REM sleep behaviour disorder (RBD) is characterised by violent behaviours (screaming, kicking, vivid dreams) during REM sleep. It has a prevalence of 1% to 2% of the general population and is especially frequent in men and the population older than 60. In the last decade, RBD has been suggested to be a prodrome of neurodegenerative disease. We analysed associated neurological diseases and responses to drug treatment in 33 patients with RBD treated in the multidisciplinary sleep disorders unit at Hospital Infanta Sofía.
Patients and methodsWe conducted an observational descriptive retrospective analysis of patients diagnosed with RBD and treated in our multidisciplinary sleep disorders unit between October 2012 and December 2015. We recorded age, sex, associated diseases, and treatments administered to these patients.
ResultsA total of 365 patients were attended at our unit, including 33 with RBD: 13 women (40%) and 20 men (60%). Mean age was 62.72 years. An associated disorder was identified in 48%, with the most common being mild cognitive impairment (69%). The percentage of patients with RBD and an associated disorder among patients older than 60 was 68%. Eighty-two percent of the patients required treatment. The most commonly used drug was clonazepam (76%), followed by melatonin (9%), gabapentin (6%), and trazodone (3%).
DiscussionIn our series, 48% of the patients had an associated disorder. The likelihood of detecting an associated disorder increases with patients’ age. The vast majority of patients required drug treatment due to symptom severity; the most frequently administered drug was clonazepam (76%).
El trastorno de conducta de sueño REM (TCSR) se caracteriza por conductas violentas (gritos, patadas, sueños vívidos) durante la fase REM del sueño. Tiene una prevalencia del 1-2% de la población general, especialmente en varones y en mayores de 60 años. En la última década se ha asociado como pródromo a una enfermedad neurodegenerativa. Nos proponemos analizar las patologías asociadas a los 33 pacientes con TCSR atendidos en la Unidad Multidisciplinar de Trastornos del Sueño del Hospital Infanta Sofía, y su respuesta al tratamiento farmacológico.
Pacientes y métodosAnálisis descriptivo, retrospectivo, observacional, de los pacientes con diagnóstico de TCSR, atendidos en la consulta monográfica de Neurología, desde octubre de 2012 hasta diciembre de 2015. Se valoran la edad, el sexo, las enfermedades asociadas, y los tratamientos empleados.
ResultadosDe los 365 pacientes valorados en la consulta, 33 presentan TCSR: 13 mujeres (40%) y 20 hombres (60%), con una edad media de 62,72 años. En el 48% se identifica una patología asociada: la más frecuente es el deterioro cognitivo leve (69%). El porcentaje de TCSR con patología asociada en mayores de 60 años se eleva al 68%. El 82% de los casos han requerido tratamiento. El fármaco más utilizado ha sido el clonazepam (76%), seguido de melatonina (9%), gabapentina (6%) y trazodona (3%).
DiscusiónEn nuestra serie el 48% de los pacientes presentan una patología asociada. La mayor edad influye directamente en la posibilidad de encontrar una patología asociada. La gran mayoría han precisado tratamiento farmacológico por la severidad de los síntomas, siendo el clonazepam (76%) el fármaco más utilizado.
Sleep disorders are very frequent, with a lifetime prevalence of up to 30%. The most common are insomnia, sleep apnoea–hypopnoea syndrome (SAHS), restless legs syndrome (RLS), and bruxism. However, prevalence of REM sleep behaviour disorder (RSBD) is much lower, affecting 1% to 2% of individuals older than 60, mainly men. The condition is characterised by violent behaviour (shouting, kicking, insulting, vivid dreams) during the REM sleep phase, with loss of muscular atonia (characteristic of this phase) and nightmares.1,2 Patients usually remember these episodes (particularly unpleasant dreams), and display tiredness upon waking and daytime sleepiness.3–5
RSBD has been recognised for over 30 years.1,2 Several studies have reported isolated cases or small case series of neurological diseases associated with RSBD, including narcolepsy, Parkinson's disease (PD), mild cognitive impairment (MCI), multiple system atrophy, Alzheimer disease (AD), Huntington disease, frontotemporal dementia, corticobasal degeneration (CBD),6–15 and progressive supranuclear palsy.16,17 Prevalence of RSBD in patients older than 60 is very low (1%–2%); however, in their 2015 criteria for prodromal PD, Berg et al.18 estimated that over 75% of patients with RSBD would progress to synucleinopathy. This is especially frequent in patients aged 60–65, in whom the risk of PD also increases (1.25% at 60 years and 2% at 65).18
The first report of a possible association between PD and RSBD was published in 1996: Schenk et al.2 published a series of 29 patients, 38% of whom were initially diagnosed with RSBD; in 2006, a group of Spanish researchers reported a series of 44 patients with RSBD, which was associated with neurodegenerative diseases in 45% of cases.6 Interestingly, the same researchers published another study reporting the progression of their series, observing that the percentage of patients presenting an associated neurodegenerative disease had increased from 45% to 82% in 2013 (especially patients with PD and Lewy body dementia).7
The purpose of this study is to analyse cases of RSBD evaluated at the multidisciplinary sleep disorder unit at Hospital Infanta Sofía, the presence of associated disorders, and the treatments used.
Patients and methodsThe Hospital Universitario Infanta Sofía, in San Sebastián de los Reyes, belongs to the public hospital network of the Region of Madrid and provides care to an assigned population of 305000.
The multidisciplinary sleep disorder unit was created in 2008, when the hospital was opened. The unit is made up by the neurology, pulmonology, paediatrics, and otorhinolaryngology departments, which share specialist sleep disorder clinics and a sleep laboratory with 3 multi-purpose hospital beds for the performance of polysomnographies (PSG), the Multiple Latency Sleep Test, respiratory polygraphs, and video-EEG monitoring.
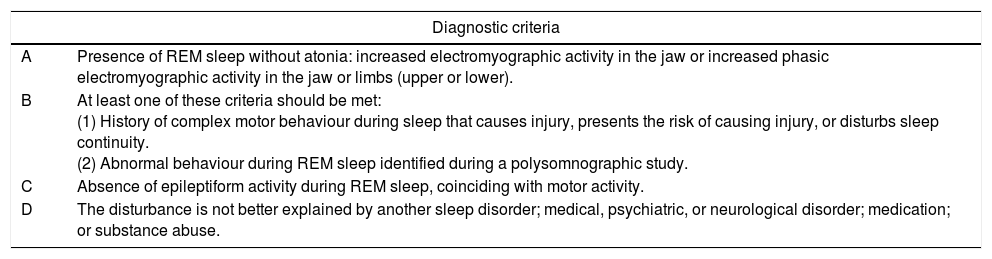
We performed a descriptive, retrospective, observational study of patients diagnosed with RSBD and treated at our hospital's neurology department from October 2012 to December 2015. Statistical analysis was performed using the SPSS software, version 19. Diagnosis of RSBD requires patients to meet the international diagnostic criteria (Table 1) and undergo a night-time PSG. Patients in whom RSBD was suspected but who did not undergo PSG were not included in the study.
REM sleep behaviour disorder diagnostic criteria.
| Diagnostic criteria | |
|---|---|
| A | Presence of REM sleep without atonia: increased electromyographic activity in the jaw or increased phasic electromyographic activity in the jaw or limbs (upper or lower). |
| B | At least one of these criteria should be met: (1) History of complex motor behaviour during sleep that causes injury, presents the risk of causing injury, or disturbs sleep continuity. (2) Abnormal behaviour during REM sleep identified during a polysomnographic study. |
| C | Absence of epileptiform activity during REM sleep, coinciding with motor activity. |
| D | The disturbance is not better explained by another sleep disorder; medical, psychiatric, or neurological disorder; medication; or substance abuse. |
Source: International Classification of Sleep Disorders, ICSD-3.
MCI and AD were diagnosed according to the criteria of the National Institute on Ageing and The Alzheimer's Association group (NIA-AA) by McKhann et al.19 and Sperling et al.20 For the diagnosis of PD, we followed the international criteria established by Suchowersky et al.21 of the American Academy of Neurology; the criteria established by Amstrong et al.22 were used for the diagnosis of CBD.
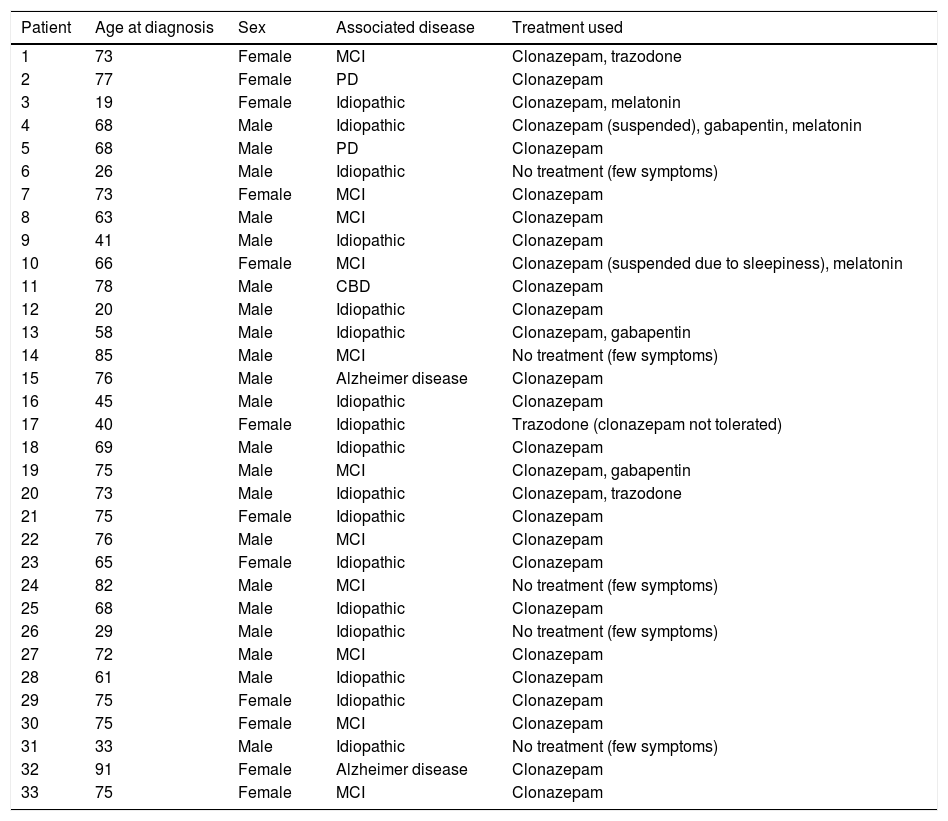
ResultsOf the 365 patients assessed at the neurology department of the multidisciplinary sleep disorder unit, 33 were diagnosed with RSBD: 13 women (40%) and 20 men (60%), with a mean age at diagnosis of 62.72 years (range, 19-91) (Table 2).
Characteristics of patients included in the study.
| Patient | Age at diagnosis | Sex | Associated disease | Treatment used |
|---|---|---|---|---|
| 1 | 73 | Female | MCI | Clonazepam, trazodone |
| 2 | 77 | Female | PD | Clonazepam |
| 3 | 19 | Female | Idiopathic | Clonazepam, melatonin |
| 4 | 68 | Male | Idiopathic | Clonazepam (suspended), gabapentin, melatonin |
| 5 | 68 | Male | PD | Clonazepam |
| 6 | 26 | Male | Idiopathic | No treatment (few symptoms) |
| 7 | 73 | Female | MCI | Clonazepam |
| 8 | 63 | Male | MCI | Clonazepam |
| 9 | 41 | Male | Idiopathic | Clonazepam |
| 10 | 66 | Female | MCI | Clonazepam (suspended due to sleepiness), melatonin |
| 11 | 78 | Male | CBD | Clonazepam |
| 12 | 20 | Male | Idiopathic | Clonazepam |
| 13 | 58 | Male | Idiopathic | Clonazepam, gabapentin |
| 14 | 85 | Male | MCI | No treatment (few symptoms) |
| 15 | 76 | Male | Alzheimer disease | Clonazepam |
| 16 | 45 | Male | Idiopathic | Clonazepam |
| 17 | 40 | Female | Idiopathic | Trazodone (clonazepam not tolerated) |
| 18 | 69 | Male | Idiopathic | Clonazepam |
| 19 | 75 | Male | MCI | Clonazepam, gabapentin |
| 20 | 73 | Male | Idiopathic | Clonazepam, trazodone |
| 21 | 75 | Female | Idiopathic | Clonazepam |
| 22 | 76 | Male | MCI | Clonazepam |
| 23 | 65 | Female | Idiopathic | Clonazepam |
| 24 | 82 | Male | MCI | No treatment (few symptoms) |
| 25 | 68 | Male | Idiopathic | Clonazepam |
| 26 | 29 | Male | Idiopathic | No treatment (few symptoms) |
| 27 | 72 | Male | MCI | Clonazepam |
| 28 | 61 | Male | Idiopathic | Clonazepam |
| 29 | 75 | Female | Idiopathic | Clonazepam |
| 30 | 75 | Female | MCI | Clonazepam |
| 31 | 33 | Male | Idiopathic | No treatment (few symptoms) |
| 32 | 91 | Female | Alzheimer disease | Clonazepam |
| 33 | 75 | Female | MCI | Clonazepam |
In our series, the percentage of men is slightly higher (60%), with a mean age of onset above 60 years. The percentage of idiopathic cases in this age group is much lower than among younger patients (32% vs 100% in the latter group).
Patients were mainly referred from the neurology department (29 patients, 88%); 2 (6%) were referred from the psychiatry department and 2 (6%) from the pulmonology department. Of the patients referred from the neurology department, 12 (36%) had previously been attended by the clinics specialising in movement disorders and cognitive disorders, and received a previous definite diagnosis.
Description of the associated pathologySeventeen patients (52%) were considered idiopathic, since they did not meet criteria for other pathologies and no specific cause or trigger factor could be identified. Associated pathologies could be identified in 16 patients (48%); the most frequent was MCI (11 cases, 69%), followed by AD (2 patients, 6%), PD (2 patients, 6%), and CBD (1 patient, 3%).
Associated pathologies were observed in 68% of patients older than 60 years.23 However, none of the 8 patients younger than 60 years presented any associated pathology.
Treatments usedTwenty-seven patients (82%) required treatment due to the severity of the disorder. Clonazepam was the most widely used (25 patients, 76%), but was suspended in 3 patients due to excessive daytime sleepiness. Melatonin was used in 7 patients and maintained in 3 (9%); it was suspended in 4 patients due to ineffectiveness (not to adverse reactions or interactions). A coadjuvant drug to clonazepam was necessary in 3 patients (9%): one with insomnia, treated with trazodone, and 2 with associated periodic limb movements (PLM), treated with gabapentin. Good symptom control was achieved in all cases. Six patients (18%) did not require treatment as symptoms were very mild.
DiscussionIn our series, we observed that 88% of patients were transferred from the neurology department, mainly from general neurology clinics (42%), and from specialist movement disorder and cognitive disorder clinics (36%). Only those patients with clinical uncertainties are transferred, as they are already diagnosed with other disorder. In daily practice, many patients under follow-up for well-established neurodegenerative diseases such as PD or AD report such sleep disorders as RSBD. Given the known association with these diseases, and depending on severity (episodes of night-time agitation, insomnia, changes to the sleep-wake cycle, aggressiveness, difficulty on the part of the caregivers taking patients to hospital), patients are not routinely referred to undergo such specific tests as night-time PSG.7–16 In our series, this leads to a selection bias, since the PSG is offered to patients from these specialist clinics whose cognitive/behavioural level and social support from their caregivers initially enable us to correctly perform the test (PSG considered adequate when performed in our laboratory for a period of at least 6hours of sleep).4,23
As in the previously described series, the diseases most frequently associated with RSBD in our sample were PD, AD, CBD, and MCI, although the number of cases of PD and AD are lower in our series, as is mentioned above. We include a case of CBD related to RSBD, which is very rarely reported in the literature (only 3 other cases) despite CBD being a known RSBD-related tauopathy.12,13 RSBD is also associated with other non-neurological causes, such as drugs (with antidepressants being the most frequent), toxic substances (alcohol), encephalitis (infectious, paraneoplastic), or hormonal alterations.24,25 Pharmacological aetiology was suspected in only 2 cases in our series; no clear improvement was observed after suspending antidepressant treatment (serotonin reuptake inhibitors).
As expected, RSBD is more frequent in men than in women in our series, although the mean age of our patients (62.72 years) is lower than that reported in other series. Iranzo et al.6,7 retrospectively assessed 44 consecutive patients (39 men and 5 women) with an older mean age (74 years) and described the associated pathologies (but not the treatment used). We believe that our mean age is lower because understanding of the disease has increased since 2006, leading us to search for the condition earlier.
Patients with a final diagnosis (suggestive symptoms and confirmation through night-time PSG) receive specific treatment for this condition, regardless of the existing neurodegenerative disease (where present). The first option described in the literature is clonazepam dosed at 2-4mg (with improvements in 80%-90% of cases) and melatonin dosed at 3-12mg, when symptoms are significant.26–29 Response to pramipexole or donepezil has been described in isolated cases and small series.30–32 The most frequently used drugs do not prevent or modify progression in cases preceding neurodegenerative diseases.
The 2 patients with associated PD in our series responded well to levodopa/carbidopa plus low doses of pramipexole. However, this treatment had little effect on the sleep disorder; therefore, in both cases, clonazepam was added at low doses (0.5-1mg at night) to specifically treat RSBD.33,34 Low doses of levodopa/carbidopa were administered to the patient with CBD; this had little effect on both the baseline disease and the RSBD, for which reason clonazepam was also added.
The great majority of patients in our series (82%) required pharmacological treatment due to symptom severity.26 The main reasons for requesting treatment were the intensity of screaming and abrupt movements (which frighten patients’ partners; 60%), followed by excessive daytime sleepiness (20%), and concern about the diagnosis (20%). Although the most frequently used drugs are usually clonazepam and melatonin, the use of the latter was limited in our study.26–29 One factor directly (expressed by patients) influenced the reduced use of melatonin: the cost of the treatment. Effective doses are more than 3mg daily, with a mean monthly cost above €20-25; melatonin is not currently subsidised by the Spanish healthcare system, and some patients have had difficulties paying for it and voluntarily discontinued treatment (regardless of its efficacy). Only 3 patients (9%) currently continue using melatonin treatment. The other 2 drugs used (gabapentin and trazodone)35 were chosen due to patients’ comorbidities (insomnia and PLMs) and were effective for treating both entities and for reducing the intensity of RSBD episodes.
As in our series, several articles report an association between RSBD and PLMs: in 2002, Fantini et al.36 published a series of 40 patients with RSBD associated with PLMs; in 2016, Lo Coco et al.37 published a series of 41 cases of PLMs and RSBD in patients with amyotrophic lateral sclerosis. PLMs associated with RSBD were more frequent among these patients than in the healthy control group. However, these series do not mention the treatments used.
In our case, we observed a direct relationship between the possibility of diagnosing a disease associated with RSBD in older patients (older than 60 years), with MCI being the disorder most frequently associated with RSBD, without ignoring the risk of PD.17,38,39 The great majority of patients required pharmacological treatment; clonazepam was the most effective drug in monotherapy (76%), with melatonin being the second-line treatment. Gabapentin and trazodone were used in 9% of patients, achieving good symptom control in all cases.
Conflicts of interestThe authors have no conflicts of interest to declare. No funding has been received by any of the authors from any pharmaceutical laboratory with regards to this article.
Please cite this article as: Abenza Abildúa MJ, Miralles Martinez A, Arpa Gutiérrez FJ, Lores Gutiérrez V, Algarra Lucas C, Jimeno Montero C, et al. Patologías asociadas al trastorno de conducta de sueño REM. Descripción de una serie hospitalaria. Neurología. 2019;34:159–164.