Children with congenital hypothyroidism (CH) are at risk of developing mild cognitive impairment despite normal overall intellectual performance. These deficits may be caused by disease-related and treatment-related factors. This study explores the impact of abnormal thyroid function during the first 3 years of life on attention performance at school age.
MethodsWe included 49 children diagnosed with CH and receiving treatment for the condition: 14 boys (mean age 9.5±2.8 years) and 35 girls (9.6±2.6 years). The number of episodes of normal, under-, and overtreatment were estimated based on TSH levels during their first 3 years of life (at 12, 18, 24, 30, and 36 months). Children were assessed using a computerised version of a sustained attention test. General linear models were calculated with the attention index as the dependent variable and sex, aetiology, and number of episodes of normal, under-, and overtreatment as independent variables.
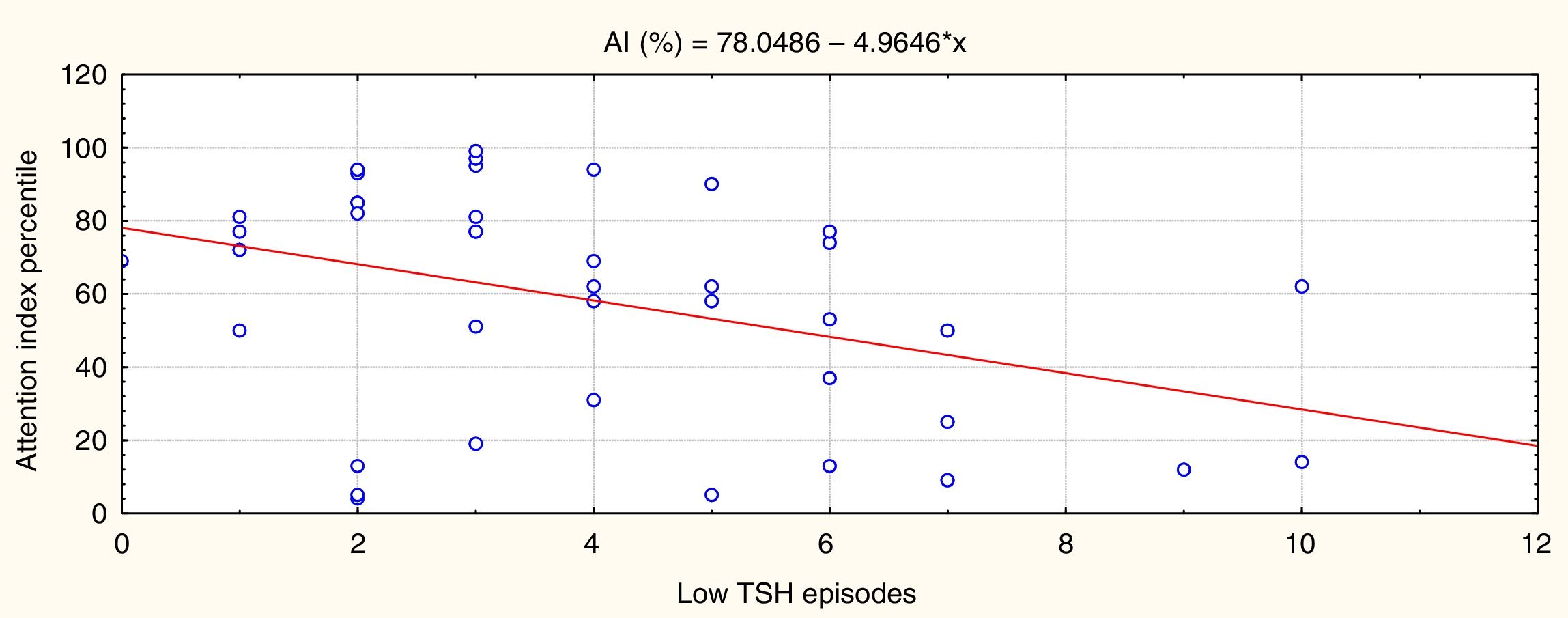
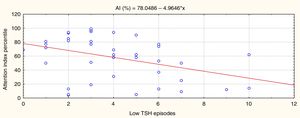
ResultsHigher numbers of episodes of overtreatment (low TSH level) were associated with poorer attention performance at school age (P=.005, r=–0.45).
ConclusionsChildren with CH should be monitored closely during the first 3 years of life in order to prevent not only hypothyroidism but also any adverse effects of overtreatment that may affect attentional function at school age.
Los niños con hipotiroidismo congénito (HC) están en riesgo de presentar déficit cognitivos sutiles, a pesar de tener un rendimiento intelectual global dentro de rangos normales. Estos déficits pueden ser consecuencia de condiciones inherentes a la enfermedad y a factores relacionados con el tratamiento. El presente estudio explora el efecto de las desviaciones del estado de eutiroidismo durante los primeros 3 años de vida en el rendimiento atencional durante la edad escolar.
MétodosFueron evaluados 49 niños con HC diagnosticado y bajo tratamiento, de ellos 14 fueron niños (9,5±2,8 años de edad) y 35 niñas (9,6±2,6 años de edad). Se calculó el total de episodios de sobre, infra y normotratamiento a partir de los valores de TSH durante los primeros 3 años de vida (medidos a los 12, 18, 24, 30 y 36 meses de edad). Los niños fueron evaluados mediante una versión computarizada del test de atención sostenida. Se calcularon los modelos lineales generales usando el índice de atención como variable dependiente y el género, la etiología y los episodios de sobre, infra y normotratamiento como independientes.
ResultadosEl número de episodios de sobretratamiento (TSH baja) se asoció a un peor rendimiento atencional en la edad escolar (p=0,005, r=–0,45).
ConclusiónDebe realizarse un seguimiento estrecho en los 3 primeros años en pacientes con HC para evitar no solo el hipotiroidismo, sino también los efectos adversos de episodios de hipertratamiento que pueden comprometer el procesamiento atencional en edad escolar.
According to a consistent body of literature, although children with congenital hypothyroidism (CH) have an intelligence quotient within normal ranges and may attend traditional primary education, they present mild cognitive deficits characterised by difficulties with visuospatial and auditory processing, mild memory impairment, poor sensorimotor performance, subclinical electroencephalographic alterations, and especially attention deficits.1–5
In 2004, Álvarez et al.6 proposed the double effect hypothesis, according to which CH is accompanied by cognitive deficits due to: (1) deficits associated with characteristics inherent to the disease7; and (2) deficits related to treatment.8 The inherent characteristics include duration and severity of foetal hypothyroidism, the initial biochemical severity (serum T4 levels below 42nmol/L),9 aetiology,10,11 and genetic factors.12,13
There is less agreement regarding treatment-related factors. The initial optimal dose of levothyroxine is the most controversial aspect, with a lack of consensus as to whether a high initial dose (15μg/kg/day) is beneficial and harmless or whether a lower initial dose is preferable.14–24 The issue is that no simple, direct relationship has been established between the initial dose and cognition due to methodological differences between studies into this association.
To illustrate these contradictions, we may consider 2 studies using event-related potentials. Martí et al.25 found that although the structural and anatomical organisation of the auditory system does not differ between children with CH and controls, N200 latency was longer in CH. This prolongation was correlated with initial overtreatment, suggesting that the information processing speed of the central nervous system might be subclinically impaired with high doses of levothyroxine. In contrast, a study of 20 Cuban children with CH reported that an initial high dose of levothyroxine sodium was associated with better overall intellectual performance, but with lower scores in cognitive tests assessing attention and visual memory processes.26
The findings of a preliminary collaborative study by Spanish and Cuban institutions may explain the above contradiction.27 When children received an initial levothyroxine dose of 10 to 15μg/kg/day, the number of episodes of overtreatment (TSH values below 0.5μIU/L in the first 6 months of life), and not variations to the initial dose within the mentioned range, were associated with poorer performance in sustained attention when children reached school age. That is to say, it was necessary to avoid not only hypothyroidism, but also episodes of hyperthyroidism during this sensitive, early period of brain development. However, whether this association was extended over a longer period of time including the first 3 years of life remained to be determined, since pruning of visuomotor areas starts in this period, which also includes a stage of increased synaptogenesis.
Subjects and methodsWe studied 49 patients: 14 boys (mean age [SD], 9.5 [2.8] years) and 35 girls (9.6 [2.6] years). Patients were diagnosed using a neonatal screening programme for CH. TSH levels were measured using dried blood spot sampling in the first 48hours after birth and time-resolved immunofluorescence (DELFIA®).28 The cut-off point for suspicion of the condition was a TSH level ≥10μIU/mL29; diagnosis was confirmed by measuring plasma levels of free thyroxine (FT4) and TSH; aetiological diagnosis was established with an emergency thyroid scintigraphy (99Tc).
Aetiology of CH was athyreosis in 7 children, sublingual thyroid in 28, and ectopic thyroid in 14.
Hormone replacement therapy was started with oral levothyroxine (LT4) at the recommended dose of 10 to 15μg/kg/day.30 Treatment started at 8.2 (7) days. Normal values during this period were 0.5 to 4.5μIU/mL for TSH and 0.8 to 2.5ng/dL for FT4.31 TSH values were monitored for the first 3 years of life, with tests performed at 12, 18, 24, 30, and 36 months of age. We calculated the episodes of overtreatment by counting the number of times that TSH was below 0.5μIU/mL and episodes of undertreatment by counting the number of times that TSH was above 4.5μIU/mL, as well as the number of times levels were within the normal range.
The study complies with the applicable ethical standards of the Declaration of Helsinki for medical research on humans; the patients’ guardians signed informed consent forms.
Neurocognitive variablesWe designed the Neurohipot 1.0 digital battery, which assesses the potentially impaired neurocognitive processes in children with CH.25 We calculated the contrasting validity of the battery by comparing healthy children, children with learning disorders, and children with CH. Results for sustained attention were obtained by measuring the attention index, which is an indicator of the proportion of correct responses, errors, and omissions during a 12-minute visual continuous performance task (correct responses–errors–omissions/correct responses+omissions+errors, with results ranging from –1 to +1). We used the continuous performance task (CPT) paradigm.32 Seven hundred stimuli (70 targets and 630 distractors) were presented, with an exposure and concealment time of 500ms for each. The test was administered by morning, in a controlled environment (neuropsychology laboratory); it was conducted by trained professionals using a computer (21.5×28.5cm screen). Cases were evaluated according to a UNICEF user manual on standards for assessing children aged between 7 and 15 years. Results of these evaluations were compared with the normative data established for the battery (UNICEF).33 These standards were established by evaluating 220 healthy school-aged children stratified by sex and age. The test has a sensitivity of 95%.
Statistical analysisWe used the statistical software Statistica 8.0 for Windows (StatSoft, 2010) to perform an analysis of variance to determine the differences between mean TSH values and between the number of normal, under-, and overtreatment episodes according to CH aetiology. General linear models were calculated with the attention index as the dependent variable, gender and aetiology as categorical predictor variables, and episodes of normal, over-, and undertreatment as continuous predictor variables. Statistical significance was established at P<.05 and the effect size was calculated using the Cohen method (large, d≥0.8; medium, d=0.5-0.7; and small, d=0.2-0.4).
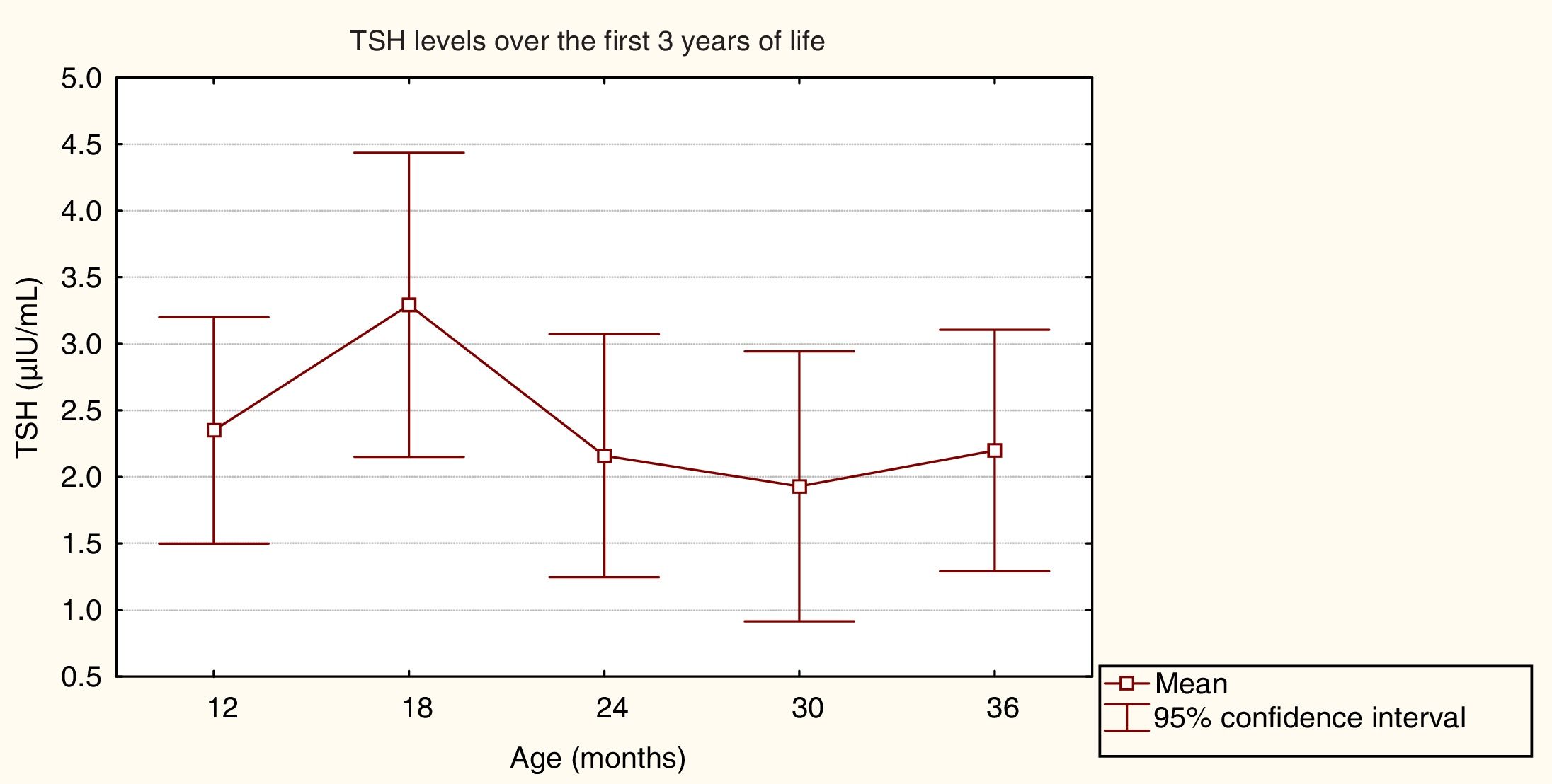
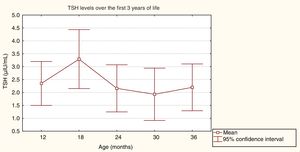
ResultsResults from general follow-up and endocrine analysisMean initial TSH values (SD) varied according to CH aetiology: 133.3 (81.6)μIU/mL for patients with athyreosis, 76.9 (28.6)μIU/mL for sublingual thyroid, and 46.4 (32.2)μIU/mL for ectopic thyroid. Initial FT4 values were 0.2 (0.1)ng/dL for athyreosis, 0.8 (0.4)ng/dL for sublingual thyroid, and 1.0 (0.7)ng/dL for ectopic thyroid. The mean initial dose of LT4 was 11.2 (2.3)g/kg/day. Fig. 1 shows the mean TSH values during the first 3 years of life.
An analysis of variance detected no statistically significant differences between mean TSH values, which were within the normal range (F=0.25, P=.91).
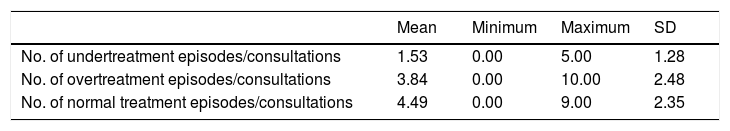
Table 1 shows the number of over- and undertreatment episodes. We counted the times TSH levels were above, below, and within the normal range and divided the number by the number of consultations the patient attended over 3 years to obtain an index evaluating the number of under- or overtreatment episodes. As could be observed, the number of times that the hormone was within the normal range was higher than the number of under- or overtreatment episodes.
Proportion of episodes of undertreatment, overtreatment, and normal treatment.
| Mean | Minimum | Maximum | SD | |
|---|---|---|---|---|
| No. of undertreatment episodes/consultations | 1.53 | 0.00 | 5.00 | 1.28 |
| No. of overtreatment episodes/consultations | 3.84 | 0.00 | 10.00 | 2.48 |
| No. of normal treatment episodes/consultations | 4.49 | 0.00 | 9.00 | 2.35 |
These results were also analysed according to CH aetiology (Table 2). The analysis of variance showed that there were no differences in the number of episodes of increased TSH levels according to CH aetiology (F=0.91, P=.4). However, episodes of decreased TSH levels (overtreatment) were significantly associated with CH aetiology (F=3.30, P=.04). Patients with athyreosis (P=.02) and sublingual thyroid (P=.04) showed a significantly higher number of these episodes than those with ectopic thyroid. Furthermore, patients with ectopic thyroid showed a significantly higher number of episodes of normal TSH levels than the other 2 groups (F=7.47, P=.002).
Mean number of episodes of increased, decreased, and normal TSH levels according to CH aetiology.
| Aetiology | Mean no. of episodes of increased TSHa | N | SD |
|---|---|---|---|
| Agenesis | 2.00 | 7 | 1.53 |
| Sublingual | 1.57 | 28 | 1.07 |
| Ectopic | 1.21 | 14 | 1.53 |
| Aetiology | Mean no. of episodes of decreased TSHb | N | SD |
|---|---|---|---|
| Agenesis | 5.14 | 7 | 2.79 |
| Sublingual | 4.14 | 28 | 2.45 |
| Ectopic | 2.57 | 14 | 1.95 |
| Aetiology | Mean no. of episodes of normal TSHc | N | SD |
|---|---|---|---|
| Agenesis | 3.29 | 7 | 1.79 |
| Sublingual | 3.89 | 28 | 2.08 |
| Ectopic | 6.29 | 14 | 2.23 |
Statistically significant results are shown in Figs. 2 and 3. Fig. 2 shows that episodes of decreased TSH levels (overtreatment) had a significant inverse association with attention performance (P=.005, r=–0.45).
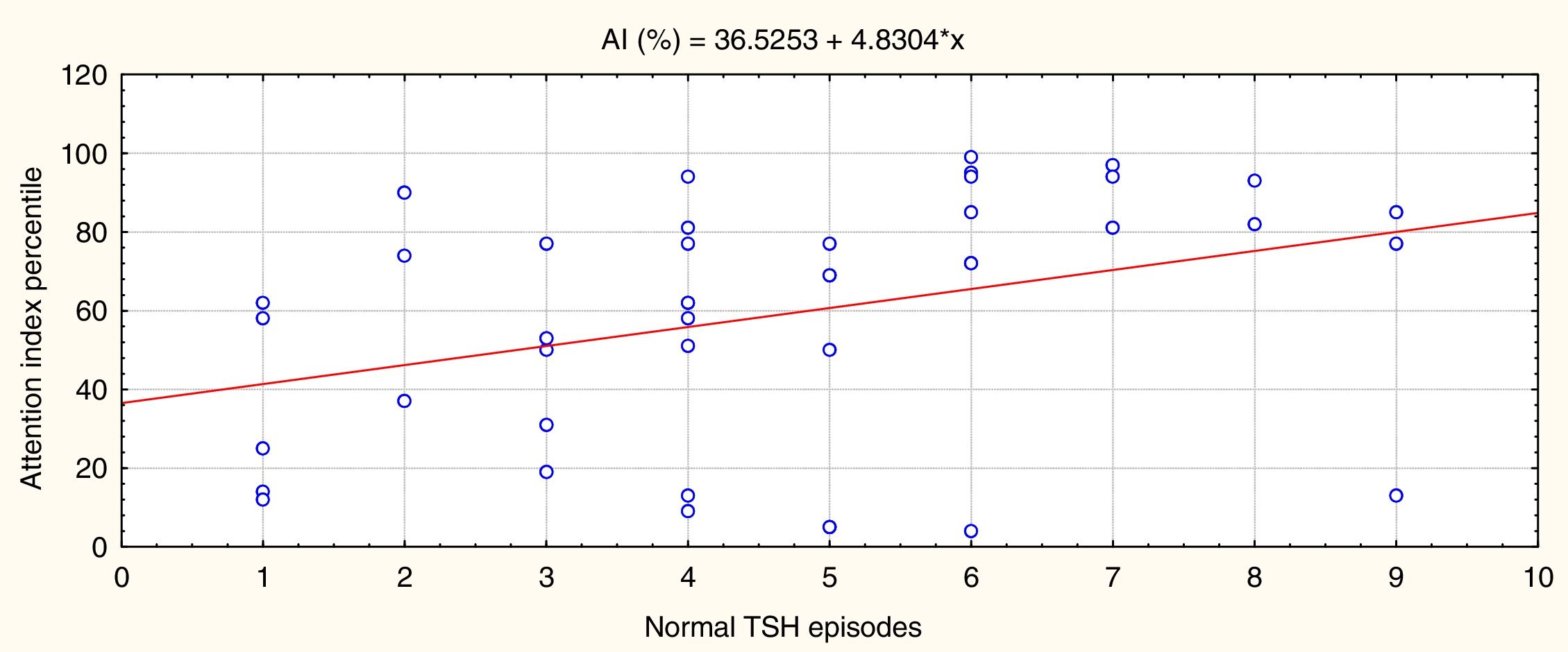
Fig. 3 shows that episodes of normal TSH values were significantly and directly associated with attention performance (P=.02, r=0.38).
In summary, the more times that TSH values were within the normal range in the first 3 years of life, the better the attention performance in school-aged children; the more episodes of overtreatment detected, the poorer their performance. No such association was identified for episodes of increased TSH levels as few episodes were recorded, and the range was narrow. These results are independent of sex and CH aetiology.
DiscussionOur results suggest that episodes of overtreatment with levothyroxine during the first years of life have an impact on cognition, requiring us to prolong monitoring of TSH levels to keep them within the normal range. In clinical practice, it was established as a risk factor for cognition in children with CH in the International Consensus on CH, held in Rome,30 and rigorous monitoring of the thyroid function and diagnostic re-evaluation are recommended if the thyroid is ectopic after 3 years of treatment.
The subtle sustained-attention deficit found to be related with episodes of overtreatment manifests without hyperactivity11,12; therefore, it may go unnoticed. It does not cause severe cognitive damage with a serious impact on academic performance, but does represent a potential limitation to children's abilities, persisting into adolescence and adulthood.
The neurobiological value of this result should be analysed from the perspective of the brain as the target organ for molecules with a modulating effect on basic cognitive processes.
Time is a key concept in neurobiology, with the action of thyroid hormones on cognition and neurodevelopment being a clear example. The effect of hormones on neurodevelopment is conditioned by the stage of the body's life cycle and the predominant types of brain plasticity characterising that stage. Therefore, hormonal influence is epigenetic. As an epigenetic factor, hormones affect behaviour through plasticity processes, triggering 2 main effects: the organising and the activating effects,35,36 which frequently overlap.
The organising effect refers to the capacity of hormones to permanently influence the cytoarchitecture and structure of the brain during its development. The need for thyroid hormones varies according to brain area and neurodevelopmental stage. Thyroid hormones participate in neurogenic processes, neuronal migration, axon and dendrite formation, myelination, and synaptogenesis,37 through the regulation of specific genes and the activation or suppression of specific receptors for these hormones in neuronal nuclei.34,38 Genes regulated by thyroid hormones present temporal and regional specificity in the developing brain, as do the mentioned nuclear receptors.39 The action of thyroid hormones in the brain follows a posteroanterior sequence, with the frontal region being the last needing these hormones in the postnatal period. A lack of thyroid hormones in early gestation has been associated with problems in visual attention and processing and gross motor skills; when it manifests during the final stage of pregnancy, there is risk of alterations to contrast sensitivity and visuospatial skills. In the postnatal period, the main areas affected are language and memory. In both stages of brain development, decreased levels of these hormones have an impact on corticogenesis and neuronal migration in the visual and auditory areas and in parietal region, and affect neuronal size, structure, and migration in the hippocampus, with the resultant deficits.40–44
The activating effect is related with the activation of target cells to facilitate behaviour in specific contexts. These are transient influences determined by the concentration of hormones in a given moment. The brain needs a precise amount of hormones throughout the life cycle for neurotransmission,45,46 and any deviation outside these limits hinders normal cognitive activity.47
The occasional excess of thyroid hormones during the period studied has both effects. The organising effect occurs because most brain development takes place in the first 3 years after birth, and the cytoarchitecture is in a critical process of formation; the activating effect presents as poorer performance in focus attention tasks than that of healthy controls, as a direct consequence of the excess of these hormones in the central nervous system.47,48
Sustained attention49 is the variable most frequently reported to be impaired in CH.11,12,27,50 This is a complex system encompassing several neuronal circuits with their own anatomy and cell structure, as well as a development pattern that may or may not be modified according to age.50–52 The maturation sequence of thyroid hormones depending on these areas in the first 3 years of life is still to be defined, but the evidence suggests that the time window for this sensitive period is wider than that reported in previous studies.11,12,34 If prolonged over a longer time, this neurodevelopmental plasticity puts the brains of children with CH at greater risk. Thyroid hormone levels should be frequently monitored in children with CH in the first 3 years of life to prevent episodes of hypothyroidism and hyperthyroidism due to overtreatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the Spanish Royal Board of Trustees for Disabilities and the Spanish Association of Neonatal Screening.
Please cite this article as: García Morales L, Rodríguez Arnao MD, Rodríguez Sánchez A, Dulín Íñiguez E, Álvarez González MA. Atención sostenida en niños con hipotiroidismo congénito en edad escolar. Influencia de los episodios de sobretratamiento en los primeros 3 años de vida. Neurología. 2020;35:226–232.