The number of elderly people with multiple sclerosis (MS) has increased in line with population ageing. As the immune system presents profound changes over an individual’s lifetime, it is important to understand the differences between these patients and younger patients.
DevelopmentImmunosenescence, defined as age-related alterations naturally occurring in the immune system, particularly influences tolerance, response, and adverse effects of disease-modifying treatments for MS. Thymic involution is the most noteworthy characteristic of this phenomenon. This process leads to a reduction in the number of virgin T cells. Other effects include an inverted CD4+/CD8+ cell ratio, severe alterations in NK cell functioning, and reduced tissue repair capacity in the brain.
ConclusionsThe number of older people with MS is increasing due to population ageing, advances in disease-modifying treatments, and improved health and social care of these patients.
Ageing of the immune system increases the risk of infections, tumours, and autoimmune diseases in elderly individuals. Furthermore, neurodegeneration is accelerated in patients with MS due to the nervous system’s loss of remyelination capacity. Understanding of the changes affecting the immune system in the elderly population is essential to improving the care provided to this ever-growing patient group.
Junto con el envejecimiento de la población general, la prevalencia de ancianos con EM se encuentra en aumento. El sistema inmunológico sufre profundos cambios a lo largo de la vida, por lo que parece imprescindible conocer qué diferencias presentan respecto a pacientes más jóvenes.
DesarrolloLa inmunosenescencia, definida como la alteración del sistema inmunológico en relación con el envejecimiento natural, juega un papel esencial en la tolerancia, efectos adversos y respuesta a los tratamientos modificadores de la enfermedad. Entre las principales características de este fenómeno, la involución que sufre el timo es la más destacable. Este hecho genera una reducción en la producción de células T vírgenes. Además, se observa una ratio de linfocitos CD4+/CD8+ invertido, alteraciones severas en el funcionamiento de las células NK o una disminución en la capacidad de reparación tisular cerebral.
ConclusionesEl número de personas de edad avanzada con EM se encuentra en aumento en coincidencia con el envejecimiento de la población general y gracias al avance en los tratamientos modificadores de la enfermedad, así como a la mejora en la asistencia sociosanitaria de estos pacientes.
El envejecimiento del sistema inmunitario conlleva un mayor riesgo de infecciones, tumores y enfermedades autoinmunes en los ancianos. En la EM, además, tiene lugar una aceleración en la neurodegeneración por la pérdida de capacidad de remielinización del sistema nervioso. Conocer los cambios que tienen lugar en el sistema inmunológico de la población de edad avanzada es esencial para mejorar la atención de este grupo de pacientes cada vez más prevalente.
Multiple sclerosis (MS) is an inflammatory demyelinating autoimmune disease of the central nervous system (CNS) of undetermined aetiology. It predominantly affects young adults, and is more prevalent among women1. Approximately 80% of patients initially present the relapsing-remitting form. However, if disease-modifying therapy is not started, up to half of patients are estimated to progress to the secondary progressive form within 10 years of symptom onset2.
Due to advances in the treatment of MS and the comorbidities associated with age, the life expectancy of these patients has increased, practically reaching parity with the general population2,3. This has led to a considerable increase in the number of older patients with MS.
Symptom onset after the age of 50 years, referred to as late-onset MS (LOMS), is very rare4–7. Prevalence ranges from 4% to 9.4% of cases, according to different cohort studies4,6,8. LOMS presents several differences with MS with onset at younger ages. Retrospective studies of the clinical and paraclinical characteristics of these patients have demonstrated that they constitute a special subgroup. Most authors agree that LOMS more frequently presents a progressive course from onset, and is associated with predominantly motor rather than visual or sensory symptoms. LOMS is also associated with a considerable diagnostic delay, probably due to advanced age, vascular comorbidities, and the predominance of progressive forms4,9.
As we age, the immune system presents significant changes, both quantitatively and functionally. Age-related immune system changes are referred to as immunosenescence. This phenomenon is particularly important in patients with autoimmune diseases or who are receiving immunomodulatory treatments.
This review analyses the main changes occurring in the immune system in association with natural ageing; the demographic, clinical, and immunopathological characteristics of older patients with MS; and the challenges we face in the management of these patients.
EpidemiologyIncidence and prevalenceAccording to various epidemiological studies conducted in different countries, the incidence and prevalence of MS have increased in recent decades. This increase seems to have been more significant among women and patients older than 50 years.
Several studies have evaluated the prevalence and incidence of MS in Spain, particularly since the 1980s. A study conducted between 1986 and 1997 in Alcoi (Alicante) estimated the incidence of the disease at 2.8 cases/100 000 person-years10. An epidemiological study conducted in Ferrol (Galicia) observed that incidence had practically doubled (5.5 cases/100 000 person-years between 2001 and 2015)11. The same study reported prevalence of 109.75 cases/100 000 population (in 2015). This contradicts the earliest prevalence studies conducted in Spain, in the 1990s, which reported rates of 30-65 cases/100 000 population12–15.
MS incidence has increased among the older population. A Danish study found that the incidence of MS in women older than 50 years had increased from 1.73 cases/100 000 person-years in 1950-1959 to 7.47 cases/100 000 person-years in 2000-200916.
MortalityRecent decades have seen a considerable improvement in the prognosis of MS. Patients with MS previously presented higher mortality rates than the general population, apparently beginning in the second decade after diagnosis17. Most survival analyses find that the disease and its complications are the cause of death in approximately 50% of patients18–20. The remaining deaths are attributed to suicide, cancer, and cardiovascular and pulmonary diseases21.
The current evidence indicates a substantial increase in these patients’ life expectancy, which is now similar to that of the general population. However, some studies suggest that life expectancy among patients with MS has not yet reached this level17,22.
A recent study of a Norwegian cohort of 1388 patients diagnosed with MS between 1953 and 2012 found that the mean survival time after diagnosis was 41 years, more than double that reported in epidemiological studies conducted in the 1960s17.
Therefore, the number of older people with MS is increasing due to population ageing and probably also because of advances in disease-modifying treatments and improved health and social care of these patients. Analysis of prevalence and incidence data must take into account the contribution of advances in diagnostic techniques and changes in diagnostic criteria.
Key points- -
The prevalence and incidence of MS have increased in recent decades, particularly among women and individuals older than 50 years of age.
- -
Life expectancy is similar to that of the general population, probably due to improvements in healthcare and the use of disease-modifying treatments.
- -
The disease and its complications constitute the cause of death in up to 50% of patients.
Immunosenescence is defined as age-related changes in the human immune system, and contributes to an increase in morbidity and mortality in older adults23. This decline in the immune system has a significant influence on a range of inflammatory processes, resistance to infection, the risk of autoimmune disease and oncological processes, and vaccine responsiveness. The most important process of immunosenescence is thymic involution, which causes a progressive decrease in the production of naïve T cells. Other effects include an inverted CD4+/CD8+ cell ratio, severe alterations in natural killer (NK) cell functioning, and reduced tissue repair capacity in the brain24,25. We summarise below the most significant age-related changes in the immune system.
Effects on the thymusThe thymus is a primary lymphoid organ where T cells mature and where both positive and negative selection processes occur; it is the main source of circulating T cells. It is located in the upper anterior part of the thorax, between the sternum and the main blood vessels. The organ increases in size until puberty and subsequently presents a progressive involution, from both an anatomical and a functional perspective26. The tissue of the parenchyma is replaced by fat, although lymphocyte subpopulations are maintained qualitatively throughout life27. Natural ageing and progressive thymic atrophy result in a significant reduction in the release of T cells into the bloodstream. While the production of new T cells from the thymus decreases with age, this does not result in a reduction in the proportion of naïve CD4+ T cells in the peripheral blood, and the total count of CD4+ T cells remains stable for some time. Twenty-five percent of T cells are produced in the thymus at the age of 25 years, decreasing to 5% by 50 years of age28,29.
The thymus receives haematopoietic precursors from the bone marrow; lymphopoiesis is thought to decrease over the years. A study using mice found that young animals undergoing transplantation of bone marrow from an older animal do not efficiently produce thymocytes30. This alteration in lymphopoiesis may affect the viability or function of thymus stromal cells, compromising the support potential of the thymic microenvironment, preventing proper differentiation and maturation of T cells.
Key points- -
From puberty, the thymus presents anatomical and functional involution, with parenchymal tissue being replaced by fat.
- -
Thymic atrophy leads to a pronounced decrease in the production of naïve CD4+ T cells and a relative increase in the fraction of regulatory T cells.
- -
Reduced lymphopoiesis may affect the function of thymus stromal cells, preventing proper differentiation and maturation of T cells.
Mature T cells released from the thymus travel to secondary lymphoid organs. Thymic involution reduces the fraction of naïve T cells and increases the proportion of memory T cells. As a result, fewer naïve T cells are present in the peripheral blood to respond to recently identified antigens, and there are more regulatory T cells to inhibit the function of T cells, probably resulting in a diminished, slowed immune response in elderly individuals31,32.
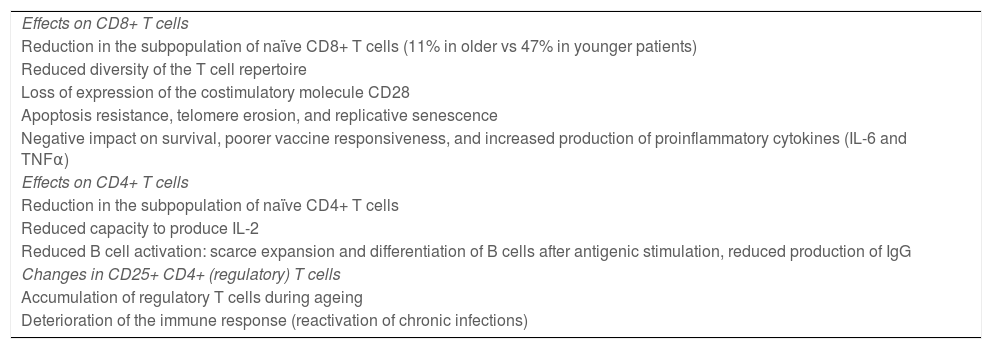
Ageing affects both CD4+ and CD8+ lymphocyte populations, although CD8+ cells are more dramatically affected (Table 1). Older adults present a reduced subpopulation of naïve CD8+ T cells (11% in elderly people vs 47% in younger individuals) and reduced diversity of the T cell repertoire23.
Effects of age on T cells.
| Effects on CD8+ T cells |
| Reduction in the subpopulation of naïve CD8+ T cells (11% in older vs 47% in younger patients) |
| Reduced diversity of the T cell repertoire |
| Loss of expression of the costimulatory molecule CD28 |
| Apoptosis resistance, telomere erosion, and replicative senescence |
| Negative impact on survival, poorer vaccine responsiveness, and increased production of proinflammatory cytokines (IL-6 and TNFα) |
| Effects on CD4+ T cells |
| Reduction in the subpopulation of naïve CD4+ T cells |
| Reduced capacity to produce IL-2 |
| Reduced B cell activation: scarce expansion and differentiation of B cells after antigenic stimulation, reduced production of IgG |
| Changes in CD25+ CD4+ (regulatory) T cells |
| Accumulation of regulatory T cells during ageing |
| Deterioration of the immune response (reactivation of chronic infections) |
The most important phenotypic alteration in senescent T cells is the loss of expression of the costimulatory molecule CD28. This phenomenon becomes more pronounced with age. Numerous studies have shown that elderly people present an expansion of CD28– CD8+ T cells in association with chronic cytomegalovirus infection33. Current evidence suggests that these cells present characteristics associated with apoptosis resistance, erosion of telomeres, and replicative senescence. It should be noted that the expansion and accumulation of these cells in older people has been associated with a negative impact on survival, poorer vaccine responsiveness, loss of bone mass, and increased production of such proinflammatory cytokines as IL-6 and TNFα34,35.
Some studies suggest that the CD4+ T cell population also presents important changes during ageing. These cells play a fundamental role in B cell activation, in the development of germinal centres, and in antibody isotope switching, which is essential to the formation of memory B cells36. Senescent CD4+ T cells have a reduced capacity to produce IL-2 after antigenic stimulation, and to correctly activate B cells. This results in diminished capacity for the expansion and differentiation of B cells and for the production of IgG antibodies32,37, with a consequent negative effect on humoural immunity.
The population of regulatory T cells increases in older age. Among regulatory T cells, a subset of CD4+ cells, generally characterised by elevated CD25 expression, controls the intensity of the immune response. These cells play a key role in controlling the immune response: they help to prevent autoimmunity by attenuating the response of T cells, and promote immune tolerance. The increase in the proportion of these cells has been associated with a deterioration of the immune response32. For instance, aged mice have been shown to present greater frequency of Foxp3+ CD4+ T cells, correlated with the spontaneous reactivation of chronic Leishmania major infection. This suggests that the accumulation of regulatory T cells in older adults may play an important role in the frequent reactivation of chronic infections observed in old age38,39.
In conclusion, the series of age-related alterations observed in the T cell subpopulation reduces the capacity to respond to new antigens and causes a delayed, diminished immune response.
Effects on B cellsMultiple studies have shown that elderly people present a reduced capacity to produce antibodies or an adequate response to new antigens and vaccines40,41.
As mentioned previously, the response of B cells to new antigens is affected by changes in CD4+ T cells; however, this is not the only explanation for their loss of capacity to generate an adequate immune response in older patients (Table 2).
Effects of age on B and NK cells.
| Effects on B cells |
| Reduced production of B cells and reduced lymphoid differentiation (in favour of myeloid lineages) |
| Reduced production of antibodies and inadequate response to new antigens and vaccines |
| Increased subpopulation of memory B cells and reduced diversity of B cells/antibody repertoire |
| Effect on natural killer cells |
| Reduced cytotoxic activity and cytokine production |
| Reduced cell production: redistribution of subpopulations with increased proportion of regulatory CD56bright cells |
| Promotes inflammation due to activation of dendritic cells and monocytes |
In adults, B cells mainly develop in the bone marrow, from haematopoietic stem cells. With ageing, the production of immature B cells by the bone marrow is reduced. It has also been reported that lymphoid differentiation decreases in older adults, in favour of myeloid differentiation42.
Despite this alteration in lymphopoiesis, the number of B cells in the peripheral blood does not significantly change43. However, as occurs with T cells, the composition of cell subpopulations does change with age, with a greater proportion of memory B cells and a dramatic reduction in B cell diversity and the antibody repertoire.
Effect on natural killer cellsNK cells are involved in innate immunity, and are responsible for such processes as cancer surveillance, defence against intracellular pathogens, and regulation of the immune system via cytokine production23. These cells develop in the bone marrow and have a cytotoxic function, secreting such cytokines as TNFα, INFγ, and IL-5. They are characterised by expression of CD56 and/or CD1644. NK cells account for 10%-15% of the entire lymphocyte population, and are classified into 2 subpopulations: CD56bright (regulatory) and CD56dim (cytotoxic) NK cells. Ageing has phenotypic and functional effects on the NK cell compartment, reducing cytotoxic activity and the production of and response to cytokines, and reducing cell proliferation, although the total number of NK cells is maintained. This is the result of a redistribution of NK subpopulations, with an increase in the proportion of CD56dim cells to the detriment of CD56bright cells45. This may cause alterations to other cells of the innate or adaptive immune systems, as CD56bright cells have a fundamental role in the activation of dendritic cells and interact with monocytes to promote inflammation46. Some studies suggest that CD56bright cells have beneficial effects in patients with MS and receiving immunomodulatory treatment47. A clinical trial with daclizumab found that the expansion of CD56bright cells observed in patients receiving the drug was correlated with a decrease in the number of gadolinium-enhancing lesions in brain MRI studies48.
As we have observed, immunosenescence not only plays a fundamental role in inflammatory disease in older patients, but also increases the risk of infection, tumours, cardiovascular diseases, and intolerance to immunomodulatory treatments in these patients. As the immune system presents profound changes over an individual’s lifetime, it is important to understand the differences between these patients and younger patients, with a view to ensuring proper monitoring of these patients and selecting the most appropriate disease-modifying treatment.
RemyelinationMS is heterogeneous both from a clinical and radiological viewpoint and with respect to the associated lesions. MS lesions are characterised by demyelination, axonal damage and loss, and reactive gliosis. Biopsies of nerve tissue have identified 4 lesion patterns according to the immunohistochemical findings. Patterns I and II correspond to immune-mediated lesions due to macrophages activated by T cells or by antibodies/complement. The latter pattern appears to be the most frequent, accounting for 50% of all cases. Patterns III and IV, however, are characterised by lesions resembling those of non–immune-mediated oligodendrogliopathies49. In addition to the great variability between individuals, there also seems to be some degree of intra-individual variability over the course of the disease.
Remyelination of MS lesions is another important process that occurs to different extents over time. Evidence in the literature suggests that the brain’s remyelination capacity is high at disease onset, with a subsequent sharp decrease with age50. A study analysing biopsies of brain lesions from patients with early and chronic MS found that remyelination was significantly more intense in the first group51. Another study examining brain tissue from 51 deceased patients found that older age at death and longer disease duration were associated with more remyelinated lesions52.
Oligodendrocyte precursor cells (OPC) play an essential role in the repair of damaged axons. Ageing is associated with a series of alterations in remyelination, including a reduction in the recruitment and differentiation of OPCs53,54. Among other factors, advanced age seems to cause deficient epigenetic modulation of the differentiation of oligodendrocytes, negatively affecting the process of remyelination55.
ConclusionsThe global increase in life expectancy has resulted in a higher prevalence of MS among older individuals. This trend constitutes a great challenge, as many of these patients present associated comorbidities that may delay diagnosis and therapeutic decision-making.
The profound age-related changes affecting the innate and adaptive immune systems may explain the increased incidence of primary forms of MS in patients older than 50 years. Furthermore, this phenomenon seems to increase the risk of infection, tumours, and cardiovascular diseases. Similarly, it may play a role in intolerance to or loss of efficacy of various immunomodulatory drugs. The decrease in remyelination capacity that occurs from the fourth decade of life also contributes to the degeneration of brain tissue in older patients with MS.
Recent years have seen significant progress in our understanding of the immune system’s role in MS. This has resulted in an expansion of disease-modifying treatment options for relapsing-remitting forms; while this advance has had little effect on the treatment of progressive forms of the disease, new treatments are expected to become available in the coming years.
It is noteworthy that all the pivotal trials have excluded older patients, with the consequence that no safety or efficacy data are available for this patient group. It is essential to study the impact of disease-modifying drugs in older patients, as this group continues to be treated according to the current treatment guidelines, in which they are not represented. On account of the growing prevalence of MS in older individuals, it is truly urgent that we conduct broader cohort studies of these patients and develop new treatment guidelines that include this population.
Furthermore, given the increased risk of infection, autoimmune reactions, and probably cancer associated with disease-modifying treatments, immunosenescence may have a cumulative effect; therefore, this process must be taken into account for the onset and maintenance of these treatments in this patient group. Thus, the decision to discontinue treatment in these patients must take into consideration such factors as age, absence of clinical/radiological disease activity in the previous years, and the specific drug in question. A randomised clinical trial currently underway (the DISCOMS trial) will assess the risk of clinical or radiological relapses after treatment suspension in patients older than 55 years56. This will contribute additional data, which may help establish a treatment approach in a subgroup of patients who are often overlooked by pharmaceutical companies, but have a particularly significant impact in the daily practice of neurologists treating patients with MS.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr Teresa Ayuso Blanco for her guidance and suggestions in the development of this study.