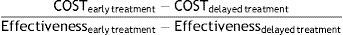The BENEFIT study has demonstrated the benefits of early treatment with interferon beta 1b (IFNβ-1b). The objective of this study was to estimate the efficiency of early vs. delayed IFNβ-1b treatment in patients with clinically isolated syndrome (CIS) suggestive of multiple sclerosis (MS) in Spain.
MethodsA Markov model reflecting the social perspective was developed with time horizons ranging from 2 years to lifetime. A cohort of 1000 patients with CIS, whose health status had been measured on the Expanded Disability Symptom Scale (EDSS), included patients who received early IFNβ-1b treatment and those who did not. Data from the BENEFIT study were used to model EDSS progression and transitions to MS. Costs were estimated from published literature. Patient utilities were derived from EQ-5D data and published data. Mortality was estimated using life tables and EDSS data. Costs (€ at 2013 rates) and outcomes were discounted at 3% per annum. A probabilistic sensitivity analysis was performed.
ResultsIn the base case, both the incremental cost utility ratio (ICUR) and the incremental cost effectiveness ratio (ICER) of IFNβ-1b vs. no treatment were dominant (more effective and less costly) from a social perspective. From the perspective of the Spanish Health System, the ICUR was € 40702/QALY and the ICER was € 13/relapse avoided.
ConclusionEarly treatment with IFNβ-1b after a CIS vs. delayed treatment is efficient from a social perspective, but it may not be efficient from the perspective of the NHS which does not take non health-related costs into account.
El estudio BENEFIT ha mostrado los beneficios del uso precoz del interferón beta 1b (IFNβ-1b). El objetivo del trabajo fue estimar la eficiencia del tratamiento precoz vs. diferido del IFNβ-1b en pacientes con un síndrome desmielinizante aislado (SDA) indicativo de esclerosis múltiple (EM) en España.
MétodosSe desarrolló un modelo de Markov desde la perspectiva social, con un horizonte temporal de 2 años hasta toda la vida. Una cohorte de 1.000 pacientes con SDA y estados de salud definidos por la Expanded Disability Syndrome Scale (EDSS) fue tratada o no con IFNβ-1b al inicio. Los datos del BENEFIT se usaron para la progresión en la EDSS y las transiciones a EM. Los costes se estimaron de la literatura. Las utilidades derivaron del EQ-5D y publicaciones y la mortalidad de tablas de mortalidad y de la EDSS. Costes (€ de 2013) y resultados se descontaron al 3% anual. Se realizó un análisis de sensibilidad probabilístico.
ResultadosEn el caso base, tanto la razón de coste utilidad incremental (RCUI) como la razón de coste efectividad incremental (RCEI) del IFNβ-1b vs. no tratamiento fueron dominantes (más eficaz y menos costoso) bajo la perspectiva social. Bajo la perspectiva del SNS, la RCUI fue de 40.702 €/AVAC y la RCEI de 13 €/recaída evitada.
ConclusiónEl tratamiento precoz con IFNβ-1b después de un SDA frente al tratamiento diferido es eficiente desde la perspectiva social, pero puede no ser eficiente desde la perspectiva del SNS al no tener en cuenta los costes no sanitarios.
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system characterised by progressive demyelination. Most MS patients present severe physical disability and cognitive impairment.1
According to a recent study conducted in La Rioja, Spain, MS has a prevalence of 65 cases per 100000 population and predominantly affects young women.2 The mean annual cost per patient with MS amounts to €24272, with direct costs representing around 60% of the total cost.3
The first manifestation of the disease in 85% of the young adults who develop clinically definite MS (CDMS) is an event resulting from isolated demyelination of the optic nerves, brainstem, or spinal cord.1,4 This event is called ‘clinically isolated syndrome’, or CIS.
Disease-modifying treatments (DMT) such as interferon beta-1b (IFNβ-1b) are the standard first-line of treatment for MS outbreaks, since they have been shown to reduce the exacerbation rate and slow disease progression in clinical trials.1,5–7
According to the Betaferon® in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT)8 trial, early treatment after a CIS suggestive of MS reduces the risk of conversion to CDMS by 41% compared with delayed treatment. Furthermore, several studies have shown that early treatment reduces the risk of disease progression by 40% to 45% compared with delayed treatment.7–10
Whereas the cost-effectiveness of treatments for MS has been thoroughly studied, this is not the case for CIS suggestive of MS.11–15
The aim of this study was to estimate the cost-effectiveness in Spain of IFNβ-1b in patients with a CIS suggestive of MS.
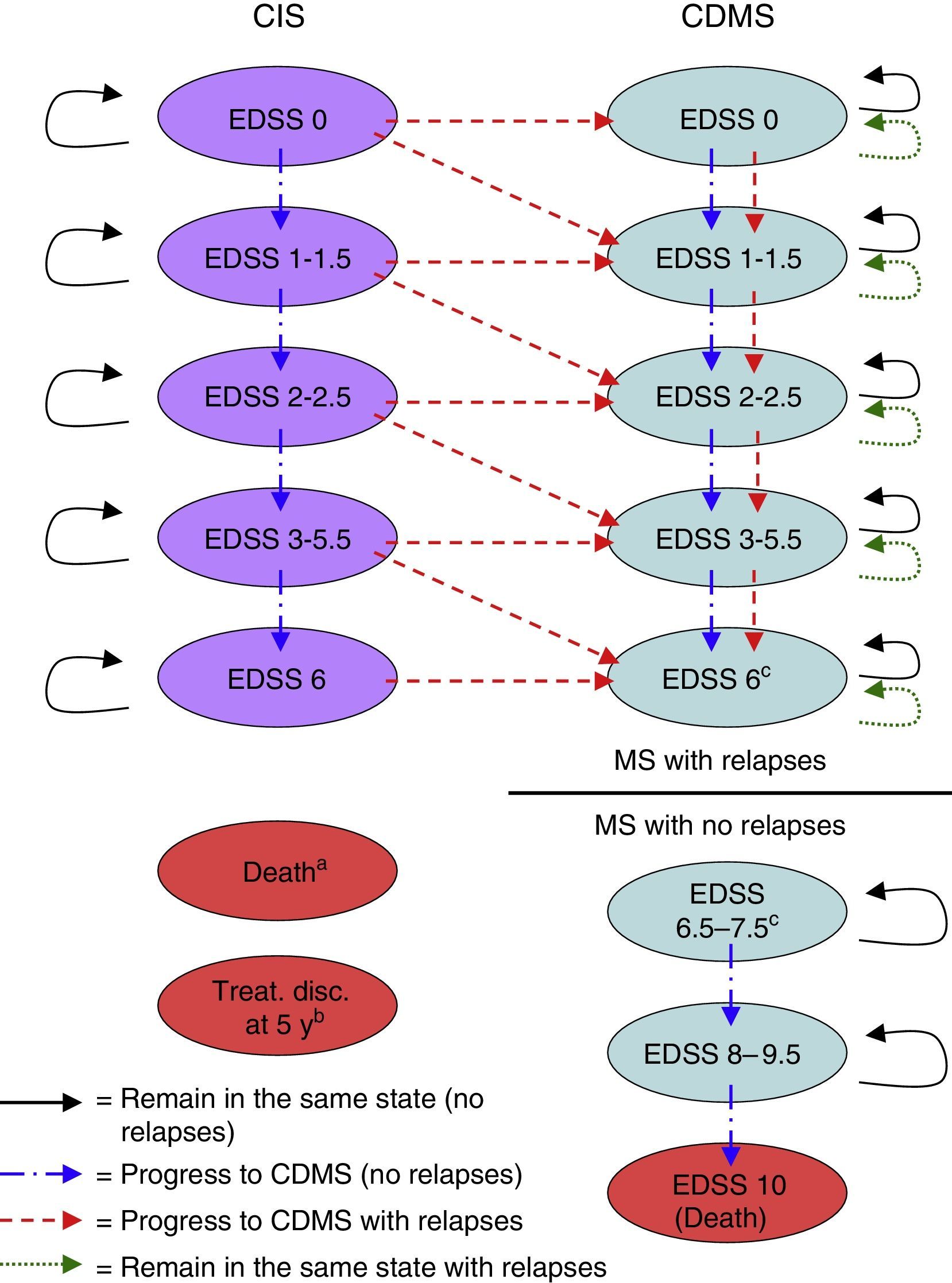
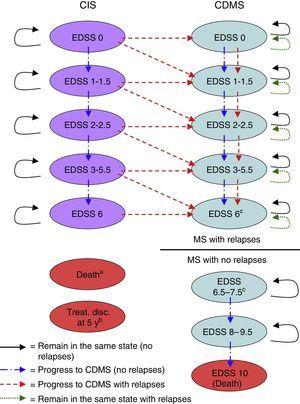
Material and methodsDescription of the modelWe used MS Excel® to create a Markov model to estimate the costs and benefits of a hypothetical cohort of 1000 patients (mean age: 30 years, 70% of whom were women, in line with the population of the BENEFIT study) with a CIS who were treated with IFNβ-1b (250mg every other day) either right after a CIS suggestive of MS (early treatment) or at onset of CDMS (delayed treatment).9,10 We used the model to simulate results for time horizons from 2 years to lifetime. Patient's lifespans were divided into 6-month cycles. During each cycle, patients may experience a wide range of clinical events including an increase in Expanded Disability Status Scale (EDSS) scores, an MS relapse, or survival and gain of life years. Each health state had a unique set of probabilities of either remaining in the same health state or changing to other health state during each cycle (Fig. 1).
Markov model health states. Treat. disc. at 5 y: treatment discontinuation at 5 years; CDMS, clinically definite multiple sclerosis; EDSS, Expanded Disability Status Scale; CIS, clinically isolated syndrome.
a A patient can transition to ‘death’ from any health state during any cycle of the model.
b Patients discontinuing treatment in the CIS branch during the fifth year are removed from the model at the end of the fifth year.
c No transitions occur from EDSS 6 health state to EDSS 6.5–7.5 health state. The user of the model decides the score threshold to consider non-recurring MS (ie, 6, 6.5, 7, or 7.5) and the EDSS 6–7.5 health states are proportionally divided according to the EDSS score chosen.
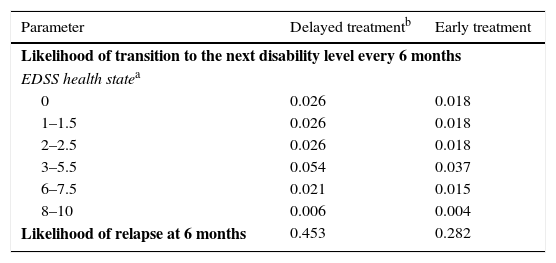
Health states were defined according to the level of disability as measured by the EDSS,16 the occurrence of a second event leading to a diagnosis of CDMS, and death. The different EDSS categories (EDSS 0; 1–1.5; 2–2.5; 3–5.5; 6; 6.5–7.5; 8–9.5; and 10) were determined by examining the data on probabilities of transition available in the literature (Table 1).5,6,17–22 These categories were later discussed by clinical experts to identify the ones that represented the main disability markers.
Main clinical data obtained from the literature.
| Parameter | Delayed treatmentb | Early treatment |
|---|---|---|
| Likelihood of transition to the next disability level every 6 months | ||
| EDSS health statea | ||
| 0 | 0.026 | 0.018 |
| 1–1.5 | 0.026 | 0.018 |
| 2–2.5 | 0.026 | 0.018 |
| 3–5.5 | 0.054 | 0.037 |
| 6–7.5 | 0.021 | 0.015 |
| 8–10 | 0.006 | 0.004 |
| Likelihood of relapse at 6 months | 0.453 | 0.282 |
Taken from The IFNB Multiple Sclerosis Study Group,5 The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group,6 Weinshenker et al.,18 Runmarker and Andersen,19 Goodkin et al.,20 Weinshenker and Ebers,21 and Weinshenker et al.22
The model included 2 types of relapses: conversion from CIS to CDMS and relapses after diagnosis of CDMS. Probability of relapse for CDMS patients in both treatment groups (early and delayed) was obtained from the literature (Table 1).17 In order to account for the effect of treatment with IFNβ-1b, we applied the reduction in relapse rate estimated in the IFNβ-1b clinical trial to both groups.5,6
Two types of mortality were included in the model: all-cause mortality and mortality due to MS. All-cause mortality was estimated using the probability of death by age and sex, which was extracted from the mortality rate tables published by Spain's National Statistics Institute.23 When patients scored 10 (death) on the EDSS, mortality was considered to be due to MS.
Patients could discontinue treatment at any time during the time horizon to reflect the real-world situation. Additionally, we assumed that IFNβ-1b treatment was suspended when a patient scored 7 on the EDSS, based on clinical expert opinion.
At the end of the model simulation, we calculated the costs and benefits of all the cycles to make cost and benefit estimations for early vs. delayed treatment. Diagnoses of CDMS were established according to the diagnostic criteria developed by Poser et al.24 and McDonald et al.25
In every cycle, patients who remained alive accrued 6 months of life which were later adjusted by the utility corresponding to their health state and occurrence of a relapse (when appropriate). Results from these calculations were expressed as quality-adjusted life years (QALYs). At the end of the model, QALYs of all cycles included in the time horizon were added up for each group to calculate the incremental cost-utility ratio (ICUR). The ICUR was calculated as the difference between early treatment and delayed treatment using the following formula:
Likewise, we calculated the incremental cost-effectiveness ratio (ICER) considering the relapses avoided for each treatment group.
We used a 50-year time horizon in the analysis, since the mean age of the patients who participated in the BENEFIT8 trial was 30 years, and the average life expectancy in Spain is around 80 years.26
The model was created from a Spanish societal perspective. Furthermore, we undertook an additional analysis from the perspective of the Spanish National Health System (SNHS). All costs were calculated in 2013 euros, and costs and results were discounted at an annual rate of 3%.27
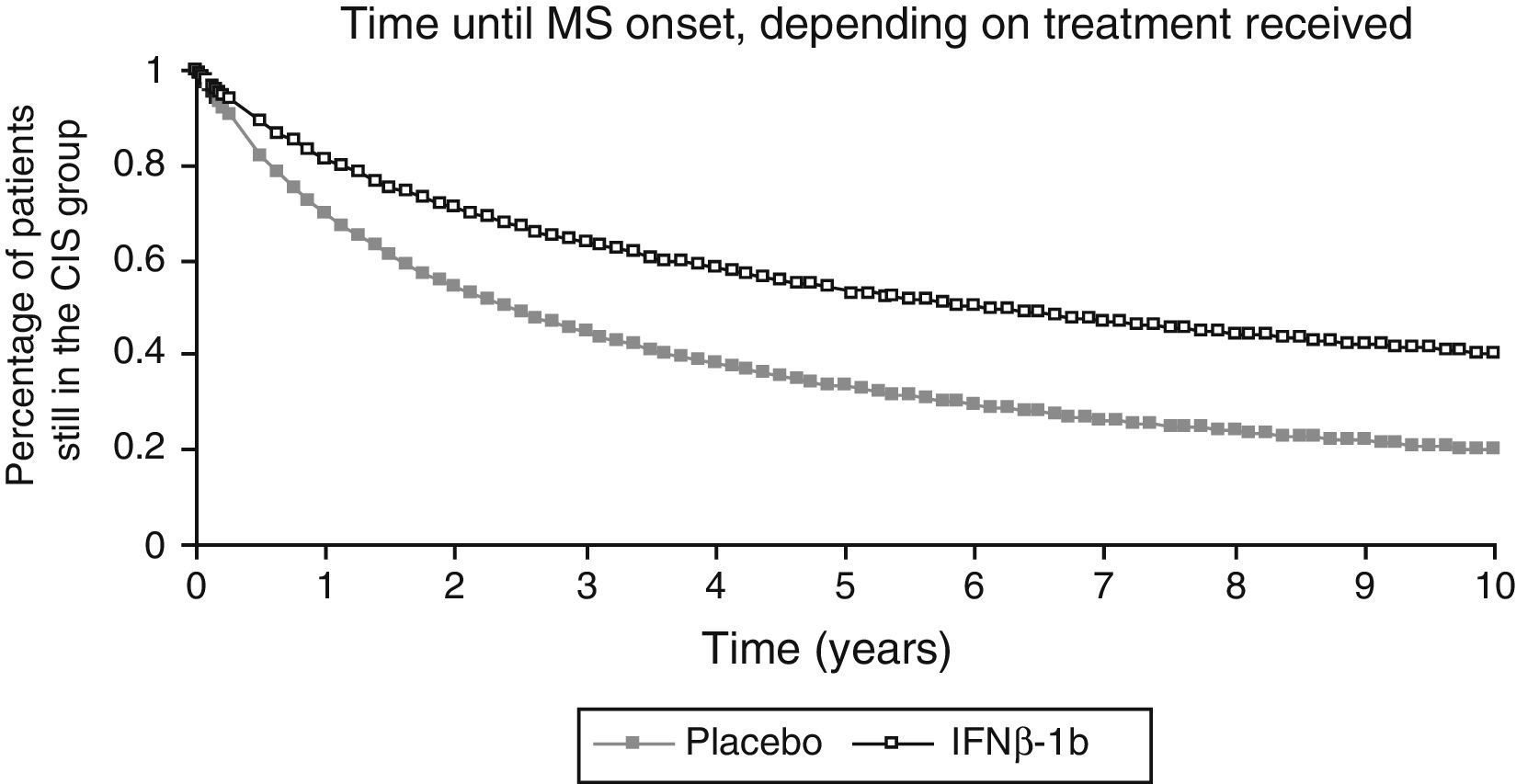
Data sourcesThe main data source for our model was the BENEFIT clinical trial.8 This trial assessed the impact of IFNβ-1b treatment after CIS (early treatment) compared with the impact of delaying IFNβ-1b treatment until diagnosis of CDMS (delayed treatment).9,10 The BENEFIT study was a prospective, placebo-controlled trial conducted in 98 centres from 20 countries. We recruited a total of 468 patients with a symptomatic CIS lasting for more than 24hours and radiological findings of one or more silent lesions to the central nervous system. Patients were randomly assigned to receive either IFNβ-1b (250mg every other day) or a placebo during the first 2 years. Patients included in the placebo group continued receiving the placebo until they were diagnosed with CDMS according to the Poser et al.24 criteria or until the end of the placebo-controlled phase (2 years). Patients who completed the placebo-controlled phase were offered the possibility of being treated with IFNβ-1b for up to 5 years from randomisation. The results obtained at the end of the study period showed that the number of patients developing CDMS was significantly lower in the IFNβ-1b group than in the placebo group (28% vs. 45%, P<.0001).9 The results obtained at 5 years showed that the risk of conversion to CDMS in patients who started IFNβ-1b treatment after a CIS decreased by 37% (P=.003) in comparison with patients treated with a placebo.10
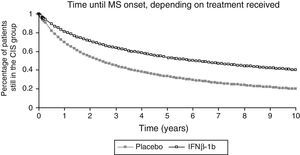
We used data from the BENEFIT trial corresponding to the first 2 years for the placebo group and the first 5 years for the IFNβ-1b group to calculate the 10-year probability of conversion from CIS to MS in both groups. We used the Kaplan–Meier survival curve for this purpose (Fig. 2). We did not extrapolate data beyond the 10-year time frame. For cycles in the model after that time frame, we used the probability of conversion for the respective tenth year cycle.
Treatment discontinuation rates were obtained from data extrapolated from the Stockholm Swedish MS Registry28 and applied to both early and delayed treatment groups. Registry data were adjusted using Weibull distributions and extrapolated to calculate the treatment distribution rates for a period of 50 years. The treatment discontinuation rate increased with time and at approximately half the time horizon all patients had discontinued treatment. This rate was similar in both groups. Furthermore, we assumed that once patients suspended treatment (not including changes in treatment), they did it until the end of the time horizon.
Healthcare and non-healthcare costs (informal care and loss of productivity) of MS treatment were estimated based on data published by Kobelt et al.29 (Table 2). Costs for patients with MS calculated according to EDSS scores were also used for patients with a CIS. We assumed one adverse event (that is, an event motivating an unscheduled medical consultation, which was assigned the unitary cost of a medical appointment) per year attributable to IFNβ-1b treatment. Annual cost of purchasing IFNβ-1b (administration not included) was obtained from the General Council of Official Colleges of Pharmacists (CGCOF) website.30
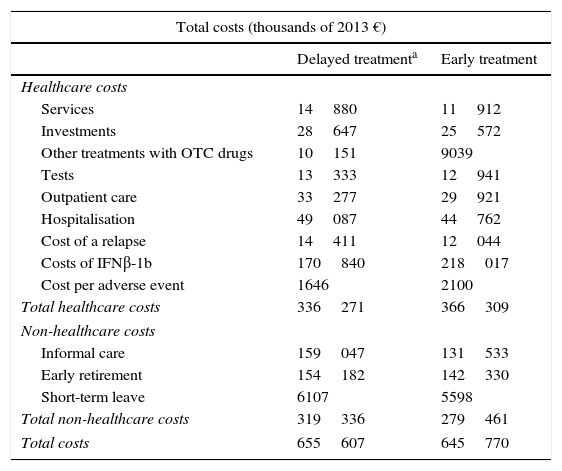
Costs (in thousands of 2013 euros).
| Total costs (thousands of 2013 €) | ||
|---|---|---|
| Delayed treatmenta | Early treatment | |
| Healthcare costs | ||
| Services | 14880 | 11912 |
| Investments | 28647 | 25572 |
| Other treatments with OTC drugs | 10151 | 9039 |
| Tests | 13333 | 12941 |
| Outpatient care | 33277 | 29921 |
| Hospitalisation | 49087 | 44762 |
| Cost of a relapse | 14411 | 12044 |
| Costs of IFNβ-1b | 170840 | 218017 |
| Cost per adverse event | 1646 | 2100 |
| Total healthcare costs | 336271 | 366309 |
| Non-healthcare costs | ||
| Informal care | 159047 | 131533 |
| Early retirement | 154182 | 142330 |
| Short-term leave | 6107 | 5598 |
| Total non-healthcare costs | 319336 | 279461 |
| Total costs | 655607 | 645770 |
We used the EuroQol-5 Dimensional Questionnaire data collected every 6 months during the BENEFIT trial to estimate the specific utility value of each EDSS health state of the model. We calculated the mean value for each EDSS health state using the conversion algorithm developed by Dolan.31 Utilities of the health states for a period beyond the follow-up phase of the BENEFIT trial were estimated using published data based on EDSS scores and occurrence of relapses.32,33
Model analysisThe analysis of the base case assessed the ICUR and ICER by comparing the differences between costs per QALYs or relapses avoided in early vs. delayed treatment from a societal perspective. This same analysis was conducted from the SNHS perspective.
A probabilistic sensitivity analysis was performed to determine the robustness of the model using a second-order Monte Carlo simulation. We used the beta distribution for the results from the BENEFIT trial for likelihood of effectiveness (EDSS/disability progression, relapse rate, conversion to CDMS) and utility values, and the gamma distribution for costs.
ResultsIn the base case (with a 50-year time horizon), both the ICUR and the ICER of early treatment with IFNβ-1b vs. delayed treatment were dominant (ie, less costly and more efficient) from the societal perspective (Table 3). Both Poser and McDonald diagnostic criteria were used.
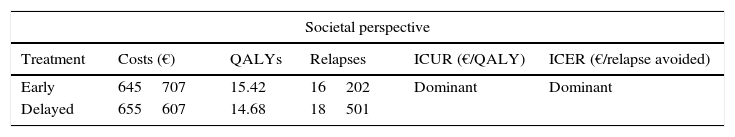
Baseline results of the cost-effectiveness analysis.
| Societal perspective | |||||
|---|---|---|---|---|---|
| Treatment | Costs (€) | QALYs | Relapses | ICUR (€/QALY) | ICER (€/relapse avoided) |
| Early | 645707 | 15.42 | 16202 | Dominant | Dominant |
| Delayed | 655607 | 14.68 | 18501 | ||
| SNHS perspective | |||||
|---|---|---|---|---|---|
| Costs (€) | QALYs | Relapses | ICUR (€/QALY) | ICER (€/relapse avoided) | |
| Early | 366309 | 15.42 | 16202 | 40702 | 13 |
| Delayed | 336271 | 14.68 | 18501 | ||
QALY, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; SNHS, Spanish National Health System.
From the SNHS perspective, ICUR was €40701.9/QALY and ICER €13.07/relapse avoided (including healthcare costs only) (Table 2).
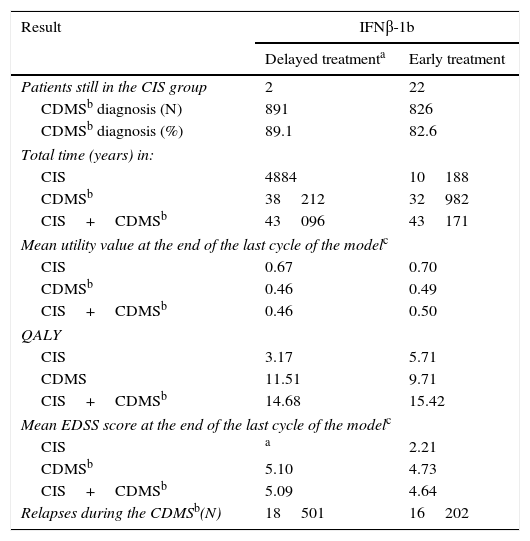
The number of relapses (conversions from CIS to CDMS and relapses after CDMS diagnosis) was lower in the early treatment group than in the delayed treatment group, which proves that early treatment with IFNβ-1b after a CIS is beneficial (Table 4).
Clinical results of the model.
| Result | IFNβ-1b | |
|---|---|---|
| Delayed treatmenta | Early treatment | |
| Patients still in the CIS group | 2 | 22 |
| CDMSb diagnosis (N) | 891 | 826 |
| CDMSb diagnosis (%) | 89.1 | 82.6 |
| Total time (years) in: | ||
| CIS | 4884 | 10188 |
| CDMSb | 38212 | 32982 |
| CIS+CDMSb | 43096 | 43171 |
| Mean utility value at the end of the last cycle of the modelc | ||
| CIS | 0.67 | 0.70 |
| CDMSb | 0.46 | 0.49 |
| CIS+CDMSb | 0.46 | 0.50 |
| QALY | ||
| CIS | 3.17 | 5.71 |
| CDMS | 11.51 | 9.71 |
| CIS+CDMSb | 14.68 | 15.42 |
| Mean EDSS score at the end of the last cycle of the modelc | ||
| CIS | a | 2.21 |
| CDMSb | 5.10 | 4.73 |
| CIS+CDMSb | 5.09 | 4.64 |
| Relapses during the CDMSb(N) | 18501 | 16202 |
EDSS, Expanded Disability Status Scale; CDMS, clinically definite multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; IFNβ-1b, interferon beta-1b; CIS, clinically isolated syndrome.
Early treatment incurred higher healthcare costs (€30038 difference), whereas delayed treatment incurred higher non-healthcare costs (€39875 difference) over the 50-year horizon. In both groups, costs due to early retirement accounted for more than 96% of the total costs resulting from loss of productivity. After adding healthcare and non-healthcare costs together, delayed treatment incurred higher total costs (€9837 difference) than early treatment (Table 2).
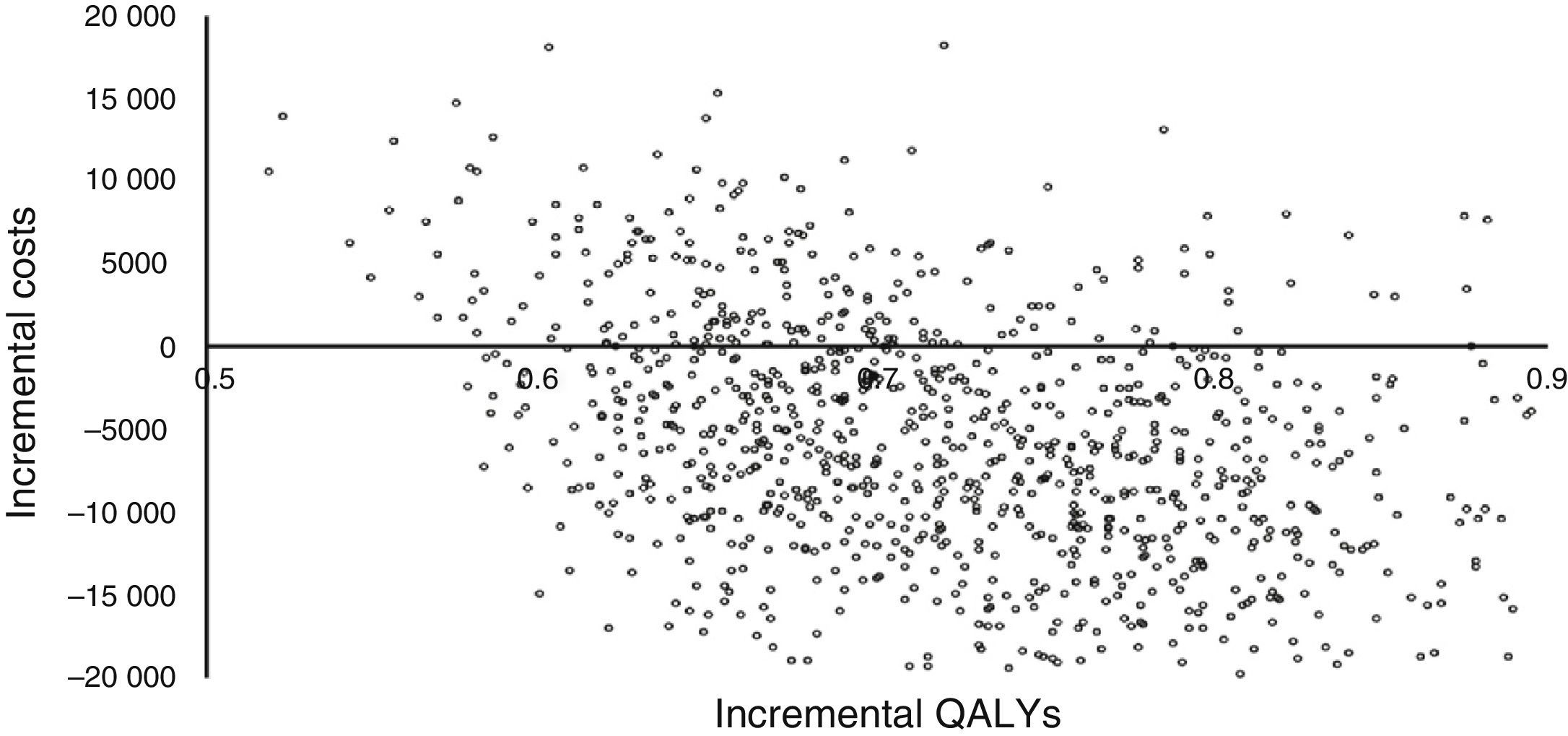
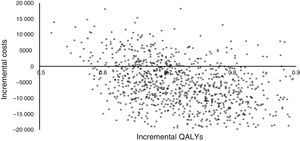
Fig. 3 shows the results of the Monte Carlo simulation used for the sensitivity analysis. Incremental costs and incremental QALYs are charted in the cost-effectiveness scatter plot as individual dots for each of the 1000 simulated patients. This figure shows that more than half of the dots are located in the right lower quadrant of the plot, which indicates lower costs and greater effectiveness, while all the dots indicating higher costs (right upper quadrant) have an ICUR of less than €30000/QALY (the cost-effectiveness threshold used in Spain).34
DiscussionOur study assessed the cost-effectiveness of early IFNβ-1b treatment of patients with a CIS vs. delayed treatment until CDMS diagnosis. Our analyses showed that from a societal perspective and using either Poser or McDonald diagnostic criteria, early treatment was associated with lower costs and greater effectiveness when compared with delayed treatment.
Although QALYs are the most common denominator in ICER, other cost-effectiveness measurements included in this model also provide useful information on the importance of early treatment. For example, early treatment also proved to be less costly and more effective when we defined effectiveness as the total number of relapses in the MS group. This supported the conclusion that early treatment has a high cost-benefit ratio in terms of relapse burden in the MS group since it delays conversion to CDMS.
There is limited information in the literature about the cost-effectiveness of early treatment for a CIS. Lazzaro et al.35 showed that early treatment with IFNβ-1b, in comparison with delayed treatment, was dominant from the societal perspective in Italy, which goes in line with our results. Iskedjian et al.36 also made an economic evaluation of a DMT after a CIS and found that treatment with IFNβ-1b was cost-effective from the Canadian societal perspective. However, in the model used in that study, results of health-related benefits were not presented as costs per QALY; therefore, those results are not directly comparable to ours. Two more studies with comparable data from the societal perspective have recently been published.37,38 Pan et al.37 published their results using the same model adapted to the USA, which showed an ICER of $46357 per QALY gained and $30967 per life year gained. However, a study adapting this model to Sweden38 found that early treatment with IFNβ-1b dominated delayed treatment when effectiveness was measured in terms of QALYs gained. These 2 reports showed results using this model; however conclusions differ when comparing Sweden and Spain on the one hand (with similar results) to the USA on the other, probably due to healthcare system as well as medical care cost differences between these 2 European countries and the USA.
Despite some limitations and uncertainties in their economic models, several studies have shown that disease progression is associated with increased costs39,40 resulting from the greater need for costly DMT, which effectively reduces relapse rates and slows MS progression, and early treatment with a DMT, which has been proposed to delay conversion to CDMS in patients with a CIS. Therefore, stabilisation of the disease at low functional grades of disability should aim to not only improve patients’ quality of life but also provide socio-economic benefits. Delaying disease progression as early as possible results in improved quality of life and functional independence, and lower costs for healthcare systems, society, and patients.41
This analysis has some limitations. Firstly, the impact of IFNβ-1b treatment on MS progression is based on data from the BENEFIT clinical trial exclusively, as no data on long-term outcomes of early vs. delayed treatment after a CIS were available in the literature. Therefore, results of our model should be extrapolated to real populations with caution. Second, and due to the lack of data on patients with CIS, we assumed that costs and utilities were similar for patients in the same EDSS category, whether they had a CIS or CDMS. In addition, there is also uncertainty as to the most suitable EDSS threshold for discontinuing DMT. Our threshold is based on data extrapolated from the BENEFIT trial and expert opinion. Furthermore, we assumed that once patients discontinued treatment, they remained untreated until the end of the time horizon. However, in real life, some patients may have changed treatment. Also, and due to the lack of long-term data on the risk of adverse events of DMT, we assumed one event per person per year, based on expert opinion. However, this may have resulted in excessive additional costs. Lastly, MS patients treated with INFB-1b may develop neutralising anti-interferon beta antibodies, which could reduce the clinical efficacy of INFB-1b. The potential impact of antibodies on DMT treatment efficacy was not included in this model due to the lack of data.
In conclusion, the results of our model show that early treatment with IFNβ-1b after a CIS vs. delayed treatment is efficient from the societal perspective, whether we use QALYs or relapses avoided as denominator (dominant in both cases). However, it may not be efficient from the SNHS perspective since the model does not consider non-healthcare costs (informal carers and medical leaves) or use QALY as denominator.
Conflicts of interestDuring the drafting of the model, the author was working for Bayer, the company marketing the drug. The contractual relationship terminated before the manuscript was prepared.
I would like to thank Txomin Arbizu for his collaboration, Juan Oliva for his wise advice, and Max Brose, because without his help, I could not have finished this study.
Please cite this article as: Piñol C. Análisis de coste-efectividad del interferón beta-1b en el tratamiento de pacientes con síndrome desmielinizante aislado indicativo de esclerosis múltiple en España. Neurología. 2016;31:247–254.
Previous versions of this study (2009 data) have been published as:
Arbizu T, Piñol C, Casado V, Caloyeras JP. Coste utilidad de interferón beta en el tratamiento de pacientes con síndrome desmielinizante aislado sugestivo de esclerosis múltiple en España. XXVIII Jornadas de la Asociación de Economía de la Salud. Málaga, 17–19 de junio 2009. Gaceta Sanitaria. 2009;23 Espec Cong 2:65.
Arbizu T, Piñol C, Casado V. Cost-utility of interferon beta-1b in the treatment of patients with a clinical isolated syndrome suggestive of multiple sclerosis in Spain. ISPOR 12th Annual European Congress. Paris, France, 24–27 October 2009. Value in Health. 2009;12(7):A370.