Nicotinic acetylcholine receptors (nAChRs) are widely expressed throughout several brain regions. Formation of the α4β2 and α7 subtypes in particular is involved in the organisation of different types of memory. Furthermore, due to their location, these receptors can control the release of various types of neurotransmitters and contribute to synaptic plasticity.
MethodsRats were divided into three groups, an experimental group (E), sham-operated group (S) and an intact group (T). In group E, stereotactic guidance was used to induce a chemical lesion with 1μg/μL of 5,7-dihydroxytryptamine (5,7-DHT) in the anteroventral part of the dorsal raphe nucleus (DRN). In the sham-operated group (S), animals underwent surgery including delivery of the same excipient solution to the same site. The intact group (T) received no treatment whatsoever. Twenty days after surgery, animals in all groups were euthanised by decapitation to evaluate the expression of α4 and α7 nAChRs by means of molecular biology techniques.
Results5-HT denervation of the rat prefrontal cortex (PFC) differentially modified the expression of α4 and α7 receptors: while α4 receptor expression increased, α7 expression decreased.
ConclusionExpression differences observed between the two subtypes may be due to their separate locations. The α4 subtype is found in postsynaptic locations and may be related to adaptive changes in postsynaptic cells, while the location of α7 is presynaptic. This explains why the lesion and the elimination of 5-HT fibres in the CPF would cause a decrease in α7 expression.
Los receptores de la acetilcolina de tipo nicotínico (R-Ach-n) son expresados ampliamente en diferentes regiones del cerebro. Particularmente, la conformación de los subtipos α4β2 y la α7ha sido involucrada con la organización de diferentes tipos de memoria. Además, debido a su localización, estos pueden controlar la liberación de diferentes tipos de neurotransmisores, así como su participación en la plasticidad sináptica.
MétodosSe conformaron 3 grupos de trabajo, un grupo experimental (E), un grupo control (C) y un grupo testigo (T). Al grupo E se le realizó la lesión farmacológica por vía estereotáxica en la región anteroventral del núcleo del rafe dorsal (NRD) con 1μg/μl de 5,7-dihidroxitriptamina. Al grupo C, se le sometió a cirugía y se le aplicó la solución vehículo y finalmente el grupo T no recibió ningún tratamiento; 20 días después de la cirugía, los animales de los 3 grupos fueron sacrificados por decapitación para el análisis de la expresión de las subunidades, α4 y α7 de los R-Ach-n mediante la técnica de biología molecular.
ResultadosLa denervación 5-HTérgica a la CPF de la rata modifica la expresión de los receptores α4 y α7 de manera diferencial. La expresión de las subunidades α4 se incrementa, mientras que las subunidades α7 disminuyen.
ConclusiónLas diferencias de expresión que tuvieron las 2 subunidades podrían deberse a la localización que presentan. La subunidad α4 se localiza en sitios post sinápticos y podría estar relacionada con cambios post sinápticos adaptativos, en tanto que la de la α7 se localiza en sitios presinápticos, por lo que la lesión y eliminación de fibras 5-HTérgicas en la CPF provoca su disminución.
Cortical electrical activity is modulated by the interaction between the cholinergic and serotonergic systems. It is also correlated with the behavioural expression of learning and memory, and both neurotransmitter systems interact in each of those functions.1 Nicotinic acetylcholine receptors (nAChRs) are particularly active in behavioural manifestations.2 It has been reported that systemic administration of high doses of mecamylamine, an nAChR antagonist, results in impaired memory in rats as demonstrated by performance in a radial-arm maze,3 whereas low doses of the same drug produce improvement.4 In addition, researchers have shown that activation of these receptors by means of agonists improves performance on memory tasks in both rabbits5 and monkeys.6 Brain structures such as the hippocampus7 and the amygdala have been shown to be involved in memory through activation of nAChRs. It is reported that use of antagonists to α7 and α4α2 receptor subtypes impairs working memory.8
Nicotinic acetylcholine receptors are pentameric structures formed by the heteromeric combination of α subunits (α2–α10) and β subunits (β2–β4). In contrast, α7 subunits form homomeric pentamers, and they are found in both pre-synaptic and post-synaptic cholinergic, GABAergic, and glutamatergic neurons.9
Both forms of nAChRs are found in the prefrontal cortex (PFC), a region involved in working memory.10 Studies have shown that nAChRs and muscarinic receptors in the rat prefrontal cortex are involved in that type of memory. Nevertheless, we lack information about how cortical nAChRs participate in memory, whether in humans or animals.11 Researchers have reported that loss of the α4 subunit is correlated with poor performance on memory tasks in patients with Alzheimer disease.12 In addition, stimulation of nAChRs with selective agonists minimises the memory impairments produced by loss of cortical nAChRs.13
Due to the distribution and location of nAChRs along presynaptic terminals, the latter are able to regulate the release of different types of neurotransmitters.14 Furthermore, these receptors are located on 5-HTergic terminals. Studies show that stimulation of these terminals regulates the release of serotonin (5-HT) in the rat prefrontal cortex.15
On the other hand, we know that when neurodegeneration affects axonal segments, intact neurons may suffer partial deafferentation, leading to cell death or induction of compensatory plasticity processes. Furthermore, these neurons will become more sensitive to the neurotransmitter initially released by the synapse (denervation hypersensitivity). This type of hypersensitivity is caused by an increase in the number of postsynaptic receptors.16 On the other hand, studies have shown that pharmacological lesion to the rat dorsal raphe nucleus (DRN) with 5,7-dihydroxytryptamine (5,7-DHT) induces plastic changes in prefrontal cortex neurons. These changes are correlated with alterations in performance of short-term memory (STM) tasks17 and decreases in baseline levels of 5-HT and acetylcholine (ACh).18 Nevertheless, stimulation of 5-HT1A receptors with their agonist 8-OH-DPAT increases ACh release. At the same time, stimulation of 5-HT2A receptors with the α-me-5-HT agonist does not affect ACh release in experimental vs control animals, whereas ACh release decreases with the 8-OH-DPAT agonist.18 In the light of these results, and considering the widespread distribution of nAChRs in the prefrontal cortex, it is important to gain a better understanding of how these receptors participate in memory-related processes. In particular, we need to command a greater knowledge of the plasticity response that occurs following pharmacological denervation of the DRN, as will be explored in this study.
Materials and methodsAll experiments were carried out according to the Guide for the Care and Use of Laboratory Animals by the National Institute of Health (NIH Publication No. 8023, 1978).
Since female rats exhibit increased activity of the enzyme that synthesises 5-HT and a greater capacity for storing 5-HT in serotonergic neurons, this study used 30 female Sprague-Dawley rats weighing 250 to 300g. Rats were kept under standard vivarium conditions with 12-hour light/dark cycles with food and water ad libitum. Furthermore, to avoid the potential influence of hormonal levels on results, animals were euthanised in anoestrus, that is, between metoestrus and dioestrus.19
Lesion of serotonergic afferents to the prefrontal cortexAnimals were divided into 3 groups: the experimental group (E), sham-operated group (S), and a control group (C). Animals from Group E (n=10) underwent stereotaxic surgery20 inducing a lesion in the anteroventral region of the DRN (8.0mm anterior–posterior to bregma, 2.0mm lateral to midline, to a depth of 7.2mm, and at an angle of 19°). A microliter syringe was used to inject 0.2μL of solution containing 1μg/μL 5,7-DHT and 1% ascorbic acid in 0.9% saline.21 Animals in the S group (n=10) also underwent surgery and were injected with a vehicle solution (1% ascorbic acid in 0.9% saline) at the same stereotaxic coordinates indicated above; group C animals (n=10) did not undergo surgery. Animals in the E and S groups were dosed with 50mg/kg intraperitoneal (IP) desipramine 30minutes after surgery in order to protect noradrenergic terminals22 since 5,7-DHT is toxic to noradrenergic neurons. Rats had been anaesthetised using an IP injection of1mg/kg dehydrobenzperidol followed by 50mg/kg ketamine 15minutes later.
At 20 days after surgery animals in all 3 groups were killed by decapitation. Brains were extracted under aseptic conditions and using sterilised instruments. The prefrontal cortex was subsequently dissected and tissue was stored at −70°C until the day it was required for total ribonucleic acid (RNA) extraction.
Obtaining and quantifying total ribonucleic acidTotal RNA was extracted using the guanidinium isothiocyanate–phenol–chloroform method.23 The resulting RNA precipitate was suspended in diethylpyrocarbonate-treated water (1%). The quantity and the quality of the extracted RNA were evaluated by determining the 260/280nm absorbance ratio; samples with an absorbance ratio between 1.7 and 2.0 were considered optimal.
Reverse transcriptase and chain reaction of semi-quantitative polymeraseMoloney murine leukaemia virus reverse transcriptase was used to synthesise complementary deoxyribonucleic acid (cDNA). We collected 2μg of RNA from each sample and added sterile water to achieve a total volume of 6μL. Samples were denatured at 70°C for 10minutes, after which they were immediately incubated in an ice bath with continuous agitation for 5minutes. We subsequently added a total volume of 14μL of the reverse transcriptase blend, consisting of 5X RT buffer; dNTP (deoxynucleoside triphosphate) 2.5mmol; dithiothreitol 1.0mmol; random primers 1μg/μL; and RNAse inhibitor (1U/μL). The mixture was incubated with reverse transcriptase (200U/μL) at 37°C during 1hour. The final stage was set for 95°C during 10minutes, after which 5μL of sterile water was added.
For the polymerase chain reaction, we used a reaction blend with the following composition: injectable sterile water, primers corresponding to the genes in question (Table 1), dNTPs (2.5mmol), 10× PCR buffer, MgCl2 (50mmol), cDNA and Taq DNA polymerase (1U/μL).
Oligonucleotide primer sequences used for semi-quantitative PCR.
| Subunit | GenBank | Sequence | Product (bp) | Cycles |
| α4 | Position 181–600 | 5′-GGCAATATCT CAGATGTGGTCCTCGT-3′ | 420 | 30 |
| NM_024354 | 5′-GCAAGATTGACTTAGTGAGCATTCAT-3′ | |||
| α7 | Position 298–570 | 5′-GTTCGTTTTCCAGATGGCCAGATTTG-3′ | 273 | 35 |
| NM_012832.3 | 5′-TGCAAGAGGCAGATATCAGCAGCTAT-3′ | |||
| β-actin | Position 684–1201 | 5′-CACCACAGCTGAGAGGGAAATCGTGCGTGA-3′ | 518 | 22 |
| NM_031144 | 5′-ATTTGCGGTGCACGATGGAGGGGCCGGACT-3′ |
Mineral oil was added to the amplification reactions in order to prevent evaporation. The thermal cycler was set for an initial cycle of 95°C for 5minutes. The machine automatically completed the cycles specific to each receptor. Each cycle was set for 95°C for 1minute, 60°C for 1minute, and 72°C for 1.5minutes, with a final extension cycle of 72°C for 5minutes. We also used the expression of the β-actin gene as a PCR control.
Amplified PCR products were analysed in a horizontal electrophoresis chamber by using a 100bp molecular weight marker in an agarose gel (1.5%) containing ethidium bromide 0.5mg/mL for 45minutes at 70volts in a 1× TBE (Tris-borate-EDTA) buffer. Band intensity was determined using a photo-documentation system equipped with analysis software (Molecular Image Gel Doc XR System Quantity One 1-D Analytical Software). We calculated and normalised levels of expression of each receptor according to the area represented by expression of a constitutive gene (β-actin). Results are expressed as arbitrary units of area by maximum intensity.
Statistical analysisResults were expressed as mean±standard error. A one-way analysis of variance test (ANOVA) was performed, and we used the Tukey test post hoc to compare the different study groups. The accepted level of significance was P≤.05.
ResultsHistologyWe performed histological analysis of the DRN for all animal groups in order to examine, in the context of this study, only those subjects with lesions limited to the region intermediate to the medial longitudinal fasciculus bundles (the zone that preferentially innervates the PFC).
The histological images taken of animals in the C group display a large number of nerve cell bodies in the region intermediate to the medial longitudinal fasciculus bundles (Fig. 1A). Analysis of the same region in animals in the S group reveals a slight decrease in the number of nerve cell bodies and moderate glial cell activity 20 days after the vehicle had been administered (Fig. 1B). In contrast, histological analysis of the E group 20 days after lesions were induced with neurotoxin 5,7 DHT revealed a massive loss of nerve cell bodies in the region intermediate to the medial longitudinal fasciculus bundles, in addition to a high number of reactive glia (Fig. 1C).
Histological images representing coronal slices of the dorsal raphe nucleus. Note the normal histological appearance in the control group (A), histopathological characteristics after microinjection of the vehicle in the sham-operated group (B), and after administration of the 5,7-dihydroxytryptamine neurotoxin used to destroy serotinergic neurons in the experimental group (C). Near total lack of neurons and a larger glial population in the experimental group compared to the sham-operated and control groups. f: medial longitudinal fasciculus; cresyl violet stain; bar=100μm.
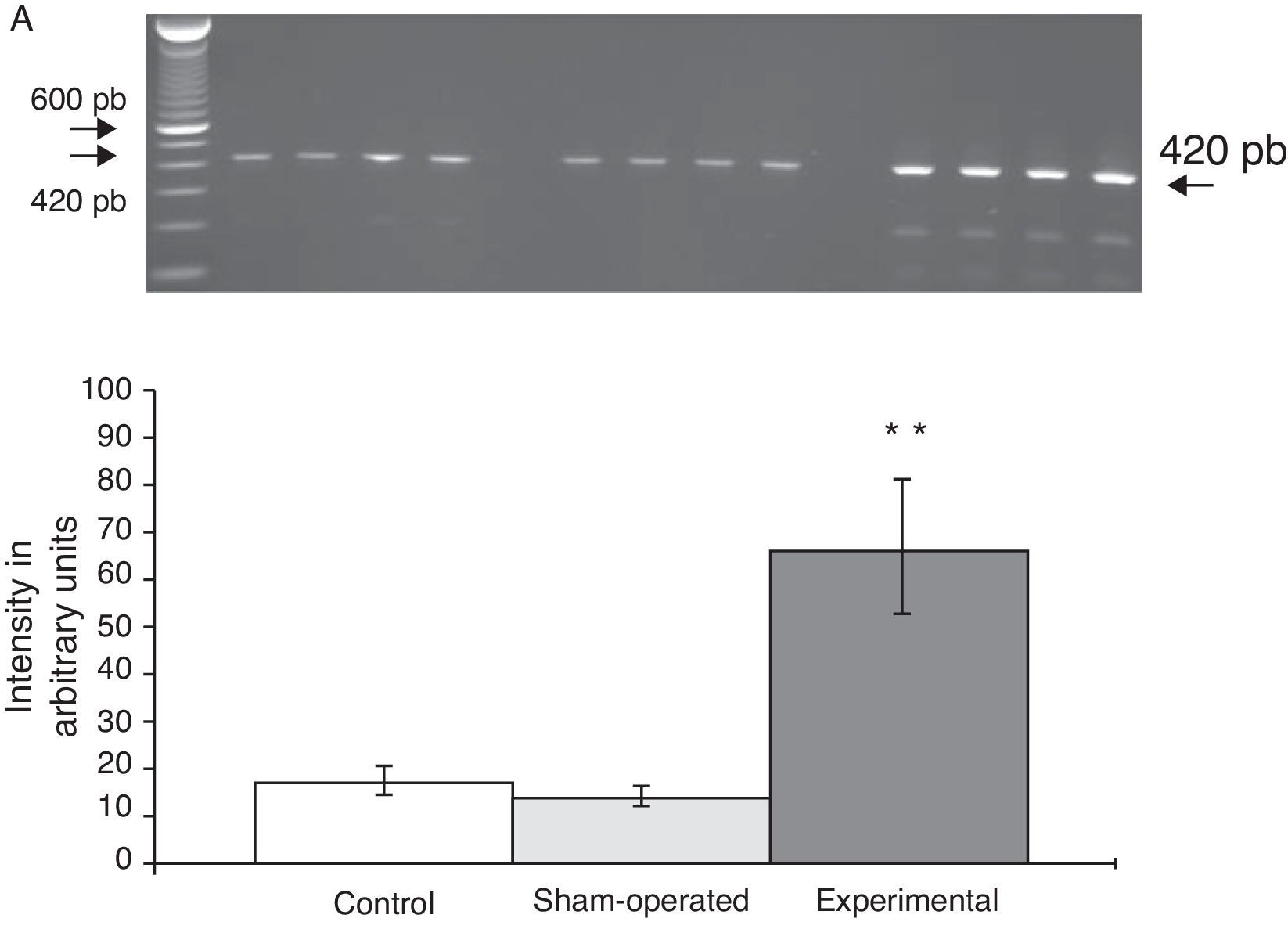
Expression of mRNA for subunit α4 in the rat PFC increased sharply (65±6) after the lesion in the DRN was induced. In comparison, values determined for animals in the C group were 15±3, and values in the S group were 13±4 (Fig. 2). At the same time, expression of subunit α7 was lower in animals with DRN lesions (15±6) than in animals in the S group (30±3) and the C group (40±5) (Fig. 3).
The PFC is the brain centre responsible for executive functions, and it also participates in planning complex cognitive activities. Specifically, it is involved in processing information stored in working memory24 through activation of nAChRs.25 These receptors are found at both presynaptic and postsynaptic locations in different brain structures.26 Results obtained in this study show that 5-HTergic denervation of the prefrontal cortex increases the expression of subunit α4 in that region. One of the most widely distributed and abundant combinations in the rat brain is the heteromeric form α4β2,26 and studies have also reported that α4β2 participates in STM (a subtype of working memory).12 Research in this area has shown that 5-HTergic denervation of the prefrontal cortex results in improved performance on tasks requiring subjects to recall information stored in short-term memory. This process correlates to morphological changes in pyramidal neurons in the PFC,17 meaning that alterations could be caused by increased expression of subunit α4. Nevertheless, an analysis of the expression of subunit α4 is needed to back up this premise. On the other hand, overexpression of the subunit may result from a compensatory plasticity phenomenon which the PFC induces to modulate the release or interaction of acetylcholine or other neurotransmitters. We do know that nAChRs in post-synaptic locations play an important role in synaptic plasticity and in regulating the effects specifically caused by acetylcholine.26
In turn, low expression of certain receptor subunits has been associated with the development of numerous neurodegenerative diseases.27
Expression of mRNA for subunit α7 decreased after 5-HTergic denervation in the PFC. On this subject, studies have reported that nAChRs in the PFC participate in short-term and long-term memory formation. Similarly, decreased levels of subunit α7 in the PFC have been correlated to a decrease or cessation of memory formation in patients with Alzheimer disease.12 Researchers using the 5-HTergic denervation model have reported changes in performance on tests of short-term memory17 and studies have also demonstrated decreases in the baseline release of 5-HT and ACh in the PFC.18 Results from our study show a decrease in the expression of the α7 receptor subunit. These subunits are widely expressed in the PFC, and when they are activated, they promote the signalling pathways needed in order to form reference and working memory.26 Furthermore, due to their homomeric structure and presynaptic location in different neurotransmitter systems, their main function is modulating acetylcholine release.26 On the other hand, the decreased expression of the α7 subunit is caused by the decrease in 5-HTergic fibre density in the PFC that follows pharmacological lesion to the DRN.28 It has been shown that release of 5-HT in the rat PFC increases when different nAChR subtypes are stimulated.15 Nevertheless, we do not know the exact combination of the nAChR subtypes involved in this effect. Some have suggested that this release of 5-HT is regulated by nAChRs, at least in the hippocampus.29 Stimulation of nAChRs may augment or decrease the release of 5-HT. In the first case, this release is promoted by the direct action of nAChRs on 5-HTergic terminals in the presence of high levels of nicotine.29 Secondly, a decrease in this activity is promoted by the release of ACh itself, by means of an inhibitory effect on the release of 5-HT. The precise mechanism involved in this interaction is currently unknown.15
Our study also found a slight decrease in mRNA expression for the α7 receptor subtype in the sham-operated group compared to the control group. This tendency may be due to the partial lesion induced by mechanical manipulation during surgery and the chemical effect of ascorbic acid on neurons in the DRN. It has been reported that ascorbic acid may cause oxidative stress and trigger apoptosis.30 These factors may explain why the PFC contained fewer α7 receptors in group S than in group C. The same effect was also observed among animals in the experimental group, but the difference was more marked.
Our study shows that pharmacological lesions to the DRN affect expression of the α4 and α7 receptors in the rat PFC. Furthermore, each subtype responds differently to the lesion. Specifically, expression of the α4 receptor subtypes increases while expression of α7 subtypes decreases. These results may provide an approximate idea of how these subtypes participate in cognitive processes. However, further experiments are needed in order to evaluate the expression of all subunits that constitute nAChRs. This will provide more detailed information about the subunits’ response to lesion processes related to cerebral plasticity, and allow us to gain more knowledge about the way they may participate in processes related to short-term memory.
The results from this study, together with those from our group, demonstrate the presence of a compensatory plasticity effect that may mediate potential changes in cortical transmission of information. Such changes underlie the behavioural expression of STM in the model of 5-HTergic denervation of the PFC.
FundingThis study was partially funded by PROMEP, dossier 103.5/07/2636 on sheet PTC-427, and by the COECYTJAL-UDG Fund as project PS-209-515.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Soria-Fregozo C, et al. La denervación 5-HTérgica córtico-frontal induce cambios en la expresión de las subunidades α4 y α7 de los receptores de acetilcolina tipo nicotínico en la corteza prefrontal de la rata adulta. Neurología. 2013;28:212–8.