First, this is a one-center retrospective and observational analysis. The risk of residual confounding cannot be ruled out in this type of study. The lack of information about associated comorbidities, changes in lifestyle - social isolation of patients with epilepsy during the pandemic, a record of adherence to VPA, the absence of VPA levels in a third of the sample and finally, and the therapeutic approach on an outpatient basis after the diagnosis of COVID-19 (for example dexamethasone), limits our findings. Sample size limitations, with only one patient admitted in people under VPA, prevents the evaluation of the effect of VPA on clinical outcomes in hospitalized patients.
This new knowledge about “antiviral mechanism of action” of VPA and its potential benefits in COVID-19, along with our reported real-world evidence, provide a novel potential therapeutic drug target for prevention of COVID-19. These findings warrant further investigation in VPA pre-exposure epidemiological studies and prospective, adequately powered, randomized trials. Valproic acid effects on COVID-19 are currently being evaluated in clinical studies (e.g. Clinicaltrials.gov NCT04513314). Nevertheless we will have to wait for stronger evidence before prescribing VPA to COVID-19 patients with pre-existing pathologies, however, VPA appear to be a candidate for further repurposing to patients with generalized epilepsy, bipolar disorders, or even migraine at risk of COVID-19.
StatementThe information of this article has not been presented in any meeting(s).
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
FundingNo external funding was received.
Conflict of interestNone of the authors has any conflict of interest to disclose.
The members of COVID19-ALC multidisciplinary research group: Esperanza Merino, Joan Gil, Vicente Boix, Ximo Portilla, Oscar Moreno-Pérez, Mariano Andrés, Jose-Manuel Leon-Ramirez, Santos Asensio, Cleofé Fernandez, Alfredo Candela, Mª del Mar García, Rosario Sánchez, Diego Torrus, Sergio Reus, Pilar González, Silvia Otero, Jose M Ramos, Beatriz Valero, Alex Scholz, Antonio Amo, Héctor Pinargote, Paloma Ruiz, Raquel García-Sevila, Ignacio Gayá, Violeta Esteban, Isabel Ribes, Julia Portilla, Cristina Herreras, Alejando Cintas, Alicia Ferradas, Ana Martí, Blanca Figueres, Marcelo Giménez, María-Ángeles Martínez, María-Mar García-Mullor, María Angeles Martínez, Irene Calabuig, Marisa Peral, Ernesto Tovar, M Carmen López, Paloma Vela, Pilar Bernabeú, Ana Yuste, José Ponce, Bertomeu Massuti, Vicente Climent, Vicente Arrarte, Fernando Torres, Laura Valverde, Laura Delegido, Cristina Cambra, Miriam Sandín, Teresa Lozano, Amaya García-Fernández, Alejandro Do Campo, Eduardo Vergara, Nicolás López, Elena Elvira, Fátima López, Fernando Dahl, Blanca Serrano, Sarai Moliner, Carmina Díaz, Dolores Castaño, Beatriz López; Antonio Picó, Joaquín Serrano, Sol Serrano, María Marín-Barnuevo, María Díaz, Cristina Gilabert, Estela Martínez, Elena Vivó, Noelia Balibrea, Miguel Perdiguero, Carolina Mangas, Lucía Medina, Oscar Murcia, María Rodríguez, Rodrigo Jover, Javier López, Marina Morillas, Mercedes Khartabil, Cristina Gil, Carlos Salazar, Eva Vera, Helena López, Vanesa Rodríguez, Sandra Baile, Norma Guerra, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabil, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, Mari-Carmen López-González, Blanca Figueres.