According to most studies, the incidence of Guillain-Barré syndrome increases with age, with a peak incidence occurring between 70 and 80 years of age. The objective of this study is to describe the incidence (overall and by sex and age group) and clinical characteristics of Guillain-Barré syndrome in Osona (Barcelona, Spain).
MethodsWe performed a retrospective, descriptive, population-based study covering the period 2003 to 2016.
ResultsThe global incidence of Guillain-Barré syndrome is 2.07 cases per 100000 person-years. Incidence increases with age, except for small peaks during childhood and between 40 and 50 years, and reaches a maximum of 6.26 cases per 100000 person-years above the age of 80. The incidences of the different variants were: AIDP, 72.1%; AMAN, 16.3%; ANSAN, 4.7%; and Miller Fisher syndrome, 4.7%. A total of 41.9% of patients had a history of respiratory tract infections, and 20.9% had a history of gastrointestinal infections. Protein in the cerebrospinal fluid was found in 76.7%. EMG findings suggested demyelination in 73.7% of the patients and axonal degeneration in 26.3%. A total of 20.9% of patients needed ventilatory support. Six-month mortality was 9.3%. Variables associated with worse prognosis were age over 80 years, delay in admission, previous gastrointestinal infection, and AMAN variant.
ConclusionsThe incidence observed in our study is in the upper range of estimated incidence rates reported in European and North American studies. The syndrome may be underdiagnosed in elderly patients; physicians must be vigilant to the possibility of the disease, which is associated with a high mortality rate if it is not treated early.
La mayoría de los estudios muestran que la incidencia del síndrome de Guillain-Barré aumenta con la edad, con un máximo entre los 70 y los 80 años y un descenso posterior. El objetivo del estudio es describir la incidencia global y específica por sexo y grupos de edad y las características clínicas del síndrome de Guillain-Barré en la comarca de Osona (Barcelona, España).
MétodosEstudio descriptivo retrospectivo de base poblacional en el periodo 2003-2016.
ResultadosLa incidencia global es de 2,07/100.000 habitantes-año. La incidencia aumenta con la edad, salvo un pequeño pico en la infancia y entre los 40 y los 50 años, alcanzando la máxima de 6,26/100.000 habitantes-año pasados los 80 años. Los porcentajes de las variantes fueron: AIDP (72,1%), AMAN (16,3%), ANSAN (4,7%) y síndrome de Miller-Fisher (4,7%). Presentaron infección previa de vías respiratorias el 41,9% e infección gastrointestinal el 20,9%. Se halló proteinorraquia en el 76,7%. El EMG mostraba un predominio desmielinizante en el 73,7% y axonal en el 26,3%. Necesitaron soporte ventilatorio el 20,9%. La mortalidad a los 6 meses fue del 9,3%. Las variables que se asociaron a un peor pronóstico fueron la edad superior a los 80 años, la demora en el ingreso, presentar infección gastrointestinal previa y la variante AMAN.
ConclusionesLa incidencia descrita en nuestro estudio se encuentra en el rango más alto de las estimadas en Europa y Estados Unidos. En ancianos pudiera estar infradiagnosticado y se requeriría de una mayor alerta ante una enfermedad con alta mortalidad si no se trata de forma precoz.
Guillain-Barré syndrome (GBS) is a dysimmune inflammatory polyradiculoneuropathy that may be triggered by a wide range of antigenic stimuli, primarily viral or bacterial infections and vaccines. It frequently manifests as ascending motor paralysis with areflexia and albuminocytological dissociation in the CSF. The classic form of GBS is acute inflammatory demyelinating polyradiculoneuropathy (AIDP). Several other variants have since been described, including acute motor axonal neuropathy (AMAN), acute motor-sensory axonal neuropathy (AMSAN), acute sensory axonal neuropathy (ASAN), and Miller-Fisher syndrome.
The incidence of GBS ranges from 0.16 to 4 cases per 100000 person-years, although most studies report incidence rates between 1.1 and 1.8 cases per 100000 person-years. In Europe and the United States, incidence is estimated to be less than 2 cases per 100000 person-years.1,2 A recent study conducted in France,3 which used diagnostic codes recorded at discharge, reports an incidence of 2.42 cases per 100000 person-years, although the authors cannot rule out over-coding of GBS. The highest incidence rates are reported in certain regions of Asia4,5 and the Caribbean (Curaçao6 and Aruba,7 with the latter showing the highest incidence rate, at 3.93 cases per 100000 person-years). In Spain, published incidence rates range from 0.85 to 1.56 cases per 100000 person-years.8–11
Most studies find that the incidence of GBS increases with age, peaking between the ages of 70 and 80 years and subsequently decreasing.1,2 Other studies report the age-related increase persisting after the age of 80.12,13 Using binomial regression, a meta-analysis of the incidence rates reported in the United States and Europe described an exponential increase in GBS incidence over time, with incidence peaking after the age of 80.2
Population-based epidemiological studies of myasthenia gravis14 and amyotrophic lateral sclerosis15 conducted in our region report high incidence rates due to the high incidence of both diseases in individuals older than 80 years; this suggests that neuromuscular disorders are underdiagnosed in the elderly.
In the context of the neuroepidemiology project of the Spanish district of Osona, we conducted a population-based study aiming to describe global and sex- and age-specific incidence rates of GBS with a view to contributing our understanding to the epidemiology of GBS in Europe and worldwide. We believe that the high incidence of GBS is explained by greater incidence rates among the oldest individuals. We also describe the clinical characteristics of GBS of our sample.
Patients and methodsHospital Universitari de Vic is located in the district of Osona, in the north of the province of Barcelona (Catalonia, Spain), and serves a population of 151907 (data obtained from the Spanish National Statistics Institute for the study period). Hospital Universitari de Vic is the reference centre of the public health system; most centres in the region belong to the Spanish public health system.
The hospital has developed a registry for the main neurological diseases, including GBS, to which all neurologists in the district contribute. The registry provides valuable data for cohort studies, whether clinical (treatments provided to these patients) or epidemiological (incidence, prevalence, and survival).
The study period was from 1 January 2003 to 31 December 2016.
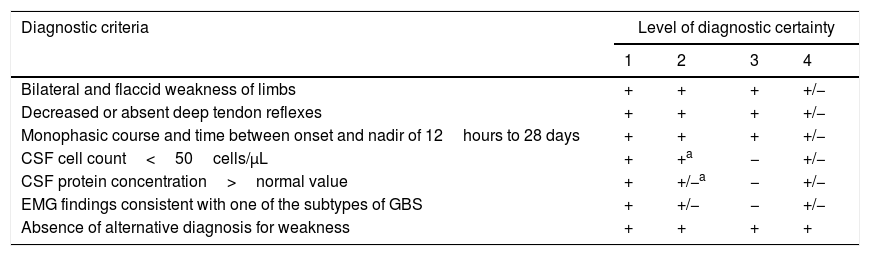
GBS was diagnosed using the Brighton criteria (Table 1).16
Brighton diagnostic criteria and level of diagnostic certainty for Guillain-Barré syndrome.
| Diagnostic criteria | Level of diagnostic certainty | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Bilateral and flaccid weakness of limbs | + | + | + | +/− |
| Decreased or absent deep tendon reflexes | + | + | + | +/− |
| Monophasic course and time between onset and nadir of 12hours to 28 days | + | + | + | +/− |
| CSF cell count<50cells/μL | + | +a | − | +/− |
| CSF protein concentration>normal value | + | +/−a | − | +/− |
| EMG findings consistent with one of the subtypes of GBS | + | +/− | − | +/− |
| Absence of alternative diagnosis for weakness | + | + | + | + |
CSF: cerebrospinal fluid; EMG: electromyography; GBS: Guillain-Barré syndrome; +: present; −: absent; +/−: present or absent.
If CSF is not collected or results are not available, nerve electrophysiology results must be consistent with the diagnosis of Guillain-Barré syndrome.
Source: Fokke et al.16
We gathered data on demographic variables (age, sex), age at disease onset, possible trigger factors in the previous 4 weeks (respiratory or gastrointestinal infection, vaccination), variant of GBS (AIDP, AMAN, AMSAN, ASAN, Miller-Fisher syndrome), presence of high CSF protein levels at admission, results of an EMG study performed at 2-3 weeks of progression (predominantly axonal or demyelinating), need for respiratory support, treatment with intravenous immunoglobulins (IVIg), and functional status at 6 months according to the modified Rankin Scale (mRS).
To calculate the incidence of GBS, we used the number of patients diagnosed with the condition between 2003 and 2016 and who lived in any of the 51 towns that form the district of Osona, and the total population (de facto population) of the district. Individuals with GBS onset occurring during the study period were considered incident cases. Sex-specific incidence was calculated using the number of men and women with GBS who were alive and resident in Osona during the study period, and the total male and female populations of Osona; age-specific incidence was calculated using the number of patients in each age group during the study period and the total population of each age group.
We conducted an epidemiological study on a patient sample drawn from an active registry of patients with GBS. Quantitative data are expressed as means (standard deviation) and relative frequencies (percentages and no. cases per 100000 population). Data were analysed with the chi-square test (Yates's correction) or the Mann–Whitney U test using Microsoft Office Excel 2007 and the IBM® SPSS® statistics software, version 23.
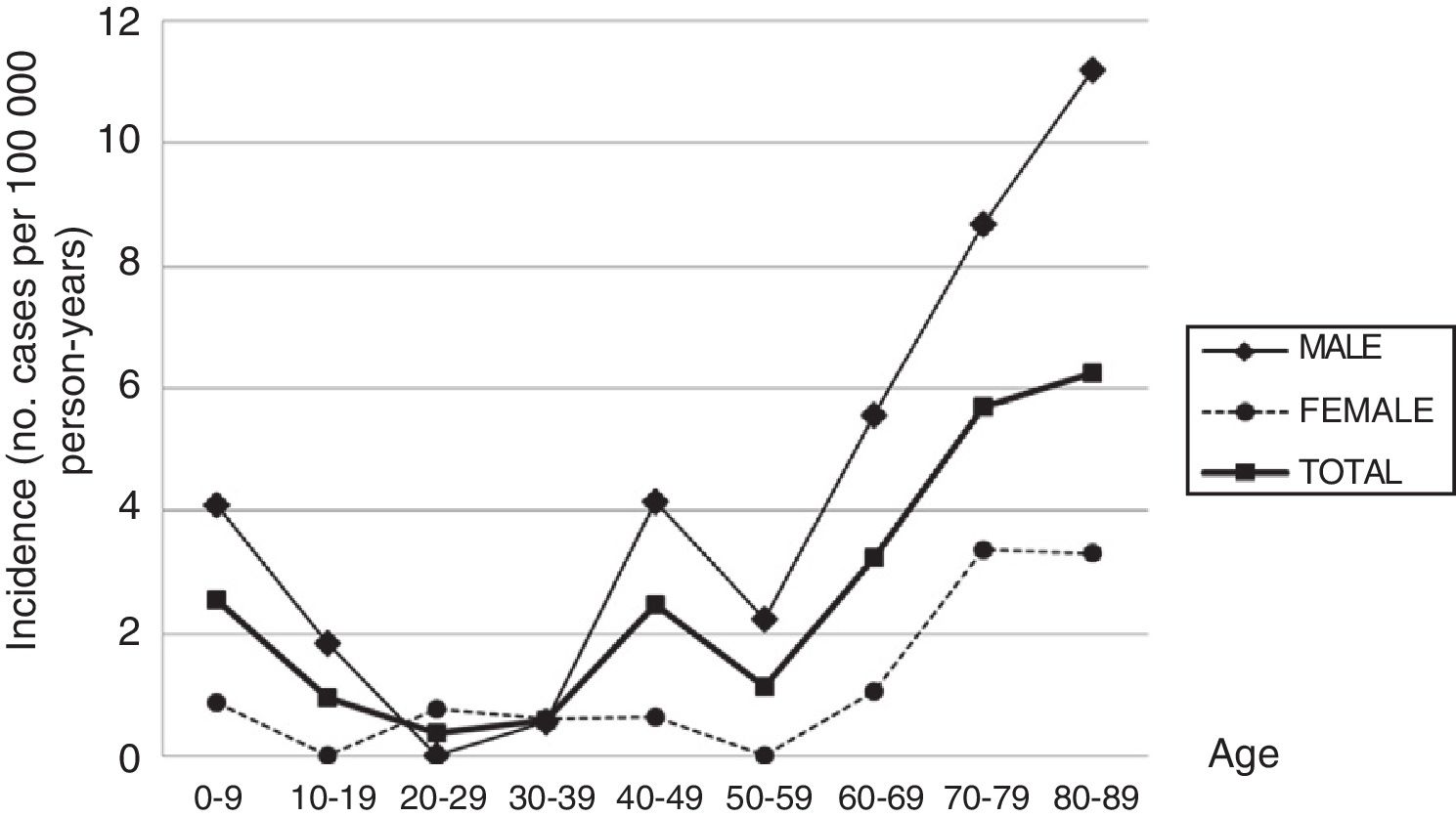
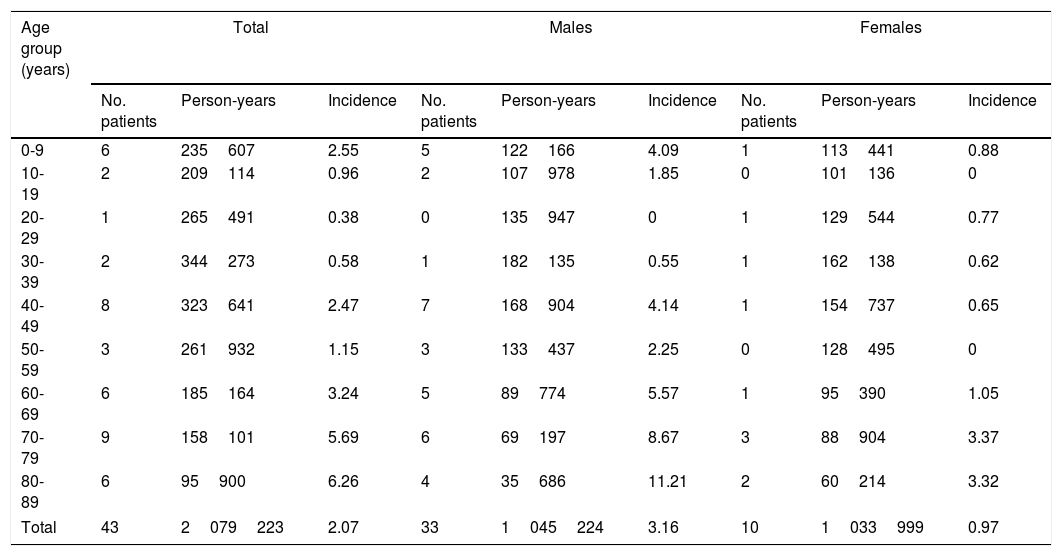
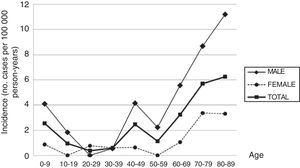
ResultsDuring the study period, 43 new cases of GBS or its variants were registered in Osona. According to the Brighton criteria, diagnostic certainty was level 1 in 58.2% of the cases, level 2 in 30.2%, level 3 in 2.3%, and level 4 in 9.3%. The incidence of GBS in the district of Osona was 2.07 cases per 100000 population for the period 2003-2016; sex-specific incidence rates were 3.16 cases per 100000 population for men and 0.97 cases per 100000 population for women. Therefore, the male-to-female ratio was 3.3:1. Mean (SD) age was 51.21 (26.9) years. Age- and sex-specific incidence rates are shown in Table 2 and Fig. 1.
Sex- and age-specific incidence of Guillain-Barré syndrome in the district of Osona between 2003 and 2016.
| Age group (years) | Total | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. patients | Person-years | Incidence | No. patients | Person-years | Incidence | No. patients | Person-years | Incidence | |
| 0-9 | 6 | 235607 | 2.55 | 5 | 122166 | 4.09 | 1 | 113441 | 0.88 |
| 10-19 | 2 | 209114 | 0.96 | 2 | 107978 | 1.85 | 0 | 101136 | 0 |
| 20-29 | 1 | 265491 | 0.38 | 0 | 135947 | 0 | 1 | 129544 | 0.77 |
| 30-39 | 2 | 344273 | 0.58 | 1 | 182135 | 0.55 | 1 | 162138 | 0.62 |
| 40-49 | 8 | 323641 | 2.47 | 7 | 168904 | 4.14 | 1 | 154737 | 0.65 |
| 50-59 | 3 | 261932 | 1.15 | 3 | 133437 | 2.25 | 0 | 128495 | 0 |
| 60-69 | 6 | 185164 | 3.24 | 5 | 89774 | 5.57 | 1 | 95390 | 1.05 |
| 70-79 | 9 | 158101 | 5.69 | 6 | 69197 | 8.67 | 3 | 88904 | 3.37 |
| 80-89 | 6 | 95900 | 6.26 | 4 | 35686 | 11.21 | 2 | 60214 | 3.32 |
| Total | 43 | 2079223 | 2.07 | 33 | 1045224 | 3.16 | 10 | 1033999 | 0.97 |
Incidence figures are expressed in cases per 100000 person-years.
In the 0-9 years age group, incidence rates were different between immigrants and Spanish individuals (12.0 vs 1.24 cases per 100000 population).
Regarding recent history of infection, 41.9% presented respiratory infections and 20.9% presented gastrointestinal infections. Only one patient had been vaccinated in the previous weeks.
High CSF protein levels were observed in 76.7% of the patients. EMG studies were performed in 86.0% of patients; neuropathy was predominantly demyelinating in 73.7% of those tested and predominantly axonal in the remaining 26.3%.
Our sample included patients with the following variants of GBS: AIDP (72.1%), AMAN (16.3%), AMSAN (4.7%), and Miller-Fisher syndrome (4.7%).
IVIg was administered to 90.2% of patients; 20.9% required respiratory support. One patient presented a relapse within 2 months of disease onset and was administered an additional cycle of IVIg, not requiring respiratory support.
No significant seasonality was observed in the incidence of GBS, although slightly fewer cases were recorded in August and September.
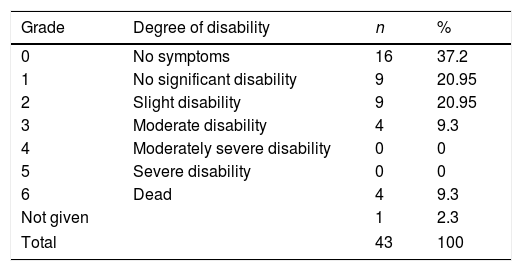
Table 3 shows the functional status (mRS) of our sample at 6 months. Four patients died within 6 months of diagnosis; in all cases, the cause of death was respiratory failure and/or pneumonia as a complication of GBS. This translates to a 6-month mortality rate of 9.3%. Excluding deaths, our sample showed a mean mRS score of 1.44 at 6 months.
Absolute and relative frequencies of modified Rankin Scale scores in a sample of patients with Guillain-Barré syndrome from the district of Osona.
| Grade | Degree of disability | n | % |
|---|---|---|---|
| 0 | No symptoms | 16 | 37.2 |
| 1 | No significant disability | 9 | 20.95 |
| 2 | Slight disability | 9 | 20.95 |
| 3 | Moderate disability | 4 | 9.3 |
| 4 | Moderately severe disability | 0 | 0 |
| 5 | Severe disability | 0 | 0 |
| 6 | Dead | 4 | 9.3 |
| Not given | 1 | 2.3 | |
| Total | 43 | 100 | |
Grade 1: able to carry out all pre-stroke activities; grade 2: unable to carry out all pre-stroke activities but able to look after self without daily help; grade 3: requiring some external help but able to walk without assistance; grade 4: unable to walk or attend to bodily functions without assistance; grade 5: requires continuous care.
Source: Van Swieten et al.17
The variables associated with poorer outcomes and mortality (mRS≥3) at 6 months were as follows: age≥80 years (50% of patients aged≥80 years scored≥3), delays in hospital admission (mean time from onset to admission was 7.9 days in patients scoring 0-2 vs 19 days in those scoring≥3; P=.015), recent history of gastrointestinal infection (33.3% of patients with gastrointestinal infection scored≥3 at 6 months), and the AMAN variant (57.1% of patients with AMAN scored≥3 at 6 months). Most patients requiring respiratory support (77.8%) showed cranial nerve involvement (P=.005). The percentage of patients requiring respiratory support was higher among those with poorer functional prognosis at 6 months, although this difference was not significant.
Patients aged≥80 years showed longer delays in hospital admission (mean of 20.7 days; P=0.012) and higher rates of poor prognosis and mortality at 6 months (50% of patients with poor functional outcomes and 50% of all deaths). No statistically significant differences were found in GBS variants, clinical variables considered in the diagnostic criteria (Table 1), or recent history of infection.
DiscussionThe incidence of GBS in our sample is higher than the rates estimated by other studies conducted in Europe and the United States.1,2 In Spain, it is also slightly higher than the incidence rate estimated in healthcare area III of the region of Aragon18 and higher than those reported by other studies.8–11,19 The fact that our study included patients from a single reference centre and that the register is updated by all neurologists in the district reduces the likelihood of overlooking incident cases, potentially explaining the high incidence reported. Despite the small study population, the long study period used (14 years) enhances the strength and validity of our results.
With the exception of the peaks observed in childhood and in men aged 40 to 49 years, the incidence of GBS increases with age, peaking after the age of 80. This age-related increase has also been reported by other authors, although most studies show a decrease after the age of 80. The age-related increase in GBS observed in our sample and in other series,12 and the decreasing incidence in patients aged over 80 reported in other studies, suggest that GBS may be underdiagnosed in elderly people. Studies of myasthenia gravis14 and amyotrophic lateral sclerosis15 conducted in the district of Osona also report the highest incidence rates in patients older than 80, which points to underdiagnosis of these neuromuscular diseases in this age group. The high incidence observed in older patients also contributes to the high global incidence rate.
The peak observed in childhood is mainly explained by the high incidence of GBS in children younger than 6. The same trend has been observed in South America,20 with incidence peaking at the age of 2 and slowly decreasing until the age of 14, as well as in some regions in Asia.21 Some European studies3,22 reveal a slight increase in the incidence of GBS among children aged 2 to 4 years. In our sample, most patients with GBS in this age group were children of immigrants from Northern Africa or South Asia. Incidence also peaked between the ages of 40 and 49 in men; this finding has occasionally been reported in the literature.23,24
Unlike in most other autoimmune diseases, the incidence of GBS is higher in the male sex. This marked male predominance is reported in most studies,1,2 although the explanation for the phenomenon is unknown.
The most frequent variant of GBS in our sample was AIDP. This is the most common variant in Europe25 and the United States,26 where it accounts for 58% and 90% of all cases, respectively; in China27 and Japan,28 in contrast, axonal variants are more frequent (30%-65%).
Recent history of infection was observed in 62.8% of patients; infections were predominantly respiratory, as is reported in other European studies.1
In Western countries, most patients are treated with IVIg or plasmapheresis. In our series, 86% of patients received IVIg and 20.9% required respiratory support. The accessibility of these treatments has reduced mortality rates; our sample showed a mortality rate below 10%, which is somewhat higher than those reported in other studies conducted in Europe29 and the United States,30 with rates approaching 5%. Mortality due to GBS is more frequent among severely affected elderly patients or patients with multiple comorbidities. The higher incidence of the syndrome observed among the oldest members of the sample partly explains the high mortality rate, since half of the deaths recorded corresponded to patients over the age of 80. On the other hand, a mortality rate calculated for such a small sample, with only 4 deaths, is of little significance.
As has previously been reported,26,31,32 age above 80 years, the AMAN variant of GBS, and delays in hospital admission were found to be associated with poorer prognosis. We also found an association between poorer prognosis and recent history of gastrointestinal infection; this variable has previously been reported in the literature and is included in a scoring system, in which age, history of diarrhoea, and disability at 2 weeks predict whether a patient will be able to walk independently at 6 months.33 Another prognostic scale includes the number of days between onset of weakness and hospital admission, presence of facial or bulbar weakness, and muscle strength (Medical Research Council sum score) to predict the need for respiratory support.34 Our study also identified facial paralysis as a predictor of the need for respiratory support.
GBS may be underdiagnosed in patients aged 80 years and older if they are not correctly evaluated, with symptoms of weakness or respiratory insufficiency being attributed to other more common age-related diseases. No differences in disease characteristics were observed between patients younger and older than 80 years, except that the latter group showed longer delays in hospital admission and poorer prognosis, with higher 6-month mortality rates.
In conclusion, our study reports one of the highest incidence rates of GBS. Incidence increases with age, although 2 small peaks occur in childhood and in men aged between 40 and 49 years, with the highest rates recorded in individuals over the age of 80. This rarely reported high incidence among the oldest members of the population may reflect underdiagnosis of the condition, demonstrating the need to be aware of the possibility that these patients may have GBS; failure to provide early diagnosis and treatment may increase mortality.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Aragonès JM, Altimiras J, Alonso F, Celedón G, Alfonso S, Roura P, et al. Incidencia y características clínicas del síndrome de Guillain-Barré en la comarca de Osona (Barcelona, España) (2003–2016). Neurología. 2021;36:525–530.