Spinal arteriovenous fistulas (SAVFs), a rare type of vascular malformation, account for 3% of all spinal cord lesions. Without early treatment, the associated morbidity is high; furthermore, SAVFs pose a major diagnostic challenge. Our purpose was to evaluate the clinical characteristics of SAVFs and review their progress after treatment to determine whether they may be too late for treatment in some cases.
MethodsWe present a retrospective series of 10 patients diagnosed with SAVFs and treated at a tertiary hospital during a 3-year period.
ResultsIn our sample, SAVFs were found to be significantly more frequent in men (80%). Mean age in our sample was 65.4 years. The most common initial symptom was intermittent claudication/paraparesis (70%). In most patients, symptoms appeared slowly and progressively. At the time of diagnosis, the most common symptoms were motor, sensory, and sphincter disorders. Mean time from symptom onset to diagnosis was 24.3 months. Initial diagnosis was erroneous in 60% of the patients. Spinal magnetic resonance imaging was diagnostic in 90% of these cases and arteriography in 100%. The most common location of the fistula was the lower thoracic region and the most frequent type was dural (seven cases). All patients were treated with embolisation, surgery, or both, and 70% improved after fistula closure regardless of progression time.
ConclusionsDiagnosis of SAVFs is difficult and often delayed, which leads to poorer patient prognosis. We should have a high level of suspicion for SAVFs in patients with intermittent claudication or paraparesis exacerbated by exercise. Early treatment should be started in these patients. Treatment should always aim to improve the quality of life or stabilise symptoms, regardless of progression time.
Las fístulas arteriovenosas espinales (FAVE) son excepcionales y representan el 3% de las lesiones espinales. Asocian gran morbilidad sin tratamiento precoz, pero el diagnóstico constituye un reto. Nuestro objetivo es evaluar sus características clínicas y revisar la evolución tras el tratamiento. ¿Puede ser tarde para tratar?
MétodosPresentamos una serie retrospectiva de 10 casos diagnosticados y tratados en 3 años en un hospital terciario.
ResultadosSe observó un predominio masculino (80%). La edad media fue de 65,4 años. El síntoma inicial predominante fue la claudicación de la marcha/paraparesia (70%). En la mayoría de los pacientes la clínica fue lentamente progresiva. Al diagnóstico, lo habitual fue la combinación de síntomas motores, sensitivos y esfinterianos. El tiempo medio desde el inicio de los síntomas hasta el diagnóstico fue de 24,3 meses. El 60% tenía un diagnóstico inicial erróneo. La RM espinal fue diagnóstica en el 90% de los casos; la arteriografía, en el 100%. La localización más frecuente fue dorsal baja y el tipo anatómico predominante fue FAVE dural (7 pacientes). Todas fueron tratadas con embolización, cirugía o con ambas y el 70% mejoró tras su cierre, independientemente del tiempo de evolución.
ConclusionesEl diagnóstico de las FAVE es difícil y generalmente tardío, lo que empeora el pronóstico de los pacientes. Se debe tener un alto nivel de sospecha ante síntomas de mielopatía o claudicación de la marcha exacerbadas con el ejercicio e intentar tratamiento precoz. Consideramos que el tratamiento siempre está indicado, independientemente del tiempo de evolución, al mejorar la calidad de vida o conseguir la estabilización.
Spinal arteriovenous fistulas (AVFs) are extremely rare. Despite accounting for 70% of all vascular malformations of the spinal cord, they represent only 3% of all spinal cord lesions.1,2 Spinal AVFs are thought to be acquired lesions and rarely present in individuals younger than 50 years, although the exact aetiology is yet to be determined. From a pathophysiological viewpoint, an AVF is an abnormal, low-flow connection, or shunt, between an artery and a vein, and is associated with potentially severe complications. Spinal dural AVFs (also known as thoracic intradural AVFs) constitute the most frequent subtype; in these, the shunt is located in the trajectory of the corresponding nerve root, within the dura mater, and is fed by radiculomeningeal arteries draining centripetally via a radicular vein into perimedullary veins. The other two subtypes, spinal pial AVFs (ventral intradural or perimedullary) and spinal epidural AVFs (extradural), are less frequent; these are supplied by a radiculomedullary or a segmental artery, respectively.3,4
Spinal dural and pial AVFs drain into perimedullary veins, leading to venous hypertension and dysregulation of spinal blood vessel flow, which results in blood–brain barrier disruption and progressive spinal cord oedema.5 Over time, the arteriovenous pressure gradient decreases, leading to spinal cord ischaemia and consequently to progressive myelopathy; the reversibility of the latter depends on progression time. The pathogenic mechanism of these shunts is yet to be understood. It has been hypothesised that progressive fibrosis or thrombosis of radicular veins (due to advanced age and accelerated by the presence of a shunt), acting until then as alternative draining routes from the arterialised perimedullary veins, may play a relevant role in the pathogenesis of hypertension as a result of the decreased outflow.6,7 Given that the thoracic and lumbar regions of the spinal cord have fewer venous drainage channels than other spinal regions, venous congestion is transmitted caudocranially along the spinal cord, which may explain why the first symptoms usually reflect dysfunction of the conus medullaris.8 These include slowly progressive non-specific symptoms (claudication during exercise, paraesthesia, etc.) followed by the onset of severe myelopathy with paraplegia and sphincter dysfunction. Much less frequently, spinal AVFs present with such acute symptoms as subarachnoid bleeding or intramedullary spinal cord haemorrhage; these conditions occur almost exclusively with spinal pial AVFs, which usually have high blood flow. In the case of spinal epidural AVFs, mass effect and compression of nearby structures (nerve roots and spinal cord) constitute the main mechanism of damage due to the large sizes they may reach; retrograde venous hypertension also plays a role.3,4
Location is typically thoracic or lumbar. The cervical region is rarely affected; involvement of cervical vertebrae below C2 is anecdotal. Spinal AVFs above C2 (craniocervical) may present with venous congestion, which may even reach the conus medullaris, or with subarachnoid bleeding; this is much more frequent at this level than in fistulas affecting the thoracic and lumbar segments and depends on the draining pattern.9
If left untreated, spinal AVFs are associated with poor outcomes.2,10–13 However, the natural history and management of the condition continue to be controversial. Recommendations follow two main approaches: surgery and endovascular therapy. We present the perspective of a neurology department where a considerable number of cases have been diagnosed in a short time. In this study, we evaluate patients’ clinical characteristics and progression after treatment, aiming to determine whether it is too late to provide treatment.
Patients and methodsWe included 10 patients aged over 18 and diagnosed with spinal AVFs between 2012 and 2015 at the neurology department of Hospital Clínico San Carlos, Madrid. Some of these patients visited our department seeking second or even third opinions after consulting other centres and specialists. We gathered clinical data from the patients’ medical histories and long-term follow-up consultations (outpatient and telephone consultations). All patients were assessed by neurologists and treated at our centre and were followed up for at least 6 months after treatment. We provide a descriptive analysis of the cases and a review of the articles retrieved from PubMed using the following keywords:
“spinal arteriovenous lesions”, “spinal arteriovenous fistulas”, “spinal dural arteriovenous fistulas”, “spinal vascular malformations”, “spinal vascular disorder”, “mielopathy”, and “spinal angiography”.
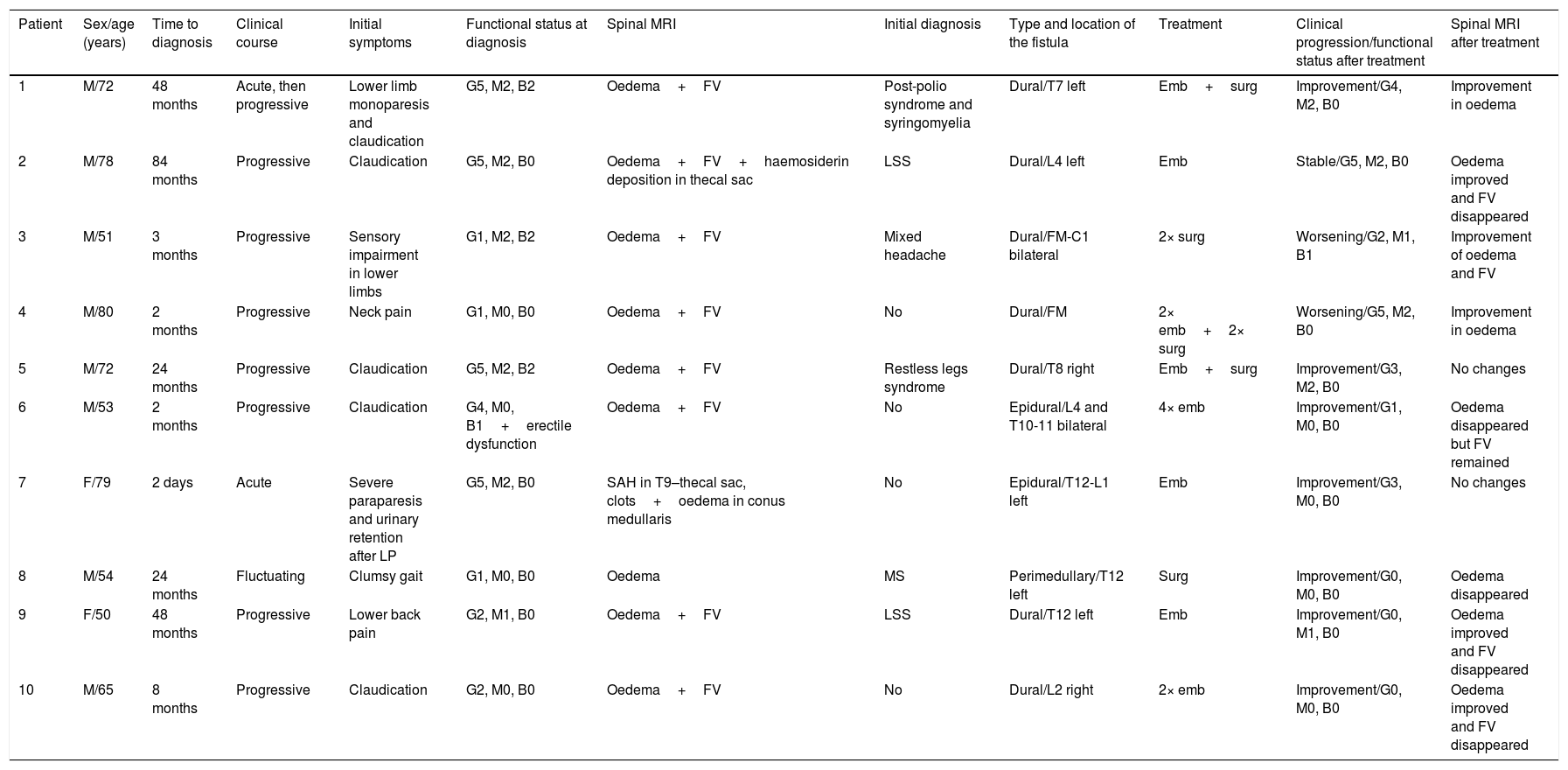
ResultsForms of presentation and clinical characteristicsOf the 10 patients included, 8 (80%) were men. Mean age at diagnosis was 65.4 years (range, 50-80). The patients’ clinical characteristics are listed in Table 1. Symptoms were slowly progressive in seven patients (70%), acute in two (20%), and fluctuating in one (10%). One patient displaying ictal onset of symptoms presented acute lower-limb monoparesis progressing to bilateral lower-limb paresis, whereas the other presented acute paraplegia due to subarachnoid haemorrhage following a lumbar puncture indicated for other reasons.
Clinical characteristics and progression of the 10 patients diagnosed with spinal AVFs.
| Patient | Sex/age (years) | Time to diagnosis | Clinical course | Initial symptoms | Functional status at diagnosis | Spinal MRI | Initial diagnosis | Type and location of the fistula | Treatment | Clinical progression/functional status after treatment | Spinal MRI after treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/72 | 48 months | Acute, then progressive | Lower limb monoparesis and claudication | G5, M2, B2 | Oedema+FV | Post-polio syndrome and syringomyelia | Dural/T7 left | Emb+surg | Improvement/G4, M2, B0 | Improvement in oedema |
| 2 | M/78 | 84 months | Progressive | Claudication | G5, M2, B0 | Oedema+FV+haemosiderin deposition in thecal sac | LSS | Dural/L4 left | Emb | Stable/G5, M2, B0 | Oedema improved and FV disappeared |
| 3 | M/51 | 3 months | Progressive | Sensory impairment in lower limbs | G1, M2, B2 | Oedema+FV | Mixed headache | Dural/FM-C1 bilateral | 2× surg | Worsening/G2, M1, B1 | Improvement of oedema and FV |
| 4 | M/80 | 2 months | Progressive | Neck pain | G1, M0, B0 | Oedema+FV | No | Dural/FM | 2× emb+2× surg | Worsening/G5, M2, B0 | Improvement in oedema |
| 5 | M/72 | 24 months | Progressive | Claudication | G5, M2, B2 | Oedema+FV | Restless legs syndrome | Dural/T8 right | Emb+surg | Improvement/G3, M2, B0 | No changes |
| 6 | M/53 | 2 months | Progressive | Claudication | G4, M0, B1+erectile dysfunction | Oedema+FV | No | Epidural/L4 and T10-11 bilateral | 4× emb | Improvement/G1, M0, B0 | Oedema disappeared but FV remained |
| 7 | F/79 | 2 days | Acute | Severe paraparesis and urinary retention after LP | G5, M2, B0 | SAH in T9–thecal sac, clots+oedema in conus medullaris | No | Epidural/T12-L1 left | Emb | Improvement/G3, M0, B0 | No changes |
| 8 | M/54 | 24 months | Fluctuating | Clumsy gait | G1, M0, B0 | Oedema | MS | Perimedullary/T12 left | Surg | Improvement/G0, M0, B0 | Oedema disappeared |
| 9 | F/50 | 48 months | Progressive | Lower back pain | G2, M1, B0 | Oedema+FV | LSS | Dural/T12 left | Emb | Improvement/G0, M1, B0 | Oedema improved and FV disappeared |
| 10 | M/65 | 8 months | Progressive | Claudication | G2, M0, B0 | Oedema+FV | No | Dural/L2 right | 2× emb | Improvement/G0, M0, B0 | Oedema improved and FV disappeared |
Functional status was evaluated with the Aminoff–Logue disability scale (G: gait; M: micturition; B: bowel).
Emb: embolisation; F: female; FM: foramen magnum; FV: flow voids; LP: lumbar puncture; LSS: lumbar spinal stenosis; M: male; MS: multiple sclerosis; SAH: subarachnoid haemorrhage; surg: surgery.
Most patients displayed a single type of symptom at onset: seven patients displayed motor symptoms, two had pain, and one had sensory alterations (Table 2). Claudication and exercise-induced paresis were the most frequent forms of presentation. For example, patient 10 played golf regularly and was able to determine the exact distance, measured in number of holes, at which he had to stop playing due to claudication; he recovered within minutes or hours. One of the patients who experienced pain had isolated lower back pain, whereas the other had neck pain radiating to the shoulders; pain worsened with physical activity and standing and improved with rest and in decubitus positions. The patient with sensory symptoms had paraesthesia in both legs and displayed the reverse Lhermitte sign (pain triggered by neck extension). None of the patients experienced sphincter dysfunction as the initial manifestation.
Symptoms at onset and at diagnosis.
| Symptom | At onset | At diagnosis |
|---|---|---|
| Claudication/paresis | 7 | 9 |
| Sensory alterations | 1 | 6 |
| Pain | 2 | 0 |
| Sphincter dysfunction | 0 | 6 |
| Other | 0 | 1a |
Number of patients presenting each symptom out of the total (N=10).
At the time of diagnosis, most patients presented several symptoms: paresis, sensory alterations, and sphincter dysfunction. Nine patients had motor impairment (five displayed severe gait impairment, needing canes/walkers/wheelchairs; mean Aminoff–Logue disability scale score in the sample was 3.1 points). Six patients had sensory alterations and six had sphincter dysfunction (six urethral and four anal). One patient also had erectile dysfunction (Table 2).
Time to diagnosisMean time from the initial manifestation to diagnosis of spinal AVFs (unequivocal diagnosis by spinal angiography) was 24.3 months (median, 24). Five patients were diagnosed after more than a year of symptom progression.
Initial diagnosis was erroneous in six patients. Initial diagnosis was considered erroneous when the initial spinal magnetic resonance imaging (MRI) scan revealed signs characteristic of spinal AVF (hyperintense spinal cord lesions on T2-weighted sequences, serpentine vessels, or flow voids, with or without contrast uptake) but the patient was diagnosed with other condition. The most frequent misdiagnosis was lumbar degenerative disc disease, which was diagnosed in two patients. One patient even underwent surgery for lumbar spinal stenosis, displaying no clinical or radiological improvements. Other misdiagnoses included multiple sclerosis, refractory restless legs syndrome, and mixed headache; patient 1 was diagnosed with post-polio syndrome and syringomyelia. Time to diagnosis was longer in these patients.
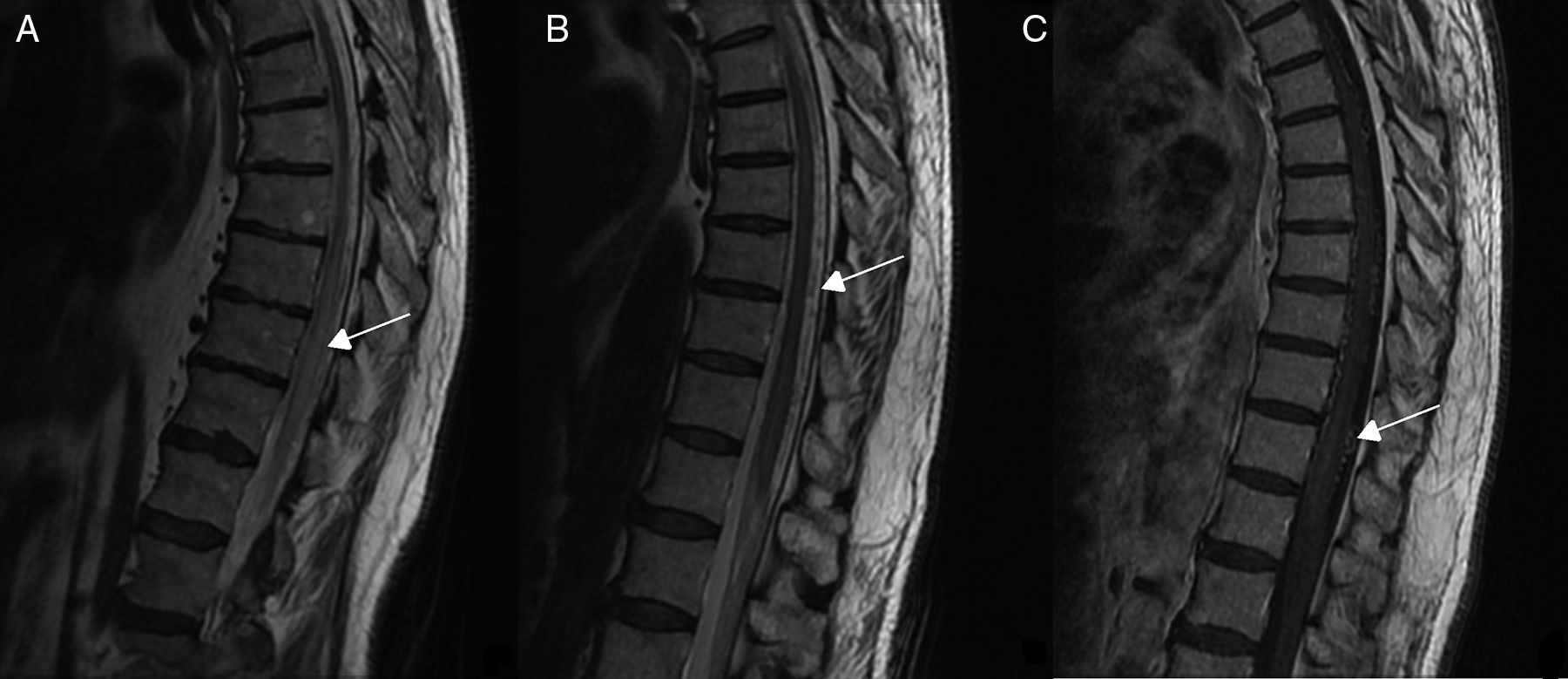
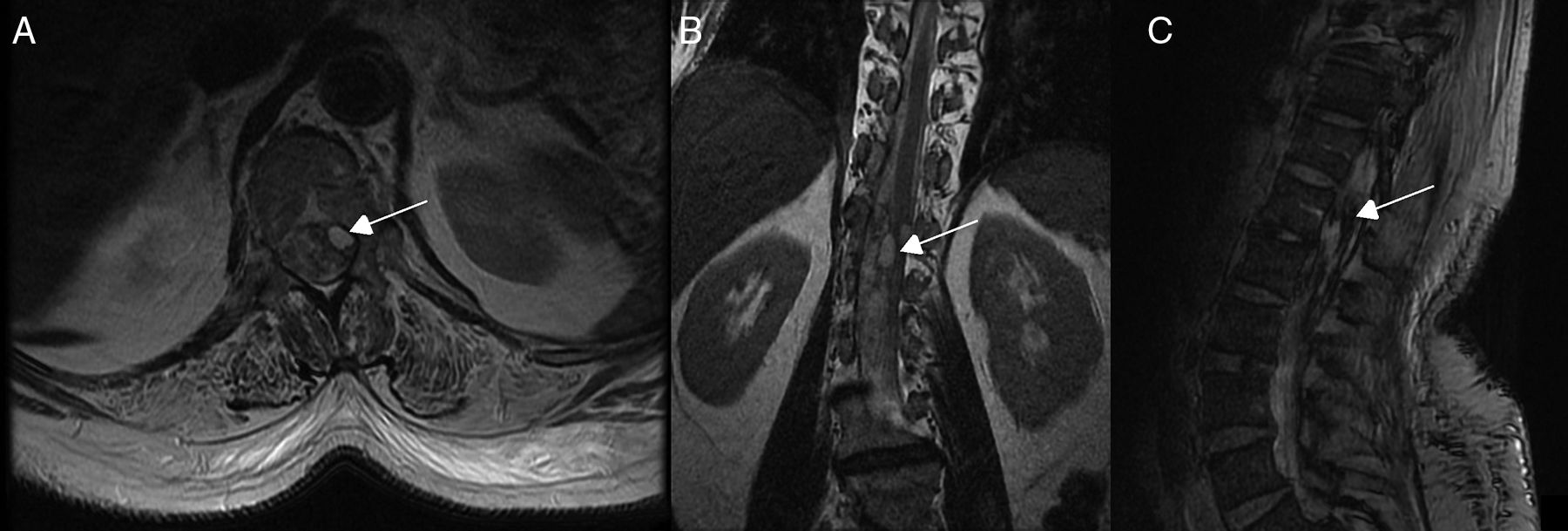
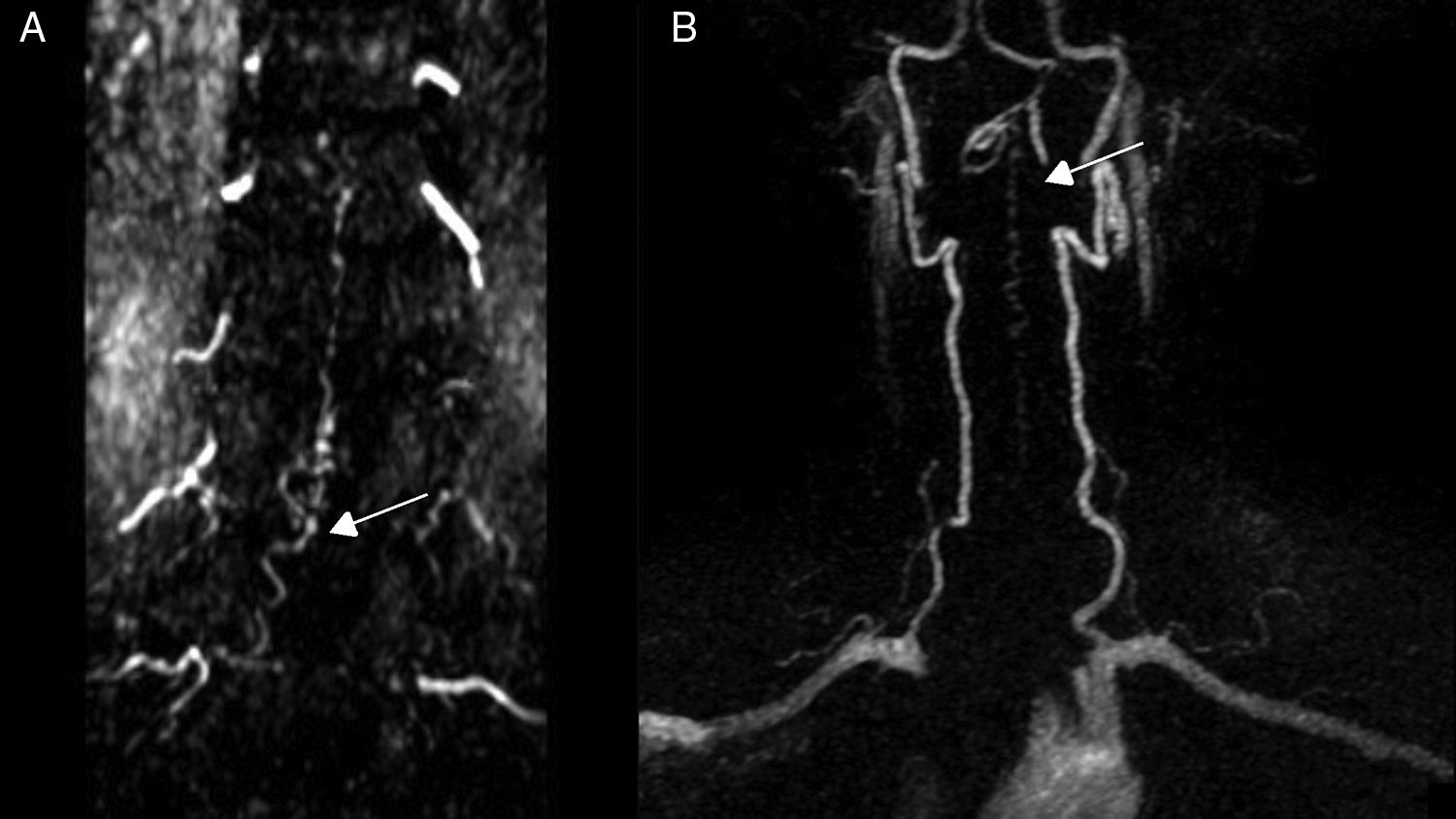
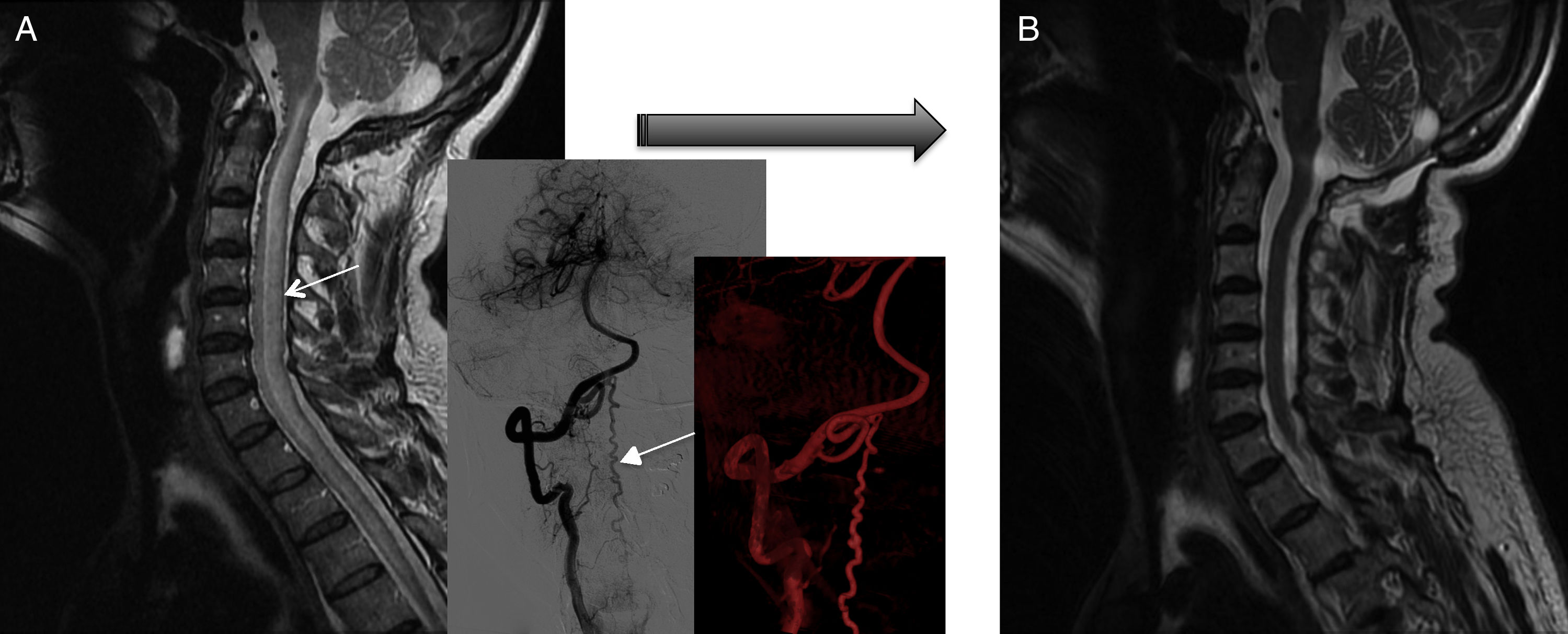
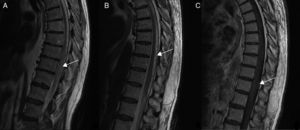
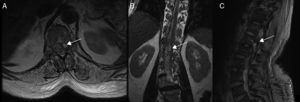
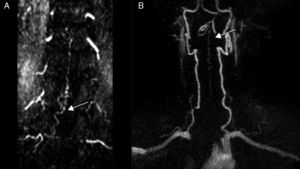
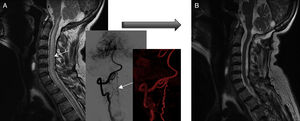
Diagnostic tests and anatomy of spinal AVFsAll patients underwent spinal MRI, which yielded a diagnosis in nine of the cases. All nine patients displayed T2 hyperintensities with a mean extension of 7.6 vertebrae; the conus medullaris was involved in eight patients. Eight of the nine patients with hyperintensities displayed flow voids with a mean extension of 6.6 vertebrae; the only patient with no flow voids had a perimedullary AVFs (Fig. 1). One patient showed signs of an old haemorrhage in the thecal sac. MRI did not yield a diagnosis only in the patient who had haemorrhage after a lumbar puncture; the bleeding resulted in numerous artefacts (Fig. 2). Time-resolved imaging of contrast kinetics (TRICKS) MR angiography sequences were taken in five patients; the exact level of the fistula could be determined in four cases (Fig. 3).
Spinal cord MRI of patient 7, showing multiple irregular artefacts in the thecal sac. (A, B) Pseudonodular hyperintensities on T2-weighted sequences. (C) Hypointensities on T2-weighted gradient-echo sequences, extending from T9 to the thecal sac; diffuse hyperintensity of CSF on T1-weighted sequences (clots distort the image of the exit of cauda equina nerve roots); and conus medullaris hyperintensity. These findings are compatible with lumbar subarachnoid haemorrhage.
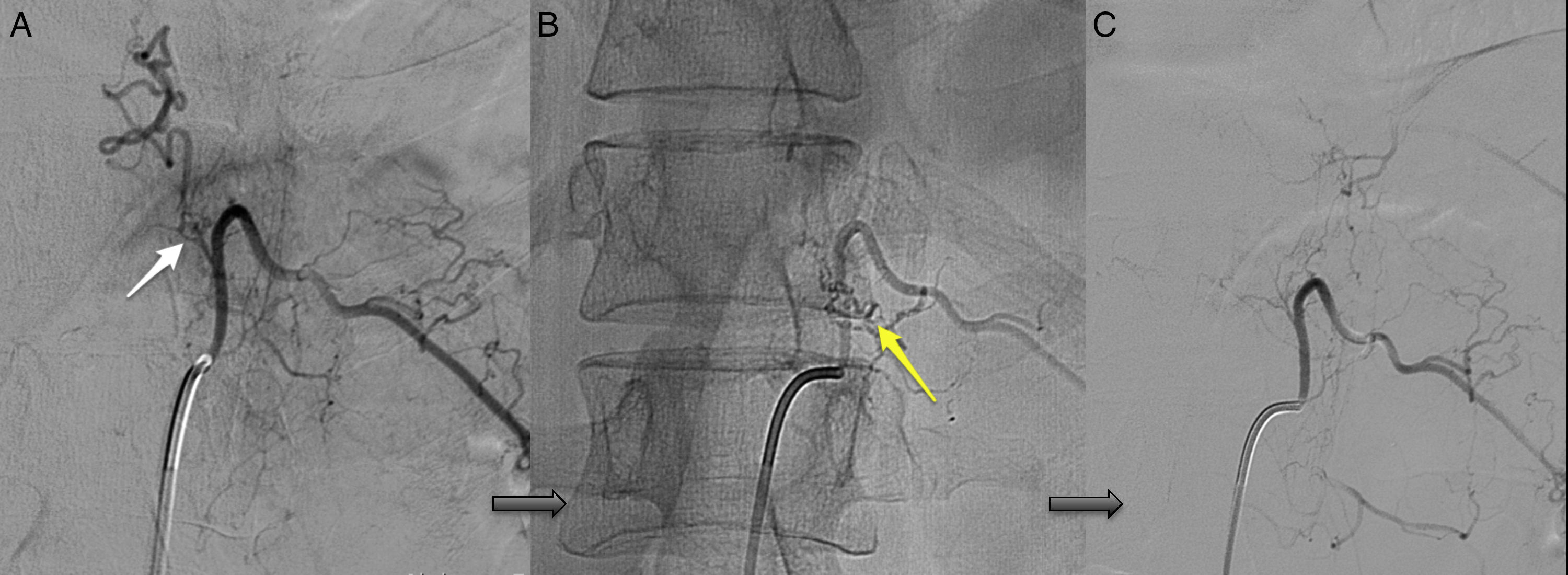
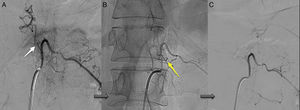
Angiography was diagnostic in all patients after a single procedure and enabled identification of the anatomical subtype: seven were dural, two epidural, and one perimedullary. Two were located in the cervical region (craniocervical junction), six in the thoracic region (below T7 in all cases), and three in the lumbar region. The most frequent location was the lower thoracic region. One patient had two fistulas, one at the level of the thoracic spine and the other in the lumbar spine; both were bilateral (Fig. 4). By laterality, five fistulas were located on the left side, two on the right side, and three were bilateral.
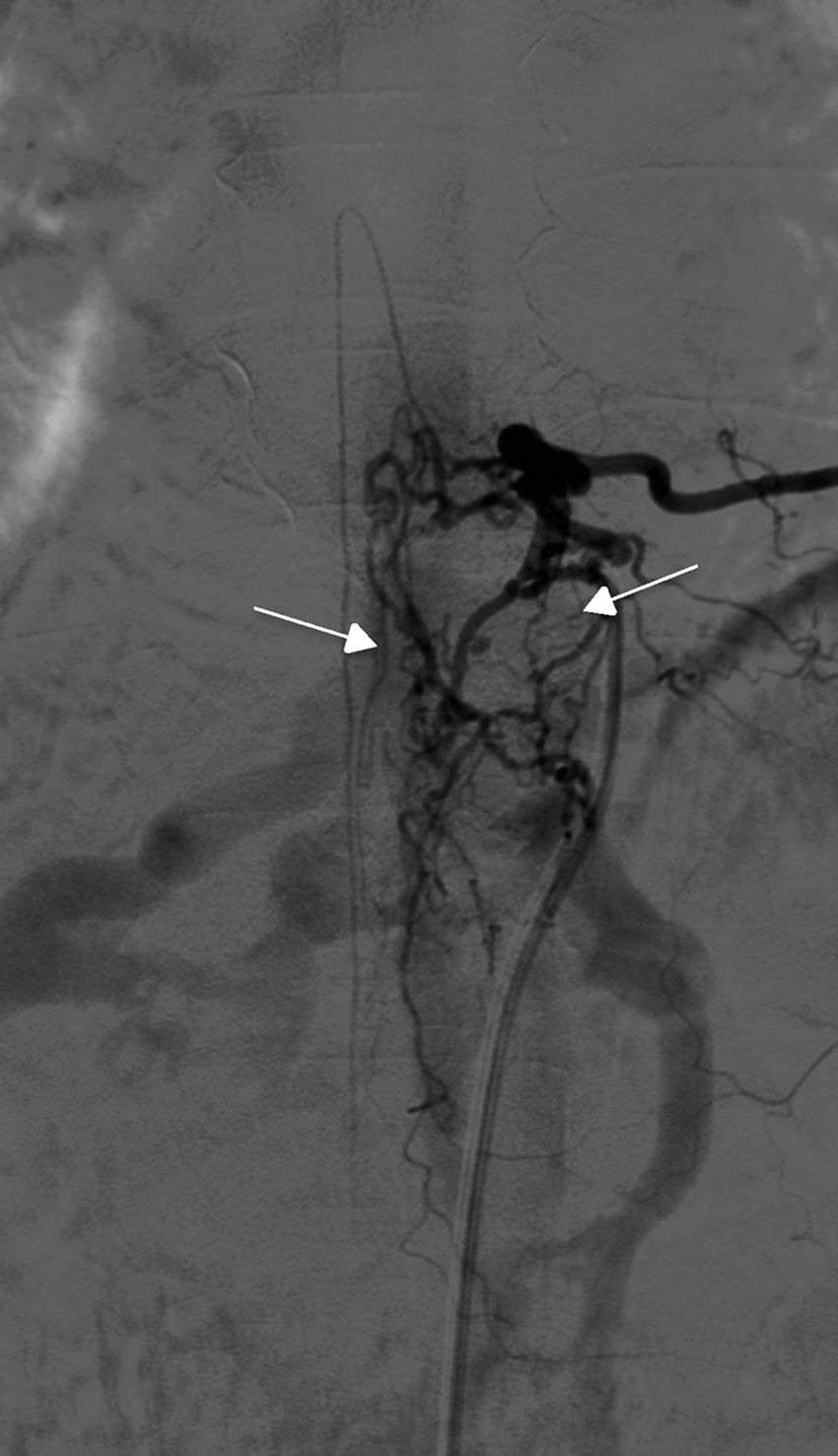
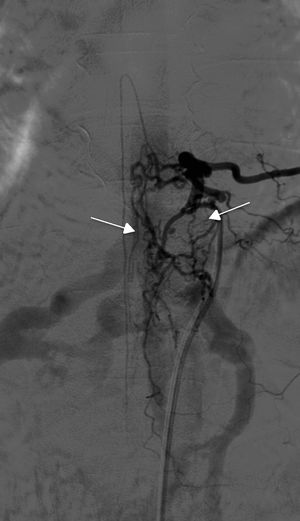
TreatmentEndovascular treatment was the treatment of choice; patients not eligible for endovascular treatment underwent surgery. Five patients were treated exclusively with endovascular embolisation (Fig. 5) and two underwent surgery. The remaining three patients needed a combination of both treatments. Surgery was the treatment of choice in a patient with a cervical spinal AVF whose angioarchitecture was too complex for embolisation, and in a patient with a spinal AVF supplied by the anterior spinal artery, which constitutes a formal contraindication for endovascular treatment. In six patients, the fistulas were closed after several attempts, either by repeating the same procedure or by combining endovascular treatment and surgery. Two patients with cervical spinal AVFs required multiple interventions: one underwent surgery on two occasions, and the other underwent two embolisation procedures and surgery. Complete obliteration with a single treatment and in a single procedure was achieved in only four patients.
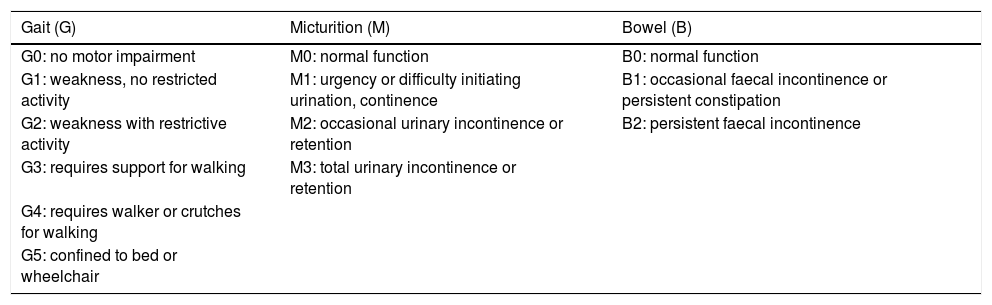
Clinical and radiological progressionTreatment outcomes were evaluated with the Aminoff–Logue disability scale; this is the most frequently used scale for follow-up of patients with spinal AVFs (Table 3). Although many of the patients displayed long progression times, fistula closure achieved significant improvements. Gait function, the most important factor for patient independence, improved significantly with treatment in seven patients (a decrease of at least 1 point on the scale) and remained stable in one patient (the patient with the longest progression time, 84 months). Only two patients showed poorer gait function after treatment; both had cervical spinal AVFs and required several interventions due to fistula reopening. All patients received rehabilitation in the form of passive or active physical therapy exercises for the affected limbs, depending on the degree of paresis and cooperation, hydrotherapy for gait, and balance exercises. Some patients also received electrical stimulation. Sphincter dysfunction improved significantly (a decrease of at least 1 point on the scale) in five of the seven patients with the condition (71.4%), especially in those with anal sphincter dysfunction.
Aminoff–Logue disability scale.
| Gait (G) | Micturition (M) | Bowel (B) |
|---|---|---|
| G0: no motor impairment | M0: normal function | B0: normal function |
| G1: weakness, no restricted activity | M1: urgency or difficulty initiating urination, continence | B1: occasional faecal incontinence or persistent constipation |
| G2: weakness with restrictive activity | M2: occasional urinary incontinence or retention | B2: persistent faecal incontinence |
| G3: requires support for walking | M3: total urinary incontinence or retention | |
| G4: requires walker or crutches for walking | ||
| G5: confined to bed or wheelchair |
B: bowel; G: gait; M: micturition.
Fistula closure was confirmed by angiography in all patients. According to MRI results, eight patients improved after complete fistula closure: spinal cord oedema decreased in all eight and venous congestion significantly decreased or resolved in five patients, although this did not result in clinical improvement in two of them (both had cervical spinal AVFs) (Fig. 6). MRI showed no changes in 2 of the 10 patients, with recurrent fistula reopening in one case and subarachnoid haemorrhage in the other. Both patients displayed significant clinical improvements, however.
Pre- and post-surgery MRI studies of patient 3. (A) At diagnosis, the MRI scan displayed spinal cord oedema extending from the cervicomedullary junction to the chest (plus angiography reconstructions). (B) Post-treatment MRI scan (after two surgeries) showing normal signal intensity. However, gait function worsened by one point on the Aminoff-Logue scale.
The demographic characteristics of our sample of patients with spinal AVFs are similar to those described in the literature: spinal AVFs are the most common in men and frequently present during the sixth and seventh decades of life.1,2 Our patients’ clinical characteristics are also comparable to those reported in other studies. Most patients experienced slowly progressive, symmetrical symptoms which were initially of a single type, mostly claudication or lower limb paresis triggered by exercise, prolonged standing, or the Valsalva manoeuvre, due to worsening of venous hypertension in the arterialised draining vein. These findings are most frequently associated with thoracic fistulas.14 Two of our patients experienced pain for months with no other symptoms; we should therefore consider the possibility of spinal AVFs in patients with persistent neck or lower back pain, with or without radicular pain, and no clear signs of degenerative disc disease. As in previous studies, sphincter dysfunction was a very infrequent initial manifestation. At the time of diagnosis, however, patients most frequently exhibited a combination of symptoms (paresis, sensory alterations, and sphincter dysfunction) and higher levels of disability.15–17 These patients usually display signs of upper and lower motor neuron dysfunction, with upper motor neuron dysfunction most closely associated with disability.14 Asymptomatic fistulas are controversial: few cases have been described of patients who were diagnosed with angiography but displayed signs or symptoms of myelopathy.18–20
Diagnosis of spinal AVFs is difficult and usually delayed by the absence of specific symptoms; time to diagnosis is long in all series.11–17 Spinal AVFs are frequently misdiagnosed for other, more prevalent diseases, such as lumbar degenerative disc disease, inflammatory myelitis, or even peripheral neuropathy, especially in older patients.4,10,11 Patients are frequently evaluated by several specialists and undergo multiple tests before the condition is diagnosed.
In our sample, spinal MRI had a high sensitivity (90%) for diagnosing spinal AVFs. TRICKS sequences were taken in some cases. Although spinal angiography is the diagnostic gold standard, TRICKS sequences constitute a promising tool due to their value in locating fistulas. These sequences constitute a variation of contrast-enhanced dynamic 3D TOFs, which enables the acquisition of multiple phases (many of these with venous filling) with a single contrast bolus, providing an optimum phase for diagnosis compared to conventional 3D TOFs. Spinal cord MRI helps neurologists decide whether to perform an angiography study (a complex, invasive procedure with potential to exacerbate venous congestion) and help determine the location of the fistula (resulting in shorter angiography studies); it provides valuable follow-up data after treatment. Given the lack of association between clinical and radiological findings, a complete MRI study should be performed when a spinal AVF is suspected. Some studies have found that the pinprick level corresponds to the level of the fistula in less than 50% of cases14 and is rarely associated with MRI hyperintensities.21 In one of the longest studies into the sensitivity and specificity of MRI for diagnosing spinal AVFs conducted to date, all patients diagnosed by angiography displayed at least one of the characteristic radiological signs of spinal AVFs: T2 hyperintensity in the area of oedema and flow voids corresponding to congestion and vessel tortuosity.18 In that study, spinal cord hyperintensities were the most frequent finding (in 90% of patients), followed by flow voids (80%); these results are similar to our own. When both signs were present, specificity for diagnosis was 97%. It is therefore essential to perform a spinal cord angiography when MRI detects either of these signs, especially when they co-occur. If neither is present, we may omit angiography and consider other diagnoses. Other less frequent findings include conus medullaris hyperintensity, contrast enhancement, or spinal cord expansion; these are less sensitive for diagnosis. In our study, however, conus medullaris hyperintensity was seen in all patients with thoracic and lumbar spinal AVFs (but not in the two patients with cervical spinal AVFs), which suggests that the sensitivity of this finding is higher than that reported in the literature. MRI is highly sensitive for diagnosing spinal AVFs, but is not perfect: according to some studies, a small percentage of patients show no alterations on T2-weighted sequences (these patients also had a lower level of disability).14,22 A high level of suspicion is therefore essential.
Several studies have attempted to establish associations between symptoms and radiological findings. More extensive hyperintensities on T2-weighted sequences before treatment have been associated with symptom worsening during exercise (this reflects the extension of the segment of spinal cord vulnerable to ischaemia when energy demand increases) and with poorer pre- and post-treatment functional status.23,24 However, no association has been established between post-surgery hyperintensities and functional status or disability after treatment.14,23 Some patients in our series showed no association between clinical and radiological signs: after fistula closure, some patients displayed significant clinical improvements (a 2-point decrease on the Aminoff–Logue scale) but no radiological improvements, and vice versa. Post-treatment MRI therefore seems to be a poor predictor of progression.
However, it is clear that poor functional prognosis is associated with longer progression times and consequently with delayed diagnosis and that post-treatment functional status is linked to pre-treatment functional status.10,12,13 An association between treatment later than 3 years from the symptom onset and poor prognosis was first described several decades ago25; treatment should therefore be administered as early as possible.
Treatment for spinal AVFs aims to resolve venous congestion by closing the arteriovenous shunt. Spinal AVFs may be treated surgically (the first line of treatment, consisting of laminectomy or laminoplasty with section/disconnection of the draining vein) or with endovascular embolisation of the supplying vessels or the draining vein. Although surgery has been shown to be more effective than embolisation (98% success vs 25-75%),15,16,21,26,27 the use of endovascular treatment is increasingly frequent on account of its lower aggressiveness, and numerous advances made, such as the development of new embolic agents (onyx) and shorter recovery and hospitalisation times.3,28–32 However, not all patients are eligible for embolisation due to variability in the distribution of spinal cord blood vessels or a common origin of the feeding artery and the artery of Adamkiewicz. Endovascular embolisation was the treatment of choice in our sample when technically viable. We cannot compare the effectiveness of embolisation and surgery since combined treatment was necessary in many cases; this is relatively common in these patients. Cooperation between neurosurgeons and neuroradiologists is essential to identify the most suitable treatment,32 with the approach chosen depending on each centre's experience with both treatments. The literature includes some cases of fistula closure using minimally invasive surgery, with microscope-assisted endoscopic interlaminar ligation of the proximal draining vein. This innovative, non-aggressive technique is suitable in some cases, but its usefulness and effectiveness for treating spinal AVFs is yet to be determined.33 If symptoms worsen, recanalisation of a spinal AVF should always be ruled out; this relatively frequent complication is associated with poorer outcomes. In these cases, it is also necessary to rule out the presence of multiple spinal AVFs, whether synchronous or metachronous.34
Craniocervical junction spinal dural AVFs, which are even more infrequent, probably constitute a different subgroup associated with a wide range of forms of presentation, from chronic myelopathy to intracranial subarachnoid haemorrhage or even brainstem dysfunction. Given the low prevalence of this type of fistula, treatment recommendations are even more controversial. No study has compared the effectiveness of surgery and embolisation for craniocervical junction spinal dural AVFs; treatment will largely depend on the angioarchitecture of the lesion and individual centres’ experience. In a recent review article, haemorrhage and surgical obliteration were found to be associated with better outcomes in these patients.35 In any case, patients with craniocervical junction spinal dural AVFs and myelopathy experiencing an exacerbation require early or even emergency treatment.
Administering preventive treatment to patients with asymptomatic spinal AVFs is a controversial approach, given the unpredictable natural history of the condition. However, as all spinal AVFs will eventually cause symptoms, early treatment, even during the asymptomatic stage, is desirable.19 We recommend close MRI monitoring and early treatment when the first symptoms or radiological alterations appear.
Steroids should not be administered to improve myelopathy symptoms: several studies suggest that neurological symptoms may worsen during or within 24h of intravenous, oral, or (less frequently) epidural administration of steroids; this worsening may be irreversible.36,37 This may be due to increased venous hypertension secondary to osmotic changes induced by water reabsorption in the kidneys; it has also been suggested that these drugs promote cerebral venous thrombosis due to increased congestion. A combination of both mechanisms is the most likely explanation.36,37 Spinal AVFs should be suspected when myelopathy symptoms worsen following administration of steroids.
Regarding treatment outcome, 70% of our patients showed significant improvements in gait; this is consistent with results reported in the literature.13,28 Craniocervical junction spinal dural AVFs were associated with poorer outcomes and more difficult management. Disability assessment scales are extremely useful during follow-up. In addition to fistula closure, early, prolonged rehabilitation was essential in all cases. Although most studies indicate that sphincter dysfunction, a frequent late-onset manifestation, is associated with poor outcomes,13,38 this complication (especially anal sphincter dysfunction) improved in most patients in our and in other series.11 Some studies have found no association between time to diagnosis and motor or sensory symptoms, but do report an association with severe bladder dysfunction. This may be due to hypertension and congestion of the conus medullaris; compared with upper spinal cord segments, this area is more vulnerable to ischaemia due to its lower number of venous channels.14 Numerous studies have suggested that patients with longer symptom progression times will have more severe disability and, consequently, poorer outcomes. However, other studies have shown that patients’ capacity for improvement after treatment does not depend on symptom duration exclusively, but rather on pre-treatment clinical status.13 Regarding anatomical location, motor improvement is significantly more likely in patients with spinal AVFs in the lower thoracic region than in any other location. This may be explained by the fact that the lower thoracic region contains more blood vessels, considering the role ischaemia plays in the pathogenesis of spinal cord injury in these patients.13
In conclusion, diagnosing spinal AVFs continues to be a clinical challenge. These fistulas are underdiagnosed but constitute a treatable cause of myelopathy. Neurologists should suspect spinal AVFs in patients with claudication or myelopathy of unknown cause. Delayed diagnosis results in poorer outcomes. However, it is never too late to treat: symptoms improve or at least stabilise in a high percentage of cases. Selecting a treatment approach will depend on each centre's experience and requires a multidisciplinary approach.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ortega-Suero G, Etessam JP, Gamazo MM, Rodríguez-Boto G. Fístulas arteriovenosas espinales del adulto. Manejo de una serie de casos desde una planta de Neurología. Neurología. 2018;33:438–448.