The apparent diffusion coefficient (ADC) in MRI seems to be related to cellularity in brain tumours. Its utility as a tool for distinguishing between histological types and tumour stages remains controversial.
ProceduresWe retrospectively evaluated children diagnosed with CNS tumours between January 2008 and December 2013. Data collected were age, sex, histological diagnosis, and location of the tumour. We evaluated the ADC and ADC ratio and correlated those values with histological diagnoses.
ResultsThe study included 55 patients with a median age of 6 years. Histological diagnoses were pilocytic astrocytoma (40%), anaplastic ependymoma (16.4%), ganglioglioma (10.9%), glioblastoma (7.3%), medulloblastoma (5.5%), and other (20%). Tumours could also be classified as low-grade (64%) or high-grade (36%). Mean ADC was 1.3 for low-grade tumours and 0.9 for high-grade tumours (P=.004). Mean ADC ratios were 1.5 and 1.2 for low and high-grade tumours respectively (P=.025). There were no significant differences in ADC/ADC ratio between different histological types.
ConclusionADC and ADC ratio may be useful in imaging-study based differential diagnosis of low and high-grade tumours, but they are not a substitute for an anatomical pathology study.
El coeficiente de difusión aparente (ADC) de la resonancia magnética parece relacionarse con el grado de celularidad de los tumores de sistema nervioso central. Su utilidad para diferenciar el grado tumoral y tipo histológico de los tumores es controvertido.
Material y métodosEstudio retrospectivo de los pacientes pediátricos con diagnóstico de tumor de sistema nervioso central desde enero-2008 a diciembre-2013. Se revisan edad, sexo, localización del tumor y anatomía patológica. Las medidas de ADC y ratio ADC (cociente ADC tumoral/ADC tejido sano) se llevaron a cabo por 2 neurorradiólogos expertos, ciegos al diagnóstico histológico. Se calcula el valor ADC y el ratio ADC y se comparan sus valores con los diagnósticos anatomopatológicos.
ResultadosSe incluyen 55 pacientes. La mediana de edad fue 6 años. Los diagnósticos anatomopatológicos fueron: astrocitoma pilocítico (40%), ependimoma anaplásico (16,4%), ganglioglioma (10,9%), glioblastoma (7,3%), meduloblastoma (5,5%), y otros (20%). El 64% fueron de bajo grado (BG) y el 36% de alto grado (AG). La media de ADC fue 1,3 en los de BG y 0,9 en los de AG (p=0,004). La media de ratio ADC fue de 1,5 y 1,2 (p=0,025) respectivamente. No hubo diferencias significativas en el ADC/ratio ADC entre los distintos tipos histológicos.
ConclusionesEl ADC y ratio ADC son una herramienta útil en la diferenciación por imagen del grado tumoral en los tumores cerebrales pediátricos, sin sustituir a la anatomía patológica.
Brain tumours are the most frequent solid tumours in paediatric patients. Between 1980 and 2012, 4448 cases of CNS tumours were entered in the Spanish Register of Paediatric Tumours.1 Brain tumours constitute the main cause of cancer deaths in this population.
The patient's age and radiological findings are crucial for diagnosing this entity. Supratentorial tumours are more common in children younger than 2 years, whereas older children present infratentorial tumours more frequently.
CT and MRI studies can orientate histological diagnosis by providing essential data on tumour location, extension, and radiological characteristics.2,3 However, conventional techniques do not allow us to differentiate between high-grade (HG) and low-grade (LG) tumours. Advanced neuroimaging techniques provide valuable radiological data on the pathophysiology and metabolism of these tumours that can be used in diagnosis and in planning the surgical approach.4
Diffusion-weighted imaging is an MRI technique that measures the Brownian motion of protons within different tissue types. According to various studies, this technique may be useful for detecting brain tumours and determining their histological grade5 and it might even have a prognostic value.6,7 Apparent diffusion coefficient (ADC) is a measure of the magnitude of diffusion. Its reported specificity for differentiating between degrees of malignity and between histological types varies from study to study.
Our purpose is to study the usefulness of ADC for determining tumour grade and histological type in paediatric patients with a diagnosis of CNS tumours in our hospital.
Patients and methodsWe conducted a retrospective study of all patients younger than 16 diagnosed with CNS tumour in the paediatric neurosurgery department at Hospital 12 de Octubre, in Madrid. We gathered the patients’ medical histories and demographic and clinical characteristics: age at diagnosis, sex, tumour location (supratentorial or infratentorial), and histological diagnosis.
Histological diagnoses of brain tumours were classified by tumour grade according to the criteria established by the World Health Organisation (WHO).8,9 WHO grade I and II tumours were considered LG tumours, whereas grade III and IV were regarded as HG tumours.
All imaging studies in our patient population were performed with a Philips Achieva 1.5T MRI scanner. We collected T1-weighted, 3D, axial T2-weighted, axial FLAIR, axial diffusion-weighted, T2-weighted perfusion, and T1-weighted contrast-enhanced sequences.
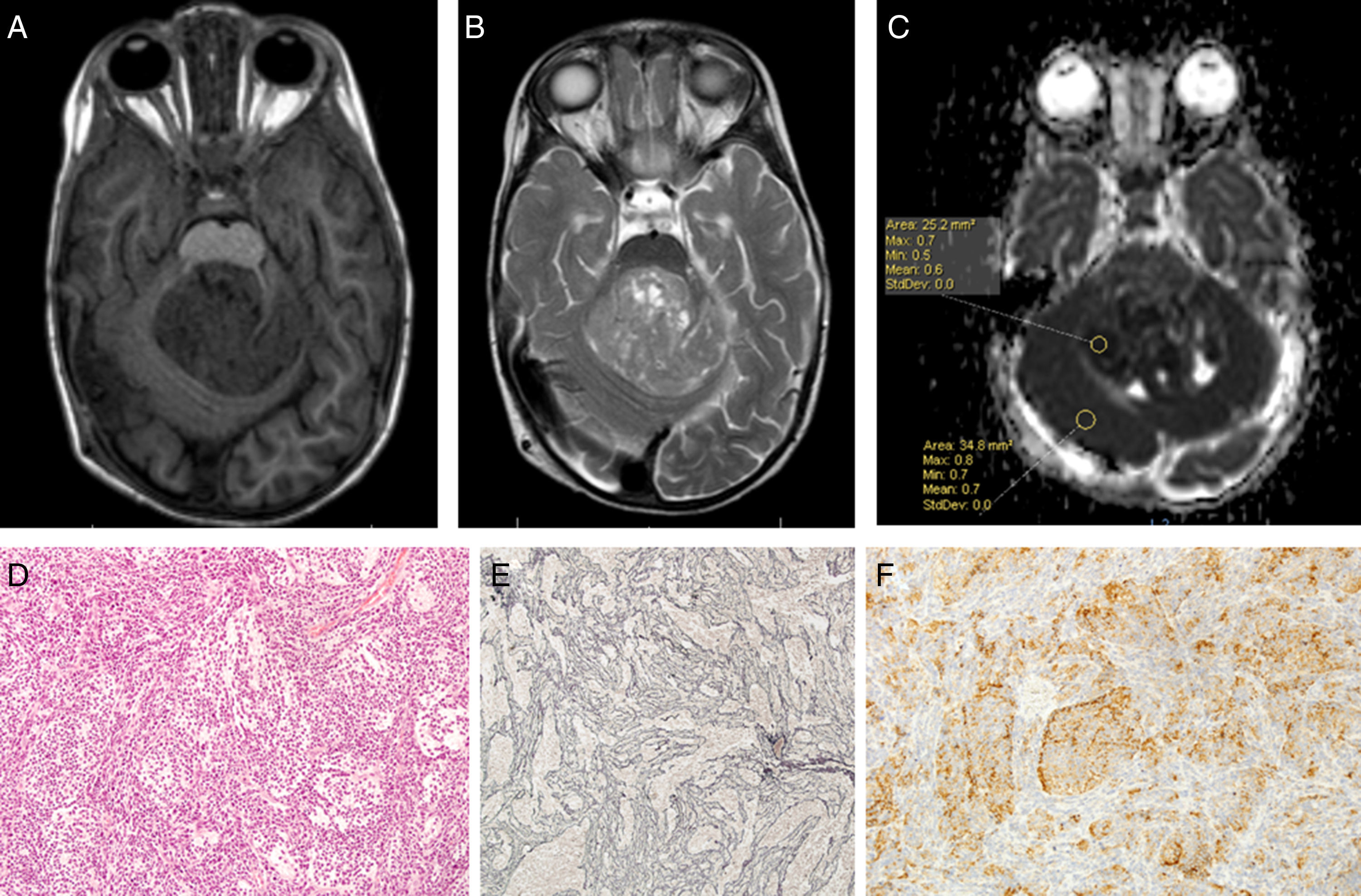
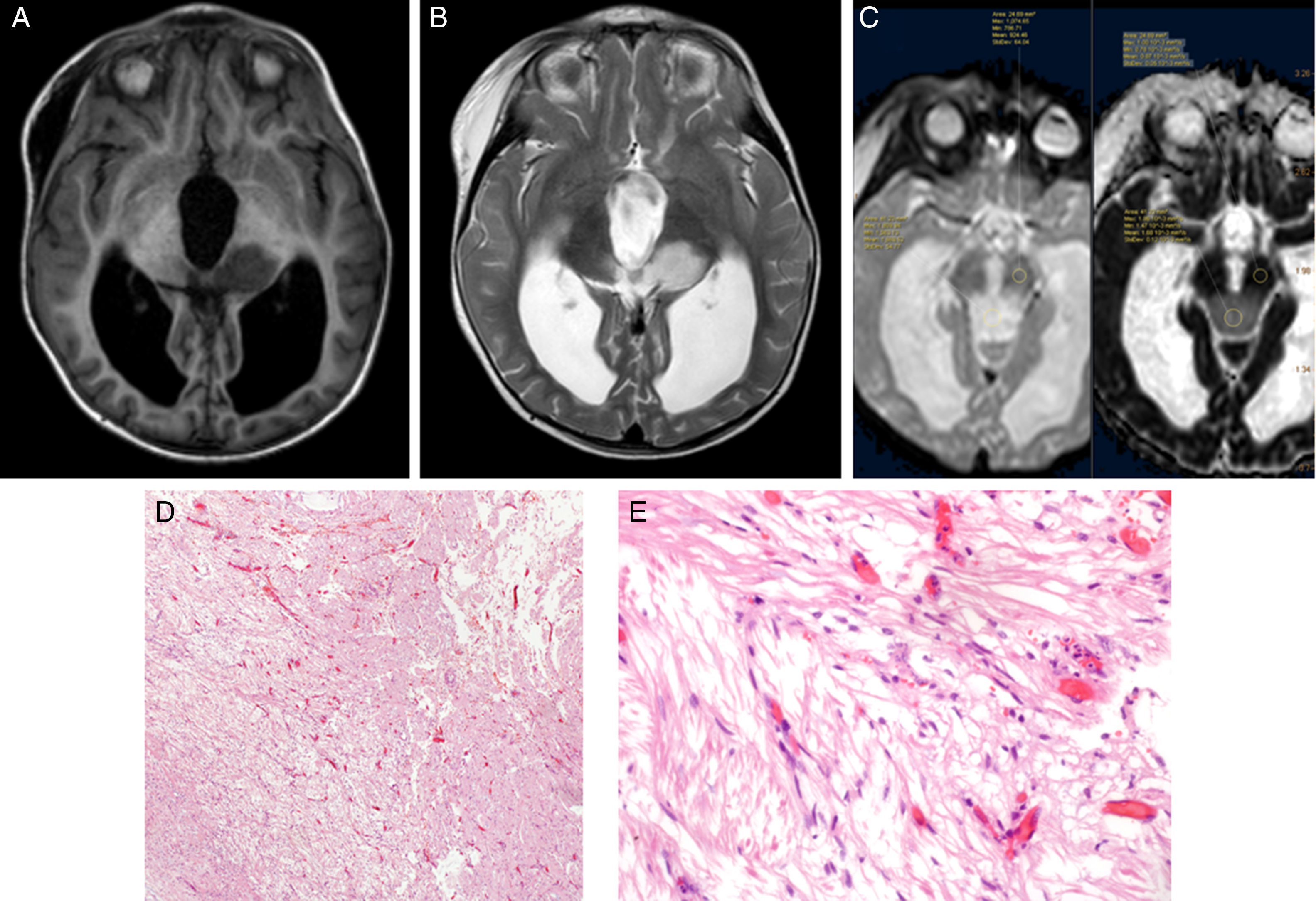
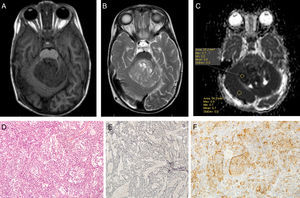
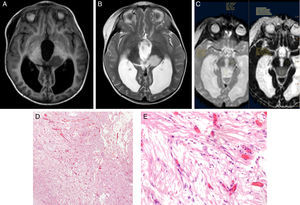
Using Philips IntelliSpace Portal software, we created an ADC map for patients undergoing an MRI study at time of diagnosis (Fig. 6).
Two expert radiologists blinded to the histological diagnosis evaluated images from the included patients. We determined the ADC in the solid portion of the tumour, excluding areas potentially affected by artefacts, that is, those containing calcium or haemosiderin, showing necrosis, or located at an interface zone. Using the selected area, we marked a region of interest (ROI) in which to determine maximum, minimum, and mean ADC values. We subsequently created another ROI in healthy contralateral white matter and measured maximum, minimum, and mean ADC values. The ADC ratio was obtained dividing mean ADC in the tumour by mean ADC in contralateral white matter. This study complies with ethical standards for personal data protection. The patients’ parents or guardians signed informed consent forms. This article provides no personal data belonging to participants (Fig. 7).
Statistical analysisWe used SPSS statistical software version 20 to test hypotheses and MedCalc version 11 for plotting ROC curves and establishing cut-off points.
The Mann–Whitney U test for independent samples was used to compare ADC and ADC ratios between LG and HG tumours.
We used the ANOVA test to compare ADC and ADC ratios between different histological types of tumours.
Differences were considered statistically significant for P-values below .05.
ResultsWe included a total of 55 patients during the study period. Ages ranged from 4 months to 16 years (mean, 5 years; median, 6 years); 74.5% of the total were boys and 25.5% were girls. The histological diagnoses of our patients were pilocytic astrocytoma, grade I (22 patients), anaplastic ependymoma, grade III (9), ganglioglioma, grade I (6), glioblastoma, grade IV (4), medulloblastoma, grade IV (3), atypical choroid plexus papillom, grade II (2), pineoblastoma, grade IV (1 patient), mature teratoma (1), pleomorphic xanthoastrocytoma with anaplastic features, grade II (1), choroid plexus carcinoma, grade III (1), dysembryoplastic neuroepithelial tumour, grade I (1), hamartoma (1), and pituitary adenoma (1). Two patients did not have a histological diagnosis since they had not undergone surgery at the time of data collection.
Thirty-four patients presented LG tumours (WHO grades I and II and any benign tumours not included in the WHO classification) and 19 patients had HG tumours. Tumours were not classified in 2 cases due to the lack of histological studies.
We excluded an additional 9 patients from the statistical study of ADC and ADC ratios since MRI diffusion-weighted sequences were not available at time of diagnosis in the following cases: 3 with pilocytic astrocytoma (including 2 in the spinal cord), 3 with anaplastic ependymoma (one was disseminated and located in the spinal cord), one ganglioglioma, one prolactinoma, and one hamartoma.
A total of 44 patients (26 with LG and 18 with HG tumours) were included in the final analysis of ADC and ADC ratios.
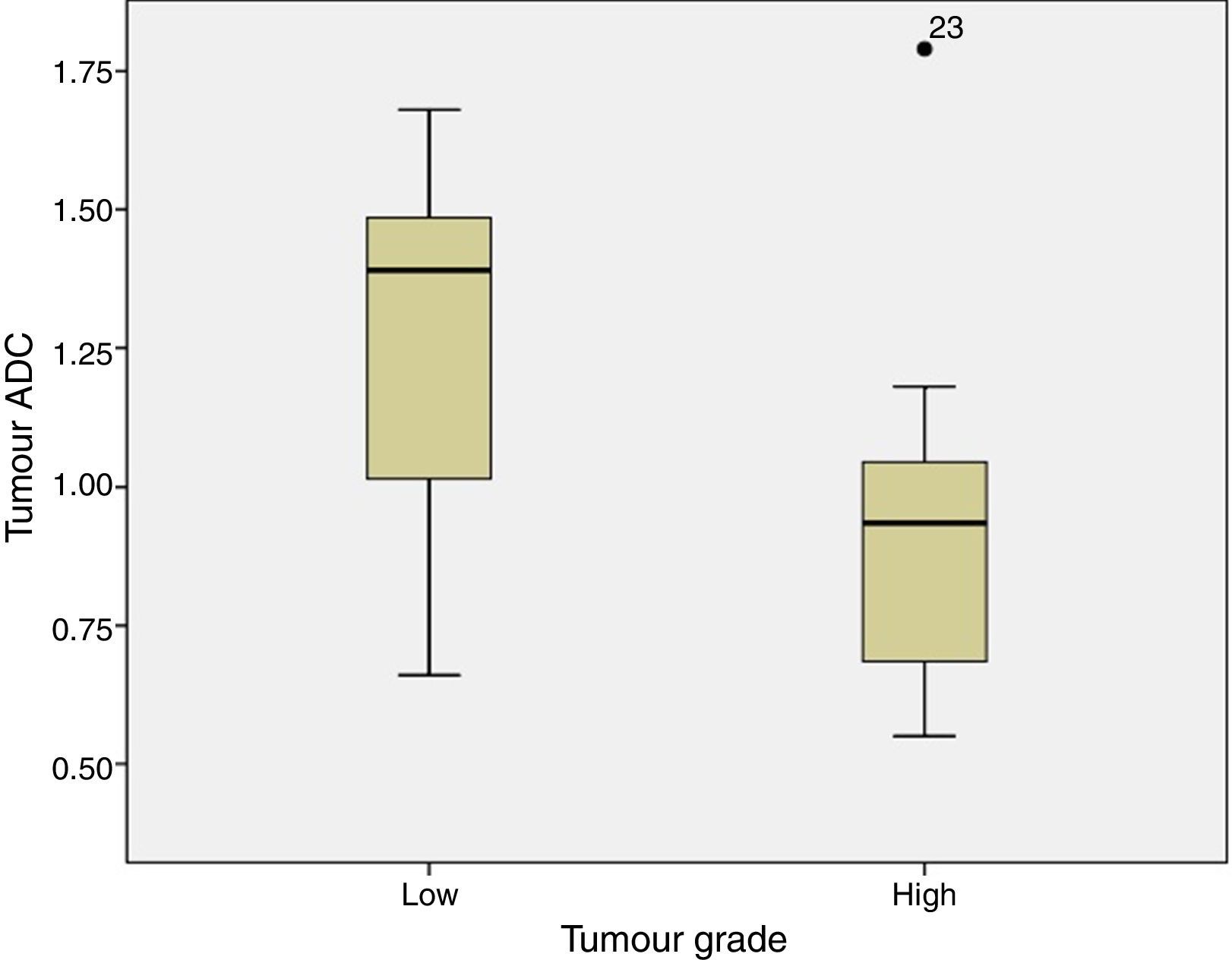
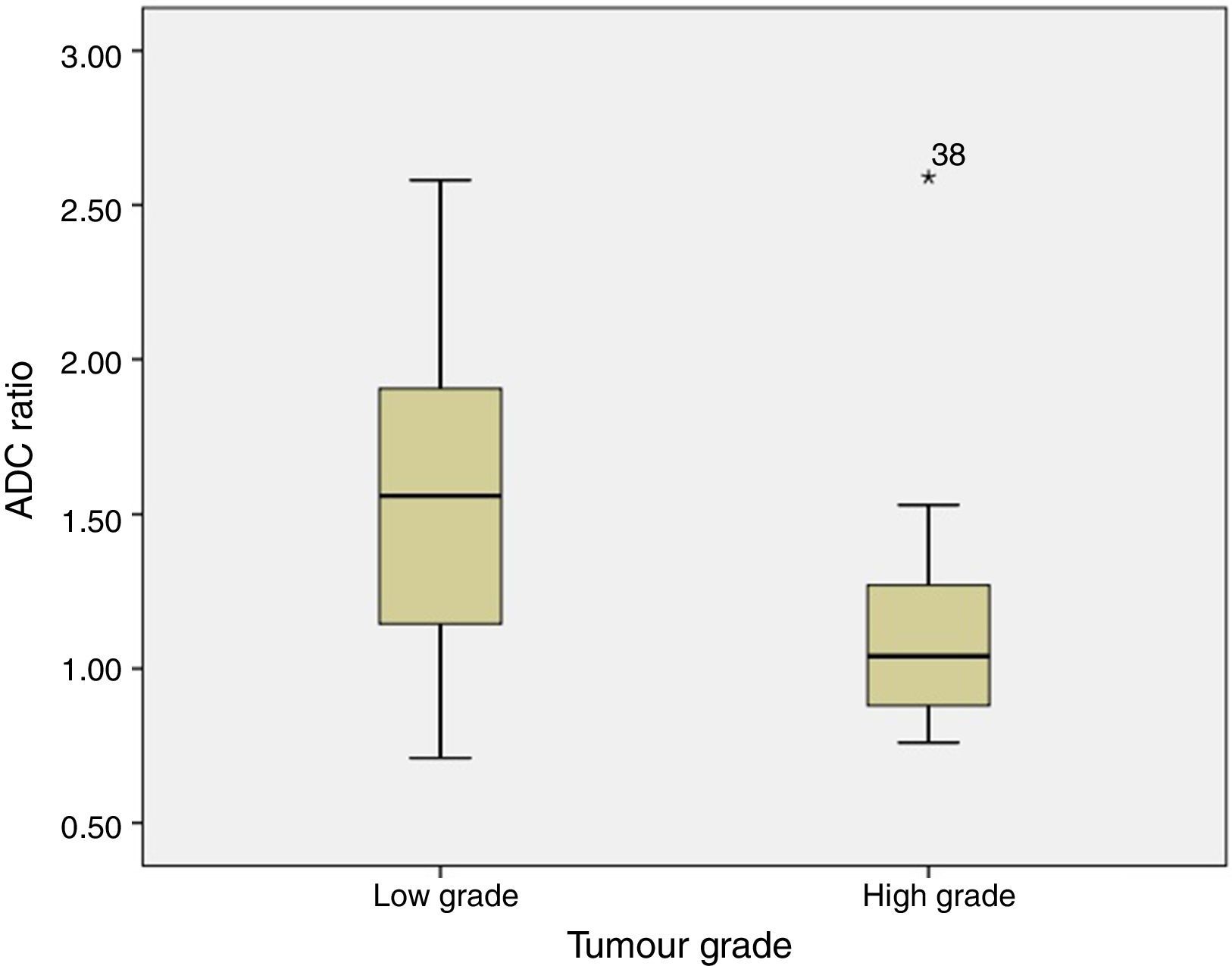
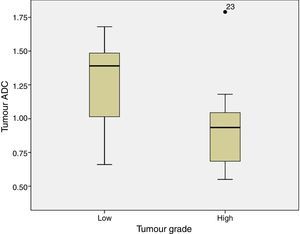
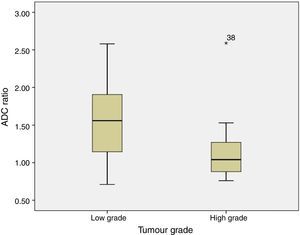
ADC was shown to be higher in patients with LG tumours than in those with HG tumours: 1.32±0.46 and 0.9±0.29×10−3mm2/s, respectively (P=.004) (Fig. 1). The same was true for ADC ratios (Fig. 2): 1.56±0.52−3mm2/s in patients with LG tumours and 1.17±0.44−3mm2/s in those with HG tumours (P=.025).
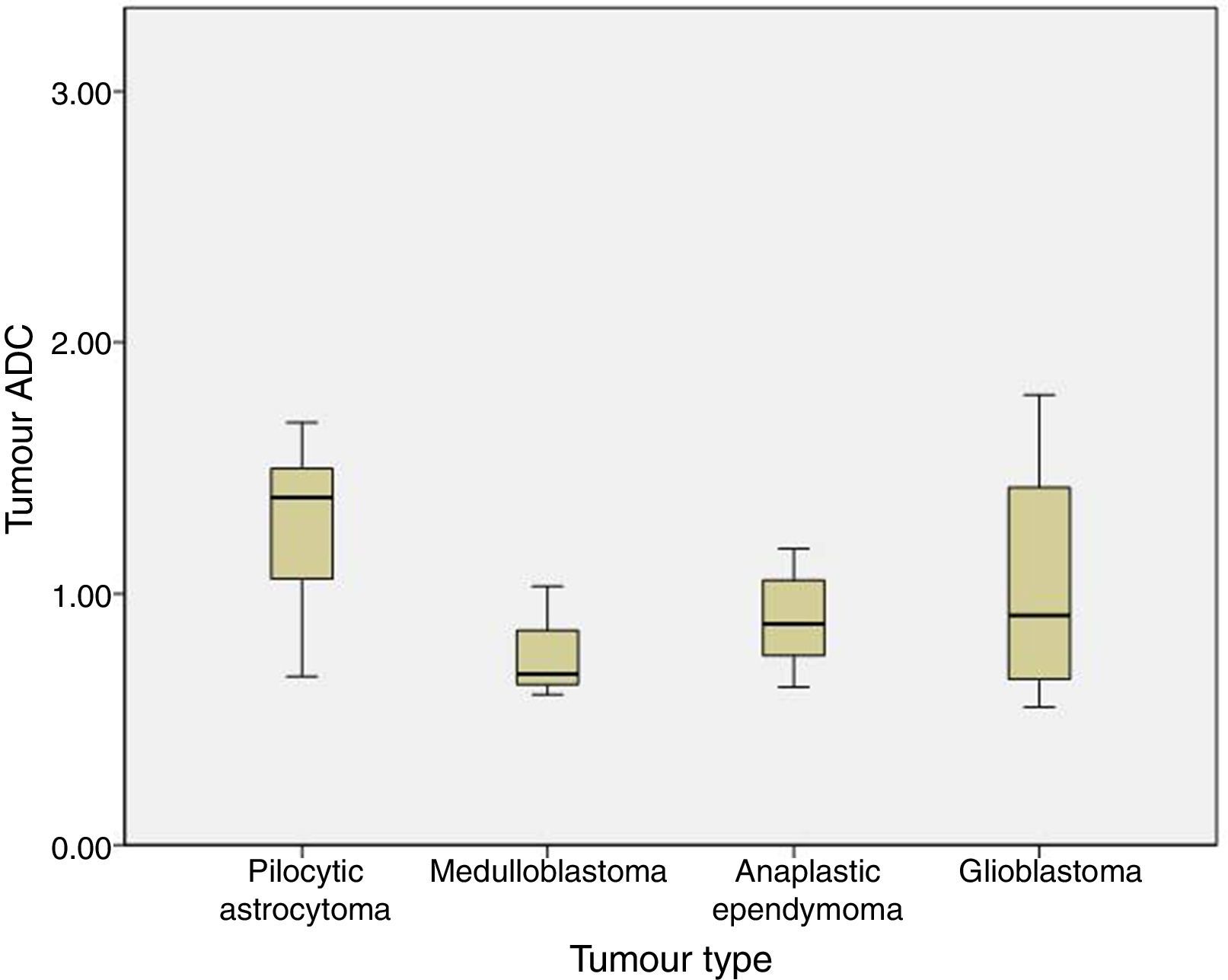
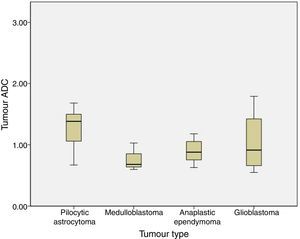
We analysed the differences in ADC and ADC ratios among pilocytic astrocytoma, medulloblastoma, anaplastic ependymoma, and glioblastoma, and found no statistically significant differences in either value among different histological tumour types. ADC was 1.29±0.31 for pilocytic astrocytoma, 0.77±0.22 for medulloblastoma, 0.9±0.21 for anaplastic ependymoma, and 1.04±0.54 for glioblastoma (P=.1) (Fig. 3).
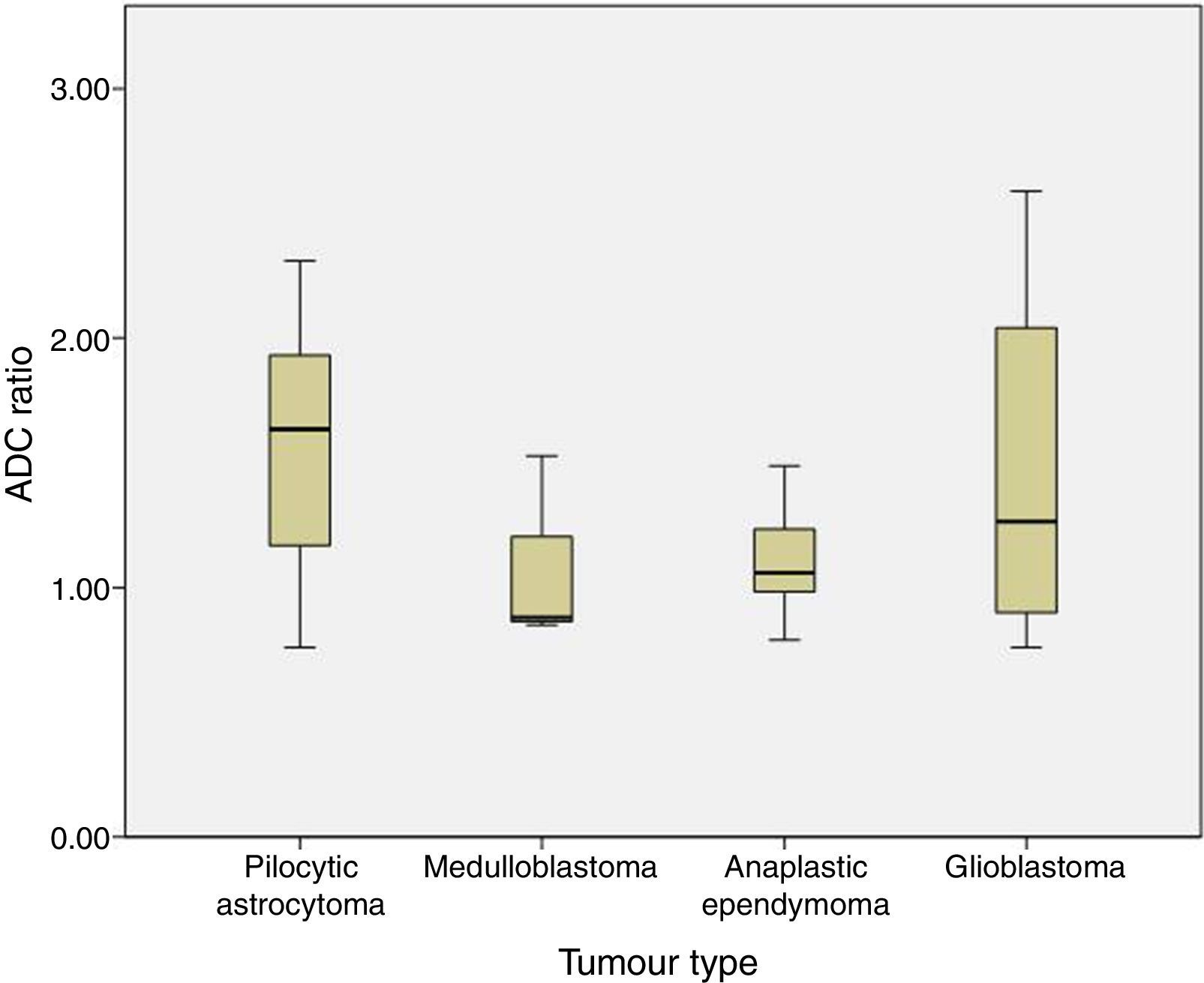
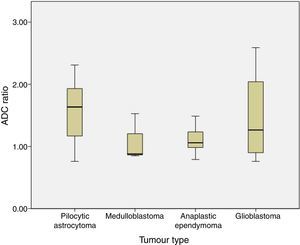
The ADC ratio was 1.58±0.47 for pilocytic astrocytoma, 1.08±.038 for medulloblastoma, 1.11±0.22 for anaplastic ependymoma, and 1.47±0.8×10−3mm2/s for glioblastoma (P=.2) (Fig. 4).
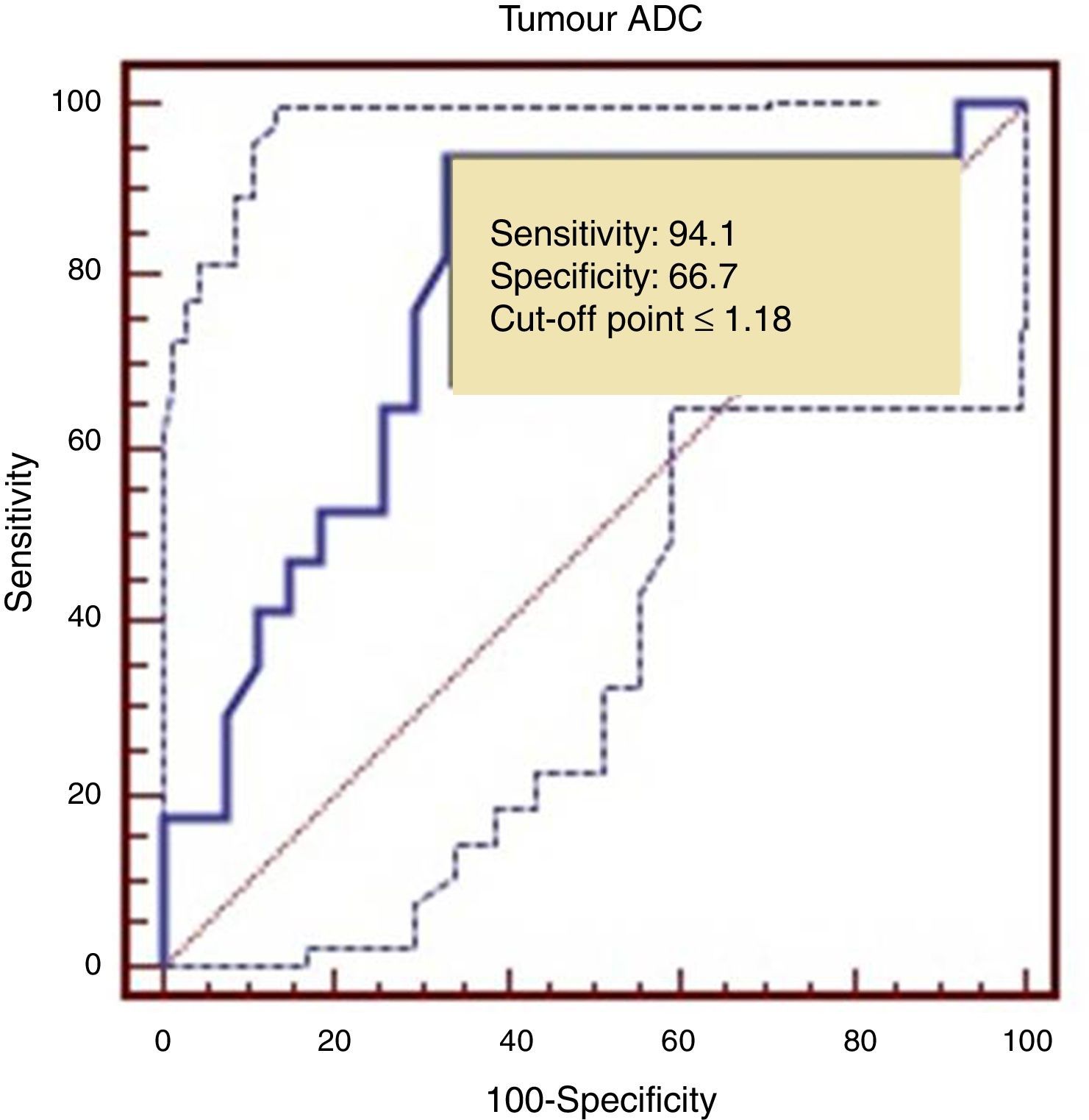
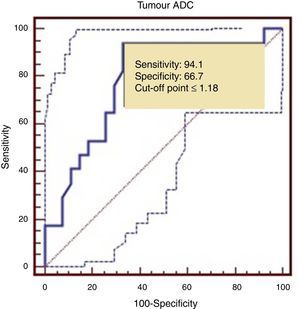
The ROC curve identified a cut-off point of 1.18 between LG and HG tumours, with a sensitivity of 94.1%, a specificity of 66.7%, and an area under the ROC curve of 0.782 (Fig. 5).
Diffusion-weighted imaging is an MRI technique that measures the random Brownian motion of protons, mainly protons in the water contained by tissue. The diffusion pattern of the water molecules depends on the microscopic structure of each type of tissue. Water molecule diffusion decreases at higher cell densities, reduced extracellular space, and an increased nuclear-cytoplasmic ratio. In the CNS, diffusion is highest in CSF. Brightness in diffusion sequences differs between white and grey matter due to the different structure of these 2 types of tissue.
However, bright areas in diffusion-weighted sequences are not exclusively caused by diffusion of water molecules; they may result from long T2 decay times, or ‘shine-through’. To minimise the influence of T2 shine-through and determine the extent of water molecule diffusion, ADC maps can be created using specific software tools.
Brain tumours with hypercellularity have been shown to exhibit more diffusion restriction.10 Cellularity and nuclear area are usually greater in HG tumours than in LG tumours.
Multiple studies have shown an inverse correlation between ADC and both tumour grade and histological type. However, the data published to date are controversial.11–14
In our series, as in other studies, both ADC and ADC ratio helped differentiate tumour grades, since both values were higher in LG than in HG tumours. This suggests that ADC maps may be used in imaging studies conducted before surgery since they help determine tumour grade. This, in turn, may be useful for planning surgery, given the importance of complete tumour resection: in many such cases, and especially in HG tumours, survival is determined by the presence of tumour remnants. Presurgery suspicion of an HG tumour may push neurosurgeons to plan a more aggressive surgery, in addition to performing an intraoperative biopsy.
Regarding tumour classification by histological type, differences in our series were not statistically significant although ADC values for medulloblastoma were lower than those for pilocytic astrocytoma or other HG tumours with lower cellularity. This may be due to the small sample size (only 3 patients were diagnosed with medulloblastoma) or to methodological differences with other studies.
In our sample, ADC and ADC ratio show overlaps for the 4 histological types that were analysed. Most studies report differences between pilocytic astrocytoma and medulloblastoma5,12 and establish a cut-off point with 100% specificity. Overlapping between the remaining types of tumours is more frequent.14 In our series, we also found an overlap between ADC values in pilocytic astrocytoma and medulloblastoma. The lowest ADC values in pilocytic astrocytoma were in patients with optic glioma (these tumours have higher cellularity and a more compact structure15 than LG gliomas at other locations) and in a patient with pilocytic astrocytoma associated with leptomeningeal dissemination (greater cellularity or aggressiveness). In medulloblastoma, the ADC value overlapping those of other histological types of tumour is 1.08 and corresponds to medulloblastoma with extensive nodularity. Not all medulloblastomas show restricted diffusion; diffusion restriction also depends on the degree of cellularity and the reticulin content of each histological subtype. ADC is lower in desmoplastic/nodular medulloblastoma than in medulloblastoma with extensive nodularity, since the former shows more restricted diffusion.16,17
Other possible causes of overlap are linked to tumour location. The studies achieving 100% specificity for differentiating between pilocytic astrocytoma and medulloblastoma/primitive neuroectodermal tumour are usually restricted to posterior fossa tumours, whereas our study included patients with both infratentorial and supratentorial tumours.
Our study methodology may also explain the differences between results from other studies and our own. In our study, the ROI was established in the region showing the greatest diffusion restriction within the solid portion of the tumour after excluding areas affected by artefacts. Other studies chose the area showing contrast enhancement within the solid portion of the tumour.18 We used a single ROI in our study since no differences have been reported between using one ROI and using the mean of 3 ROIs.19 Since ADC values in healthy, developing brains decrease with age,12 ADC is preferred to ADC ratio for assessing brain tumours in clinical practice; use of ADC ratio is limited to comparisons in multicentre studies.18
As in our study, ADC and ADC ratio are determined before surgery since postsurgical changes may alter diffusion signal. Therefore, these values cannot be used to detect tumour remnants or early recurrence.20 However, they may be helpful for diagnosing metastasis or tumour progression in medulloblastoma.21
Our results suggest that ADC is a useful tool for presurgical diagnosis of brain tumours and constitutes an important aid to MRI-based diagnosis, although it does not substitute histopathological diagnosis. Further studies should analyse the scope of this fast method that is compatible with all modern MRI machines.
Conflicts of interestThis study received no funding of any kind.
It was presented at the 7th Congress of the Spanish Society of Paediatric Haematology and Oncology (22–22 May 2014) and awarded the prize for the best oral communication.
Please cite this article as: Domínguez-Pinilla N, Martínez de Aragón A, Diéguez Tapias S, Toldos O, Hinojosa Bernal J, Rigal Andrés M, et al. Evaluación de la utilidad del coeficiente de difusión aparente en resonancia magnética para la diferenciación del grado tumoral de los tumores cerebrales pediátricos. Neurología. 2016;31:459–465.