The symptoms of minor stroke and transient ischemic attack (TIA) are temporary and mild. Despite the transient nature of the focal symptoms and the absence of visible brain lesions in some patients, many experience persistent cognitive problems subsequently. We aimed to establish the discriminant capacity of the Montreal Cognitive Assessment (MoCA) in screening for cognitive impairment (CI) within 90 days of TIA.
MethodsA total of 50 patients with minor stroke or TIA were recruited. Patients were administered the MoCA test and a formal neuropsychological test battery. CI was defined clinically according to neuropsychological test findings.
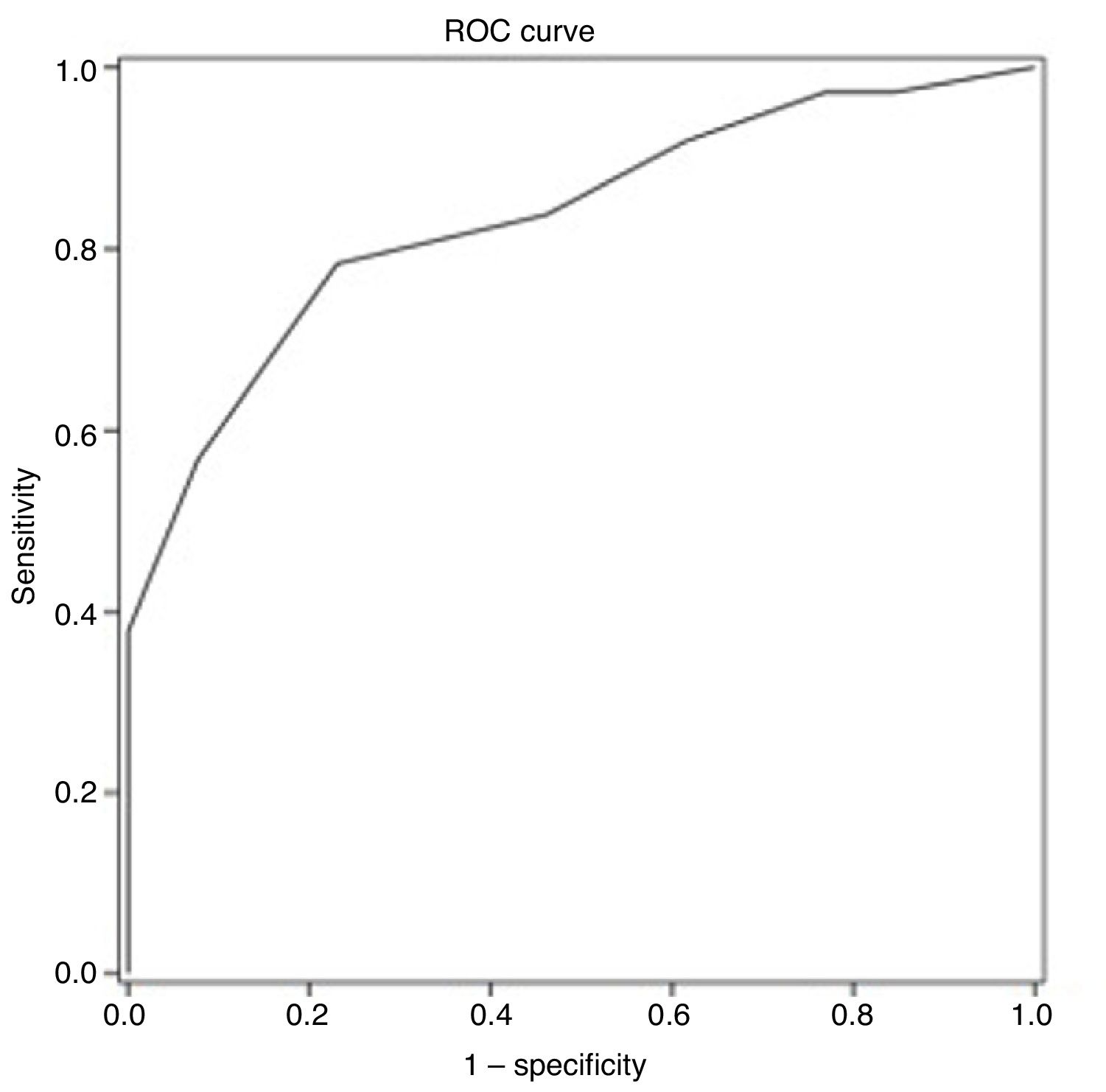
ResultsThe average age of recruited patients was 57.7±8.0 years; 70.0% were men; all patients had completed at least primary education. Thirty-seven patients (74.0%) presented CI. Receiver operating characteristic curve analysis obtained an optimal MoCA cut-off point of 25 for discriminating between patients with CI and those without, with an area under the curve of 0.835 (95% confidence interval [CI], 0.720-0.949), sensitivity of 78.4% (95% CI, 62.8%-88.6%), specificity of 76.9% (95% CI, 49.7%-91.8%), positive predictive value of 90.6% (95% CI, 81.0%-95.6%), and negative predictive value of 55.6% (95% CI, 39.5%-70.4%).
ConclusionsMore than half of the patients presented CI as determined by the formal battery of neuropsychological tests. A MoCA cut-off point of 25 is sufficiently sensitive and specific for detecting CI after minor stroke or TIA, and may be implemented as a screening technique in routine clinical practice.
Los síntomas de un ictus minor o un ataque isquémico transitorio (AIT) son leves y transitorios. A pesar de la naturaleza pasajera de los síntomas focales y la ausencia de lesiones cerebrales visibles en algunos pacientes, muchos experimentan problemas cognitivos persistentes posteriormente. Nuestro objetivo es establecer el poder discriminativo de la Evaluación Cognitiva de Montreal (MoCA) en la detección del deterioro cognitivo (DC) dentro de los 90 días posteriores al AIT.
MétodoSe incluyeron un total de 50 pacientes con ictus minor y AIT. Se les aplicó la prueba MoCA y una batería neuropsicológica formal. El deterioro cognitivo se definió clínicamente según los hallazgos de las pruebas neuropsicológicas.
ResultadosLa edad promedio de los pacientes reclutados fue de 57.7±8.0 años, siendo la mayoría de los pacientes hombres (70.0%). Todos los pacientes tenían un nivel educativo igual o superior al primario. Treinta y siete (74.0%) sujetos presentaron deterioro cognitivo. Mediante el análisis de la curva característica del receptor (ROC) se obtuvo un punto de corte del test MoCA de 25 puntos para discriminar entre sujetos con y sin deterioro cognitivo; siendo el área bajo la curva (AUC) de 0,835 (intervalo de confianza del 95%: 0.720 a 0.949), sensibilidad de 78,4% (IC95%: 62,8 - 88,6%); especificidad del 76,9% (IC95%: 49,7 - 91,8%); valor predictivo positivo del 90,6% (IC95%: 81,0 - 95,6%) y negativo del 55,6% (IC95%: 39,5 - 70,4%).
ConclusionesMás de la mitad de la muestra sufría deterioro cognitivo según lo determinado por la batería formal de pruebas neuropsicológicas. Un punto de corte de 25 en el MOCA es lo suficientemente sensible y específico para detectar DC tras un ictus minor o AIT y podría implementarse en la práctica clínica como método de cribado.
Minor stroke and transient ischaemic attack (TIA) are non-disabling cerebrovascular events with mild, short-lasting neurological symptoms. Patients with history of minor stroke or TIA are at greater risk of more severe stroke; management focuses on determining the cause of the event, providing specific secondary prevention treatment, and controlling risk factors.1
Despite the transient nature of the focal symptoms of minor stroke and TIA, and even in the absence of detectable brain lesions, these patients may experience persistent cognitive problems.2 Symptom severity, lesion volume, and infarct localisation are the main determinants of cognitive impairment after ischaemic stroke3; these factors cannot fully explain the phenomenon after a TIA or minor stroke.
The prevalence and profile of objective cognitive dysfunction are currently under study.4 Approximately one-third of patients with TIA present impairment in at least one cognitive domain within 3 months of the episode.5 While cognitive impairment may have a considerable impact on quality of life, routine management of patients with TIA or minor stroke rarely includes a cognitive assessment.6 Given that the current prevalence of cognitive impairment in these patients ranges from 21% to 70%, depending on the series, it would be useful to implement screening and follow-up strategies in routine clinical practice.4
Neuropsychological assessment continues to play an essential role in the diagnosis of dementia or any other type of vascular cognitive impairment.7 In this regard, there is a need for quick and simple tests for use during the first stage of the screening process. Several standardised tools are currently available for detecting cognitive impairment and dementia.8 The Montreal Cognitive Assessment (MoCA) is a cognitive screening tool with high sensitivity and specificity9; it has been translated and adapted to different languages and validated for use in the Spanish population.10 It is also used for detecting cognitive impairment in patients with cerebrovascular disease,11,12 with several studies finding it to be superior to the Mini–Mental State Examination (MMSE).8,13
The purpose of this study was to establish a cut-off point for the MoCA when screening for cognitive impairment within 90 days of minor stroke or TIA. Cognitive function was assessed with a structured interview and a neuropsychological test battery.
MethodsPatients were recruited consecutively from the stroke unit at Hospital Universitario de Badajoz (Spain) between 1 December 2015 and 1 December 2016. We included patients aged 18-70 years with first-ever minor stroke (NIHSS score < 3 points at admission) or TIA who underwent a complete aetiological study and a diagnostic MRI study. We excluded patients with recurrent stroke, doubtful TIA, severe depression, or a diagnosis of cognitive impairment prior to the cerebrovascular event, as well as illiterate individuals or those presenting psychiatric disorders that may interfere with the cognitive assessment.
We gathered data on demographic variables (age, sex, education level, economic status, and area of residence), vascular risk factors, and ischaemic stroke subtype (atherothrombotic, cardioembolic, lacunar, undetermined, or another cause). All patients underwent MRI studies as part of the management protocol.
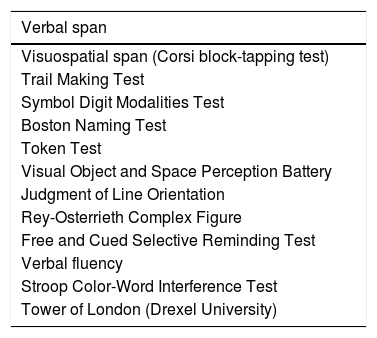
Neuropsychological assessmentDiagnosis of cognitive impairment was established using the NEURONORMA test battery, which includes 13 tools evaluating such cognitive domains as attention and information processing speed, working memory, language, visuospatial and visuoconstructive ability, verbal and visual memory, and executive function.14 Total administration time for the NEURONORMA test battery is approximately 1.5hours. We gathered raw and scaled scores for all tests. Cognitive impairment was diagnosed using the cut-off point of a scaled score of 6. Table 1 lists the tests included in the NEURONORMA test battery.
Tests included in the NEURONORMA test battery.
| Verbal span |
|---|
| Visuospatial span (Corsi block-tapping test) |
| Trail Making Test |
| Symbol Digit Modalities Test |
| Boston Naming Test |
| Token Test |
| Visual Object and Space Perception Battery |
| Judgment of Line Orientation |
| Rey-Osterrieth Complex Figure |
| Free and Cued Selective Reminding Test |
| Verbal fluency |
| Stroop Color-Word Interference Test |
| Tower of London (Drexel University) |
Diagnosis of cognitive impairment was established according to the diagnostic criteria for “mild vascular neurocognitive disorder” of the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). In our study, a cognitive domain was considered to be impaired when the patient performed poorly (scaled score < 6) on at least one of the tests assessing that domain, with the exception of attention, information processing speed, and executive function, for which impairment was defined as poor performance on at least 2 tests. Patients scoring in the normal range (scaled scores ≥ 6) for all tests, or performing poorly on only one test assessing attention, information processing speed, or executive function, were considered to have no cognitive impairment.
Description of the toolThe MoCA evaluates 6 cognitive domains. The maximum score is 30 points, and the administration time is approximately 10minutes. MoCA items are distributed as follows: 1) Memory: patients have 2 trials to learn 5 words (not scored), which they have to recall after approximately 5minutes (5 points); 2) Visuospatial function: clock-drawing test (3 points) and a cube copy task (1 point); 3) Executive function: a line-drawing task adapted from the Trail Making Test part B (1 point), a phonemic fluency task (1 point), and 2 verbal abstraction tasks (2 points); 4) Attention/concentration/working memory: a sustained attention task (1 point), a serial subtraction task (3 points), and digits forward and backward tasks (1 point each); 5) Language: a 3-item confrontation naming task with 3 low-familiarity animals (3 points) and repetition of 2 complex sentences (2 points); and 6) Orientation: orientation to time and place (6 points). MoCA scores must be adjusted for education level. In the original version, the cut-off score for cognitive impairment is 26.
Statistical analysisStatistical analysis was performed using version 21.0 of the SPSS® statistics software (SPSS Inc.; Chicago, IL, USA). Normally distributed continuous variables are expressed as means (SD) and were compared with the two-sample two-tailed t test. Non–normally distributed continuous variables are expressed as medians (IQR) and were compared with non-parametric tests. Categorical variables were compared with the chi-square test. The performance of the MoCa test was expressed with the sensitivity, specificity, predictive values, and likelihood ratios for the optimal cut-off point. We also analysed the area under the ROC curve to compare the discrimination ability of the MoCA for detecting cognitive impairment.
The study was approved by our hospital’s ethics committee; all participants gave written informed consent. Our centre has a legal copy of the tests included in the NEURONORMA test battery and response sheets for all tests.
ResultsThe study included 50 patients. Mean age (SD) was 57.7 years (8.0); most patients were men (70%). The median NIHSS score (IQR) of patients with minor stroke was 1.00 point (2.00). Stroke was atherothrombotic in 9 patients (18%), cardioembolic in 4 (8%), lacunar in 12 (24%), of undetermined cause after a complete aetiological study in 22 (44%), and due to another cause (carotid artery dissection) in 3 (6%).
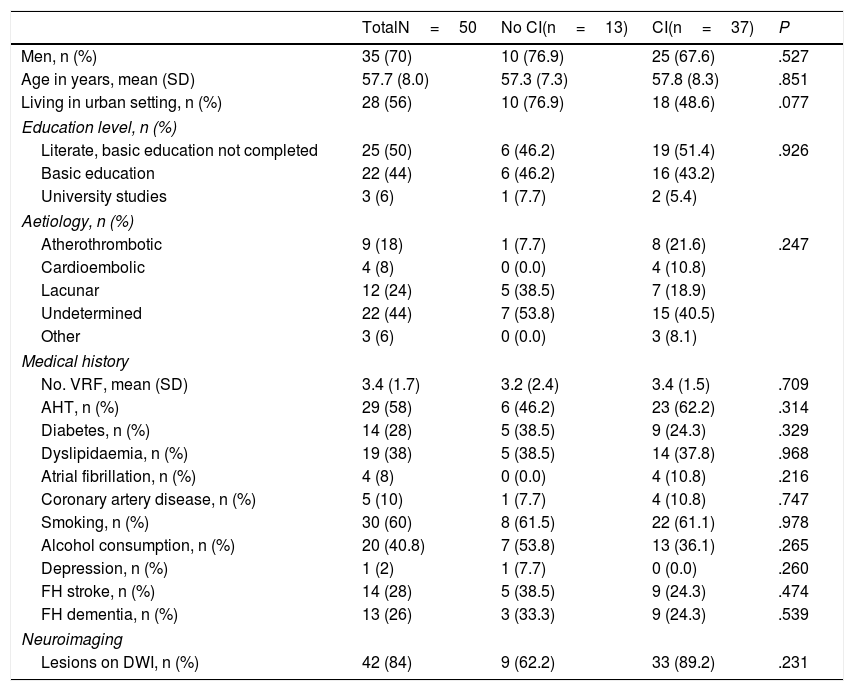
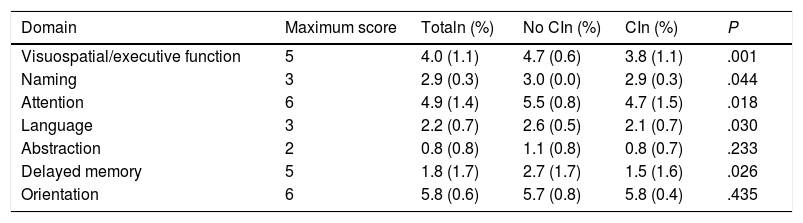
According to the results of the neuropsychological test battery, 74% (95% CI, 60.4%-84.1%) had cognitive impairment. No significant differences were observed between patients with and without cognitive impairment for sociodemographic variables, vascular risk factors, history of depression, family history, stroke aetiology, or neuroimaging results (Table 2). Patients with cognitive impairment scored a mean of 27 points (1.9) on the MoCA, whereas cognitively healthy individuals scored 22.4 (3.4); Table 3 shows MoCA scores by domain in both patient subgroups.
Clinical characteristics of our patient sample.
| TotalN=50 | No CI(n=13) | CI(n=37) | P | |
|---|---|---|---|---|
| Men, n (%) | 35 (70) | 10 (76.9) | 25 (67.6) | .527 |
| Age in years, mean (SD) | 57.7 (8.0) | 57.3 (7.3) | 57.8 (8.3) | .851 |
| Living in urban setting, n (%) | 28 (56) | 10 (76.9) | 18 (48.6) | .077 |
| Education level, n (%) | ||||
| Literate, basic education not completed | 25 (50) | 6 (46.2) | 19 (51.4) | .926 |
| Basic education | 22 (44) | 6 (46.2) | 16 (43.2) | |
| University studies | 3 (6) | 1 (7.7) | 2 (5.4) | |
| Aetiology, n (%) | ||||
| Atherothrombotic | 9 (18) | 1 (7.7) | 8 (21.6) | .247 |
| Cardioembolic | 4 (8) | 0 (0.0) | 4 (10.8) | |
| Lacunar | 12 (24) | 5 (38.5) | 7 (18.9) | |
| Undetermined | 22 (44) | 7 (53.8) | 15 (40.5) | |
| Other | 3 (6) | 0 (0.0) | 3 (8.1) | |
| Medical history | ||||
| No. VRF, mean (SD) | 3.4 (1.7) | 3.2 (2.4) | 3.4 (1.5) | .709 |
| AHT, n (%) | 29 (58) | 6 (46.2) | 23 (62.2) | .314 |
| Diabetes, n (%) | 14 (28) | 5 (38.5) | 9 (24.3) | .329 |
| Dyslipidaemia, n (%) | 19 (38) | 5 (38.5) | 14 (37.8) | .968 |
| Atrial fibrillation, n (%) | 4 (8) | 0 (0.0) | 4 (10.8) | .216 |
| Coronary artery disease, n (%) | 5 (10) | 1 (7.7) | 4 (10.8) | .747 |
| Smoking, n (%) | 30 (60) | 8 (61.5) | 22 (61.1) | .978 |
| Alcohol consumption, n (%) | 20 (40.8) | 7 (53.8) | 13 (36.1) | .265 |
| Depression, n (%) | 1 (2) | 1 (7.7) | 0 (0.0) | .260 |
| FH stroke, n (%) | 14 (28) | 5 (38.5) | 9 (24.3) | .474 |
| FH dementia, n (%) | 13 (26) | 3 (33.3) | 9 (24.3) | .539 |
| Neuroimaging | ||||
| Lesions on DWI, n (%) | 42 (84) | 9 (62.2) | 33 (89.2) | .231 |
AHT: arterial hypertension; CI: cognitive impairment; DWI: diffusion-weighted imaging; FH: family history; SD: standard deviation; VRF: vascular risk factors.
Scores for the different domains of the Montreal Cognitive Assessment in our sample of patients with minor stroke or transient ischaemic attack, by presence or absence of cognitive impairment (diagnosis established with a neuropsychological test battery).
| Domain | Maximum score | Totaln (%) | No CIn (%) | CIn (%) | P |
|---|---|---|---|---|---|
| Visuospatial/executive function | 5 | 4.0 (1.1) | 4.7 (0.6) | 3.8 (1.1) | .001 |
| Naming | 3 | 2.9 (0.3) | 3.0 (0.0) | 2.9 (0.3) | .044 |
| Attention | 6 | 4.9 (1.4) | 5.5 (0.8) | 4.7 (1.5) | .018 |
| Language | 3 | 2.2 (0.7) | 2.6 (0.5) | 2.1 (0.7) | .030 |
| Abstraction | 2 | 0.8 (0.8) | 1.1 (0.8) | 0.8 (0.7) | .233 |
| Delayed memory | 5 | 1.8 (1.7) | 2.7 (1.7) | 1.5 (1.6) | .026 |
| Orientation | 6 | 5.8 (0.6) | 5.7 (0.8) | 5.8 (0.4) | .435 |
CI: cognitive impairment.
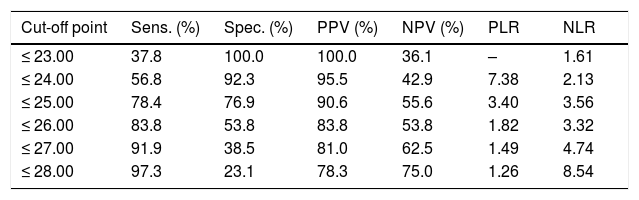
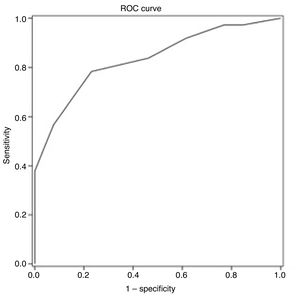
ROC curve analysis yielded an optimal cut-off point of ≤ 25 points for discriminating between patients with and without cognitive impairment (Table 4). The area under the ROC curve was 0.835 (95% CI, 0.720-0.949), with 78.4% sensitivity (95% CI, 62.8%-88.6%), 76.9% specificity (95% CI, 49.7%-91.8%), a positive predictive value of 90.6% (95% CI, 81.0%-95.6%), a negative predictive value of 55.6% (95% CI, 39.5%-70.4%), and a positive likelihood ratio of 3.40 (Fig. 1).
Discrimination indices of cognitive impairment according to results on the Montreal Cognitive Assessment 90 days after minor stroke or transient ischaemic attack.
| Cut-off point | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| ≤ 23.00 | 37.8 | 100.0 | 100.0 | 36.1 | – | 1.61 |
| ≤ 24.00 | 56.8 | 92.3 | 95.5 | 42.9 | 7.38 | 2.13 |
| ≤ 25.00 | 78.4 | 76.9 | 90.6 | 55.6 | 3.40 | 3.56 |
| ≤ 26.00 | 83.8 | 53.8 | 83.8 | 53.8 | 1.82 | 3.32 |
| ≤ 27.00 | 91.9 | 38.5 | 81.0 | 62.5 | 1.49 | 4.74 |
| ≤ 28.00 | 97.3 | 23.1 | 78.3 | 75.0 | 1.26 | 8.54 |
NLR: negative likelihood ratio; NPV: negative predictive value; PLR: positive likelihood ratio; PPV: positive predictive value; Sens.: sensitivity; Spec.: specificity.
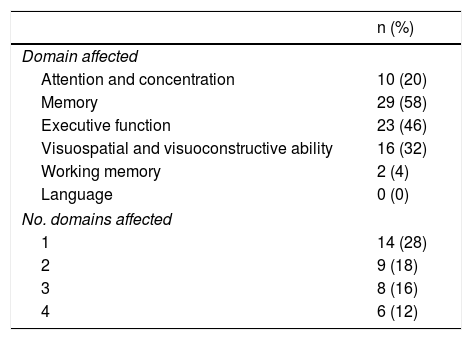
Our sample’s neuropsychological assessment results are summarised in Table 5. The most frequently affected cognitive domains were memory (29 patients; 58%), executive function (23; 46%), visuospatial and visuoconstructive ability (16; 32%), and attention/concentration (10; 20%). Fourteen of the patients with cognitive impairment (38%) presented impairment in one domain only, whereas 23 (62%) presented impairment in 2 or more cognitive domains.
Cognitive domains showing impairment (diagnosis with a neuropsychological test battery) in our sample of patients with minor stroke or transient ischaemic attack.
| n (%) | |
|---|---|
| Domain affected | |
| Attention and concentration | 10 (20) |
| Memory | 29 (58) |
| Executive function | 23 (46) |
| Visuospatial and visuoconstructive ability | 16 (32) |
| Working memory | 2 (4) |
| Language | 0 (0) |
| No. domains affected | |
| 1 | 14 (28) |
| 2 | 9 (18) |
| 3 | 8 (16) |
| 4 | 6 (12) |
This study analyses the psychometric properties of the MoCA for diagnosing cognitive impairment 90 days after a minor stroke or TIA, with clinical diagnosis as the gold standard. Our results show that the MoCA presents good psychometric properties, making it a useful tool for detecting cognitive impairment in clinical practice. The authors of the MoCA established an optimal cut-off point of ≤ 25 points for detecting cognitive impairment9; this is higher than the cut-off point calculated in our study population.
Similar studies into the usefulness of the MoCA for patients with stroke and TIA11 also establish different cut-off scores to that recommended by Nasreddine et al.9 Two studies conducted in France and in Beijing, respectively, establish the optimal cut-off point at ≤ 22 points, reporting good sensitivity and specificity.15,16 A recent meta-analysis of the accuracy of the MoCA for detecting cognitive impairment after stroke, which included 12 studies (over 2100 patients in different stages of the disease), reports areas under the curve of 0.90, 0.90, and 0.95 for cut-off scores 20/19, 21/20, and 26/25, respectively, showing high predictive validity for detecting cognitive impairment within a month of stroke. However, Youden index values were highest for lower scores.17
Several recent studies have shown that the MoCA is more sensitive than the MMSE for detecting cognitive impairment in patients with cerebrovascular disease, particularly minor stroke and TIA.15,18,19
In the Spanish general population, the prevalence of cognitive impairment in adults younger than 70 years is estimated at 3.7%-7.2%.20 According to some recent systematic reviews, minor stroke and TIA frequently cause cognitive impairment, both in mild (29%-68%) and more severe forms (8%-22%).4,21 The great variability in prevalence rates is probably due to differences in the diagnostic criteria and measurement instruments used.21
Limited data are available on the profile of cognitive impairment in these patients. However, the few studies that have addressed this topic using neuropsychological test batteries agree that these patients usually present vascular cognitive impairment, a type of cognitive impairment mainly affecting executive function, attention, and information processing speed. In other words, these patients present a non-amnestic profile secondary to subcortical and frontal damage.22,23 In our sample, the most common type was multiple-domain cognitive impairment; while memory was the most frequently affected domain, patients also presented problems with executive function, visuospatial and visuoconstructive ability, attention, and concentration. The profiles detected after analysing MoCA domains usually coincide with the results of other neuropsychological tests.24–26
Cognitive impairment after minor stroke or TIA may be explained by tissue damage, although this hypothesis should be confirmed in more specific studies.5 Despite their transient nature, minor stroke and TIA may cause permanent brain damage, altering the pathways involved in cognitive function and ultimately leading to cognitive impairment.27 This hypothesis may partially be supported by the presence of ischaemia on MR images; lesions compatible with recent infarction are frequently found on DWI sequences.28 In contrast with the traditional time-based definition of TIA, the proposed new tissue-based definition discriminates between patients with and without signs of recent infarction3; only the latter meet the diagnostic criteria for TIA. In our sample, 84% of patients showed lesions on DWI sequences, although no significant differences were observed between patients with and without cognitive impairment. Future studies should compare cognitive function between patients with transient symptoms with and without DWI lesions.
Our study presents certain limitations, mainly the small size of the sample and the fact that our patients were younger than those participating in other studies (we excluded patients older than 70 years due to the possibility of degenerative cognitive impairment). The MoCA has a considerable education bias and is strongly influenced by age; our results should therefore be interpreted with caution, as they may not be applicable to other patient profiles.
Very few studies have validated the MoCA in the Spanish population.10 Furthermore, the test has not been validated in Spanish patients with stroke, which underscores the relevance of our study. Several institutions, including the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network, have proposed the MoCA as a screening tool for cognitive impairment in patients with stroke,7 and many clinical trials are beginning to use this tool to replace the MMSE.29
In conclusion, the preliminary results for the use of the Spanish-language version of the MoCA in patients with minor stroke or TIA suggest that the tool may be effective for detecting cognitive impairment in these patients. More than half of our sample had cognitive impairment, according to results from a neuropsychological test battery. A cut-off point of 25 on the MoCA is sufficiently sensitive and specific for detecting cognitive impairment after a minor stroke or TIA; the test may therefore be used in clinical practice as a screening tool. In conclusion, the MoCA is quick and easy to administer, assisting in early detection of cognitive impairment in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramírez-Moreno JM, Bartolomé Alberca S, Muñoz Vega P, Guerrero Barona EJ. Detección del deterioro cognitivo con la Evaluación Cognitiva de Montreal en pacientes españoles con ictus minor o ataque isquémico transitorio. Neurología. 2022;37:38–44.