Disintegrins are low molecular weight proteins (4–15kDa) found in the venom of some snake species, these proteins act as integrin inhibitors. Integrins are membrane cell surface receptors formed by α–β subunits. These integrins modulate cell–cell and cell–extracellular matrix interactions. β1 and β3 integrins play important roles in angiogenesis and metastatic processes, suggesting that disintegrins may have utility in the development of new anticancer therapies. This review aims to show recent advances in disintegrin research and the evaluation of their biological activity in both in vitro and in vivo studies.
Since the approval of captopril, the first protein-based medication isolated from snake venom in 1975, snake venoms have become a valuable natural pharmacopoeia from which new drugs may be developed.1 Snakes are the vertebrae group with the greatest number of venomous species, which supposes a vast reservoir of different molecules,2 since snake venom contains complex mixtures of hundreds of pharmacologically active molecules, mainly peptides and proteins.3 Disintegrins are low molecular weight polypeptides (4–15kDa) found in the venoms of some species. These selectively block integrins (family of adhesion receptors),4 which perform important functions in processes like metastasis, diabetes, osteoporosis and inflammation.5–9 One of the therapeutic applications of great interest for disintegrins is the development of new anti-angiogenesis, anti-metastasis, anti-proliferative and apoptosis-inductor agents for the treatment of cancer. Mortality in this pathology is frequently caused by metastasis; in this process, cancer cells interact with adjacent cells and extracellular matrix (ECM) proteins in order to proliferate and migrate to different tissue and settle there, forming a new tumor,4 for which the interactions between cancer cells and ECM are essential. Moreover, certain types of cancer do not respond in an adequate way to current treatments, thus the importance of finding natural antagonist molecules which allow the modulation of interactions between cancer cells and ECM. The present revision shows the latest findings on snake venom disintegrins from different viper species (Family: Viperidae), and its biological activity in vitro and in vivo.
HistoryDisintegrins were discovered in 1987 by Tur-Fu Huang et al., who described a low molecular weight peptide isolated from the venom of the Asian snake Trimeresurus gramineus. This peptide, called tigramine, inhibits interaction between fibrinogen and glycoprotein IIb/IIIa (known as integrin αIIbβ3) expressed in platelets.10
Later in 1991, with the resolution of the echistatin structure (2ECH) isolated from the venom of Echis carinatus (Gariba snake)11 structure–activity association studies of the disintegrins were initiated; those same studies led to the sequence description denominated: integrin binding domain, which is formed by three amino acids – arginine, glycine and aspartate (RGD). Despite the fact that domains different from RGD were observed, a characteristic which ought to be mentioned is that aspartate (D) is conserved in most variations found thereupon (KGD, MGD, VGD, WGD, MLD). This amino acid may be responsible for the binding of integrins with β subunit, while the N-terminal amino acid of the binding domain to integrin may determine specificity through interactions of the α subunit.12 With the exception of the cysteines and binding domains, a variability between the disintegrins sequences was observed.13 Like with other toxins, in addition to the binding domain, the inhibitor activity of disintegrins also depends on the location of the cysteines and their sequences, since these lead to the formation of disulphide bridges (S-S). The first report about S-S bridge repair in disintegrins (albolabrin) was published by Calvete in 1991.14 On this basis, despite the high degree of similarities observed between disintegrins, they were able to prove that they have different patterns concerning their S-S bridges,15 which allowed their current classification.
Classification of disintegrinsDisintegrins come from the proteolytic processing of P-II metalloproteases (SVMP)2 and conform to a family of polypeptides whose main characteristics are: their cysteine content, their low molecular weight (4–15kDa)4 and their actions as very selective integrins (β1 and β2).11 Different criteria have been taken into account in order to classify them according to their size and the number of S-S bridges; it is possible to divide them into five groups: (1) short disintegrins, which have anywhere between 41 and 51 amino acids, as well as four S-S bridges, (2) medium disintegrins, which have around 70 amino acids and six S-S bridges, (3) long disintegrins, with about 84 amino acids and seven S-S bridges, (4) dimeric disintegrins and (5) heterodimeric disintegrins. Dimeric disintegrins contain subunits of 67 amino acids with 10 cysteines involved in the formation of four intrachain disulfides and two interchain cysteine linkages.2
According to the variability in their integrin-binding domains, disintegrins are classified into three main families: RGD, MLD and R/KTS.16 Based on their inhibitor action, the classification includes RGD disintegrins, which block the α8β1, α5β1, α8β1, αVβ1, αVβ3 and αIIbβ3 integrins.17 MLD, which block α4β1, α4β7, α3β1, α6β1, α7β1 and α9β1 integrins, disintegrins from the VGD and MGD families which block α5β1 integrins and KGD, which inhibit αIIbβ3 with a high selectivity degree; WGD has been reported as a powerful inhibitor of RGD-dependent integrins such as α5β1, αVβ3 and αIIbβ3. Moreover, the adhesive function of the disintegrins mentioned above is also blocked by the MVD disintegrins, while the disintegrins with KTS and RTS domains inhibit α1β1.17–19 Disintegrins are molecules of particular interest for many researchers due to their ability to inhibit cell interaction. The modulator properties of MLD and KTS disintegrins have been reported in researches about tumor angiogenesis and metastasis, immunosuppression of insulin-dependent diabetes mellitus (IDDM) and asthma, as well as in neurodegenerative in vitro studies and apoptosis.16
A group of emergent disintegrins is one from the recombinant chimerical (Table 1). In 2011, Minea et al., synthesized the first recombinant chimerical disintegrins, which they named vicrostatin (VCN), this was expressed in Escherichia coli (DE3) using recombinant technology. One of the advantages was that they were able to obtain quantities larger than 200mg of purified and active disintegrins per liter of culture.20
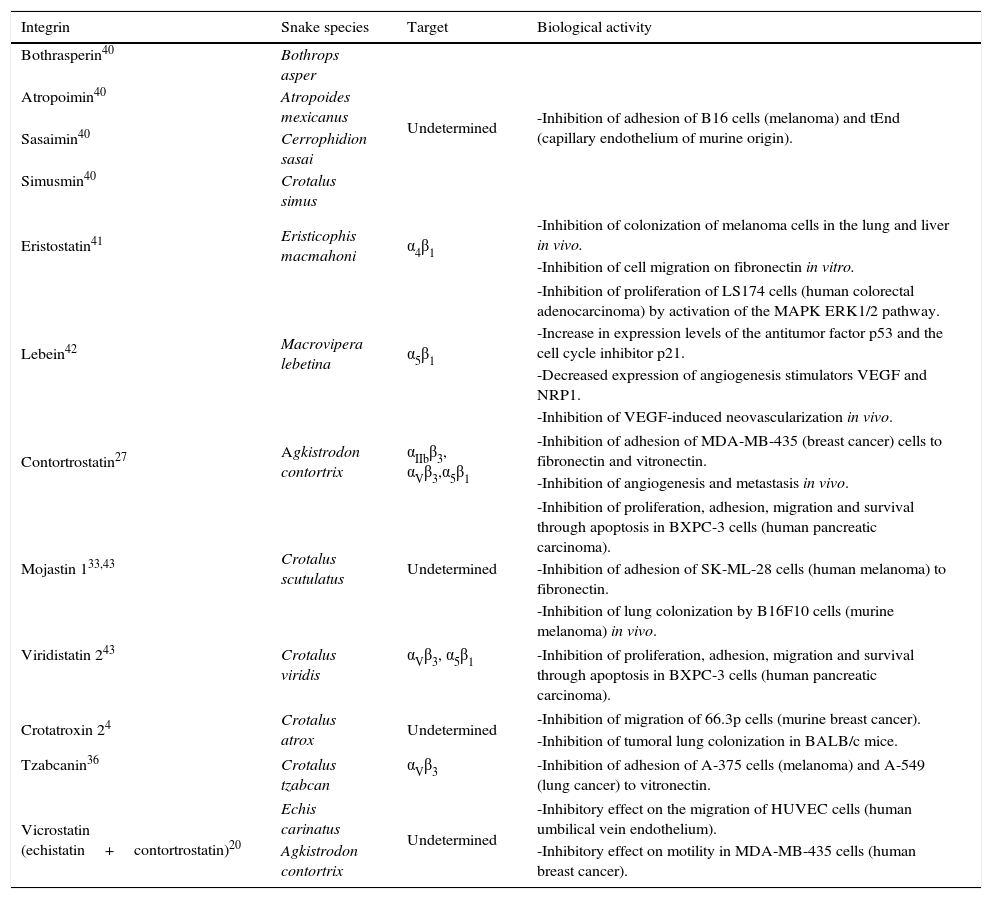
Integrin target and biological activities of main snake venom disintegrins.
| Integrin | Snake species | Target | Biological activity |
|---|---|---|---|
| Bothrasperin40 | Bothrops asper | Undetermined | -Inhibition of adhesion of B16 cells (melanoma) and tEnd (capillary endothelium of murine origin). |
| Atropoimin40 | Atropoides mexicanus | ||
| Sasaimin40 | Cerrophidion sasai | ||
| Simusmin40 | Crotalus simus | ||
| Eristostatin41 | Eristicophis macmahoni | α4β1 | -Inhibition of colonization of melanoma cells in the lung and liver in vivo. |
| -Inhibition of cell migration on fibronectin in vitro. | |||
| Lebein42 | Macrovipera lebetina | α5β1 | -Inhibition of proliferation of LS174 cells (human colorectal adenocarcinoma) by activation of the MAPK ERK1/2 pathway. |
| -Increase in expression levels of the antitumor factor p53 and the cell cycle inhibitor p21. | |||
| -Decreased expression of angiogenesis stimulators VEGF and NRP1. | |||
| -Inhibition of VEGF-induced neovascularization in vivo. | |||
| Contortrostatin27 | Agkistrodon contortrix | αIIbβ3, αVβ3,α5β1 | -Inhibition of adhesion of MDA-MB-435 (breast cancer) cells to fibronectin and vitronectin. |
| -Inhibition of angiogenesis and metastasis in vivo. | |||
| Mojastin 133,43 | Crotalus scutulatus | Undetermined | -Inhibition of proliferation, adhesion, migration and survival through apoptosis in BXPC-3 cells (human pancreatic carcinoma). |
| -Inhibition of adhesion of SK-ML-28 cells (human melanoma) to fibronectin. | |||
| -Inhibition of lung colonization by B16F10 cells (murine melanoma) in vivo. | |||
| Viridistatin 243 | Crotalus viridis | αVβ3, α5β1 | -Inhibition of proliferation, adhesion, migration and survival through apoptosis in BXPC-3 cells (human pancreatic carcinoma). |
| Crotatroxin 24 | Crotalus atrox | Undetermined | -Inhibition of migration of 66.3p cells (murine breast cancer). |
| -Inhibition of tumoral lung colonization in BALB/c mice. | |||
| Tzabcanin36 | Crotalus tzabcan | αVβ3 | -Inhibition of adhesion of A-375 cells (melanoma) and A-549 (lung cancer) to vitronectin. |
| Vicrostatin (echistatin+contortrostatin)20 | Echis carinatus | Undetermined | -Inhibitory effect on the migration of HUVEC cells (human umbilical vein endothelium). |
| Agkistrodon contortrix | -Inhibitory effect on motility in MDA-MB-435 cells (human breast cancer). | ||
Snake venom is without a doubt a great source of pharmacologically active compounds, and a good example of this are disintegrins, from which two anti-platelet agents were developed and are currently in the market: Tirofiban (Aggrastat®) developed from echistatin isolated from the venom of E. carinatus, and Eptifibatide (Integrillin®) developed from barbourin isolated from the venom of the pygmy rattle snake (Sistrurus miliarius barbouri).1 Tirofiban is a non-peptide synthetic inhibitor which acts on GpIIb/IIIa glycoproteins. A disadvantage of this antagonist is the fact that they lack specificity; thus, they inhibit functions from other RGD-dependent integrins. Nevertheless, with the substitution of lysine for arginine in the RGD domain, a molecule was produced, one with a high selectivity degree for GpIIb/IIIa,21 which led to the development of Eptifibatide, derived from the KGD domain of the barbourin, which acts as an antagonist of the fibrogenic platelet receptor and is used to reduce the risk of acute cardiac ischemic events in patients with unstable angina or in cases of heart attacks.22,23 During the development of phase III in the clinical trials of Eptifibatide, a significant decrease in coronary events in patients with low, medium and high-risk acute coronary syndromes was observed; the latter without a significant increase in hemorrhage. Eptifibatide also presented better pharmacokinetic characteristics, including short plasma half-life and a fast onset on their antiplatelet action.12
Another field of application for disintegrins is radiology, where disintegrins with radiation emission γ (99mTc, 125I), β particles (64Cu), positrons (18F) and infrared radiation are used as a tool for the visualization of tumor-dependent angiogenesis (αVβ3).24,25 As a result of the important role played by cell adhesion and signaling processes, selective integrin blockades have become desirable targets in order to treat pathological conditions where these receptors play a key role, such as acute coronary ischemia and thrombosis (integrin αIIbβ3), tumor metastasis, osteoporosis and rheumatoid arthritis (integrin αVβ3), bacterial infections and vascular diseases (integrin α5β1), inflammation, autoimmune diseases (integrins α4β1, α7β1, α9β1) and tumor angiogenesis (integrins α1β1, αVβ3).26
In fact, the utility of these molecules for blocking tumor neovascularization has been explored by the selective blocking of integrins α5β1, αVβ5 and αVβ3 with RGD disintegrins (Table 1) and the blocking of integrins α1β1 with KTS and RTS desintegrins.27,28
Since its discovery in the late 80s, approximately 100 disintegrins have been isolated and studied.29 The utility of these molecules for blocking tumor neovascularization has been explored by the selective blocking of integrins α5β1, αVβ5 and αVβ3 with disintegrins (Table 1) and the blocking of integrins α1β1 with KTS and RTS desintegrins.27,28 The utility of these molecules for blocking tumor neovascularization has been explored by the selective blocking of integrins α5β1, αVβ5 and αVβ3 with disintegrins (Table 1) and the blocking of integrins α1β1 with KTS and RTS disintegrins. The hypotheses based on the structural similarity between the integrins expressed in platelets (αIIbβ3) and those that are expressed in breast cancer cells (αVβ3) have led to important findings.27 One of the most studied disintegrins is contortrostatin (CN), isolated from the venom of the snake Agkistrodon contortrix. CN binds via integrins to MDA-MB-435 cells (breast cancer cells) and inhibits their adhesion to fibronectin and vitronectin, without affecting the adhesion of these cells to laminin and matrigel, the above without evidence of cytotoxicity. Inhibition was also observed in 74% of tumor growth generated by transfection of MDA-MB-435 cells into the mammary tissue of female BALB/c mice which were treated with CN via intra-tumor at a dose of 5mg/rat/day.27 CN produces an inhibitory effect (85%) on angiogenesis in rats treated with a dose of 10–30mg/day.27 CN also inhibits metastasis in 65% of BALB/c mice without the presence of adverse effects like internal subcutaneous hemorrhaging.27
As the i.t. administration is not extrapolated to the clinical setting, Swenson et al.30 encapsulated CN in liposomes (CNL) for intravenous (IV) administration, and observed a 94% inhibition of angiogenesis in vivo, in addition to a higher mean plasma half-life for CNL compared with unencapsulated CN. There was also a passive accumulation of disintegrin at the tumor site, and CNL did not produce platelet reactivity or trigger an immune response.30 Recently, other disintegrins (including pictistatin 1 and 2) have been obtained from the venom of Agkistrodon contortrix pictigaster. Of these two disintegrins, only pictistatin 1 showed inhibitory activity of platelet aggregation.31 The venoms of the serpents of the genera Crotalus and Sistrurus (rattlesnakes) are another important source of these molecules. Mojastin 1 and 2 are RGD disintegrins isolated from the venom of the Mojave rattlesnake Crotalus scutulatus, and have been shown to have platelet aggregation inhibitory activity in whole human blood.32 In its recombinant version, r-mojastin 1 developed by Lucena et al., has an inhibitory effect on the adhesion of SK-ML-28 (human melanoma) cells to fibronectin. Another variant, r-mojastine-GST, blocks the adhesion of SK-ML-28 and T24 cells to fibronectin at concentrations of 16 and 200nM, respectively. Lucena et al.,33 also evaluated the inhibitory activity of these recombinant disintegrins on cell migration in vitro by a woundhealing assay following a 24-h incubation with r-mojastin-1 and r-mojastine-GST at concentrations of 5μM, observing that both molecules inhibited the migration of SK-ML-28 cells at 32 and 40% respectively. On the other hand, neither of the two recombinant disintegrins inhibited the migration of T24 cells. The anti-metastasis activity of r-mojastin-1 and r-mojastin-GST was evaluated via the injection of B16F10 cells (murine melanoma) into BALB/c mice, and found that only r-mojastin-1 inhibited the colonization of tumor cells in the lungs by 51.5% at doses of 1000mg/kg.33 Another recombinant disintegrin is r-viridistatin 2 (Table 1) (GenBank ID: JQ071899), obtained from the prairie rattlesnake Crotalus viridis by Lucena et al.,34 which was evaluated in vitro in six cell lines (T24, HT-800, SK-MEL-28, CaCo-2, MDA-MB-231 and B16F10).34 The results showed that r-viridistatin 2 inhibited the adhesion of T24, SK-MEL-28, HT-1080 (Human fibrosarcoma), CaCo-2 (human colorectal adenocarcinoma) and MDA-MB-231 to fibronectin, laminin and type IV collagen in a dose-dependent manner. The highest degree of inhibition was observed in the presence of fibronectin, with T24 and SK-MEL-28 cells with inhibitory concentrations (IC 50) of 11 and 12nM, respectively. R-viridistatin 2 decreased the migration of T24 and SK-MEL-28 cells by 62 and 96%, respectively. R-viridistatin 2 also inhibited lung colonization by B16F10 cells in B71B/c mice by 71%. Crotatroxin 2, a RGD disintegrin isolated from the venom of the western diamondback rattlesnake Crotalus atrox by Galán et al.,4 showed inhibitory effects on platelet aggregation in whole human blood and significantly inhibited the migration of 66.3p (murine mammary carcinoma) cells to the lungs by 63% in BALB/c mice. Recently, Saviola et al.,35 isolated and characterized tzabcanin from the venom of the Mayan rattlesnake Crotalus tzabcan.35 The results showed that tzabcanin inhibited the adhesion of A-375 cells (human melanoma) and A-549 cells (human lung adenocarcinoma) to vitronectin in a dose-dependent manner. In addition, by competitive binding assays and flow cytometric analysis, the αvβ3 integrin was identified as the tzabcanin binding site (Table 1).36
Studies on disintegrins of Mexican snakesMexico has great potential for the discovery of new disintegrins, as it is the second largest in the world in diversity of reptiles and amphibians (herpetofauna). In Mexico, snakes of the Viperidae family are represented by nine genera (Agkistrodon, Atropoides, Bothriechis, Bothrops, Cerrophidion, Crotalus, Mixcoatlus, Ophryacus, Porthidium and Sistrurus) and 63 species, 34 of which are endemic, i.e. only found in Mexico.37 However, despite the great potential that this represents with respect to the study of disintegrins and their biological activity, the research related to these molecules in our country is a little-explored field. To date, disintegrins of the venom of only two endemic snake species have been described. One of them is basilisin, isolated from the venom of the West Coast rattlesnake Crotalus basiliscus by Scarborough et al. (1993). This disintegrin binds selectively to integrins α5β1 (fibronectin receptor) and αvβ3 (vitronectin receptor), and has an inhibitory effect on platelet aggregation and adhesion of M-21 cells (melanoma) to vitronectin.38 On the other hand, Borja et al. (2016) isolated morulustatin from the venom of the Tamaulipan rock rattlesnake Crotalus morulus, for which inhibitory activity against platelet aggregation was reported.39
ConclusionsIt has been proven that isolated disintegrins from different species of snakes have the ability to inhibit biological processes such as platelet activation, adhesion, cellular migration and survival. In the case of both disintegrins (basilisin and morulustatin) obtained from the venoms of snakes which are endemic to our country, it is important to mention that these snakes represent just 5.8% of Mexican species. Thus, the discovery, identification and evaluation of the pharmacological activity of disintegrins present in the venom of Mexican species which have not been studied yet may lead to the development of new therapeutic agents with the potential to improve the treatment of diseases such as cancer, (tumor angiogenesis, metastasis), inflammatory processes, osteoporosis and bacterial infections.
Conflict of interestThe authors have no conflicts of interest to declare.