Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, representing 25% of childhood cancers, with a peak prevalence between 2 and 9 years. Conversions of the leukemic cell lineage throughout the duration of the disease is a rare manifestation, accounting for 6–9% of relapsed cases and being more frequently observed in pediatric patients. We present a case of a patient with a lineage switch from lymphoblastic leukemia to myeloid leukemia.
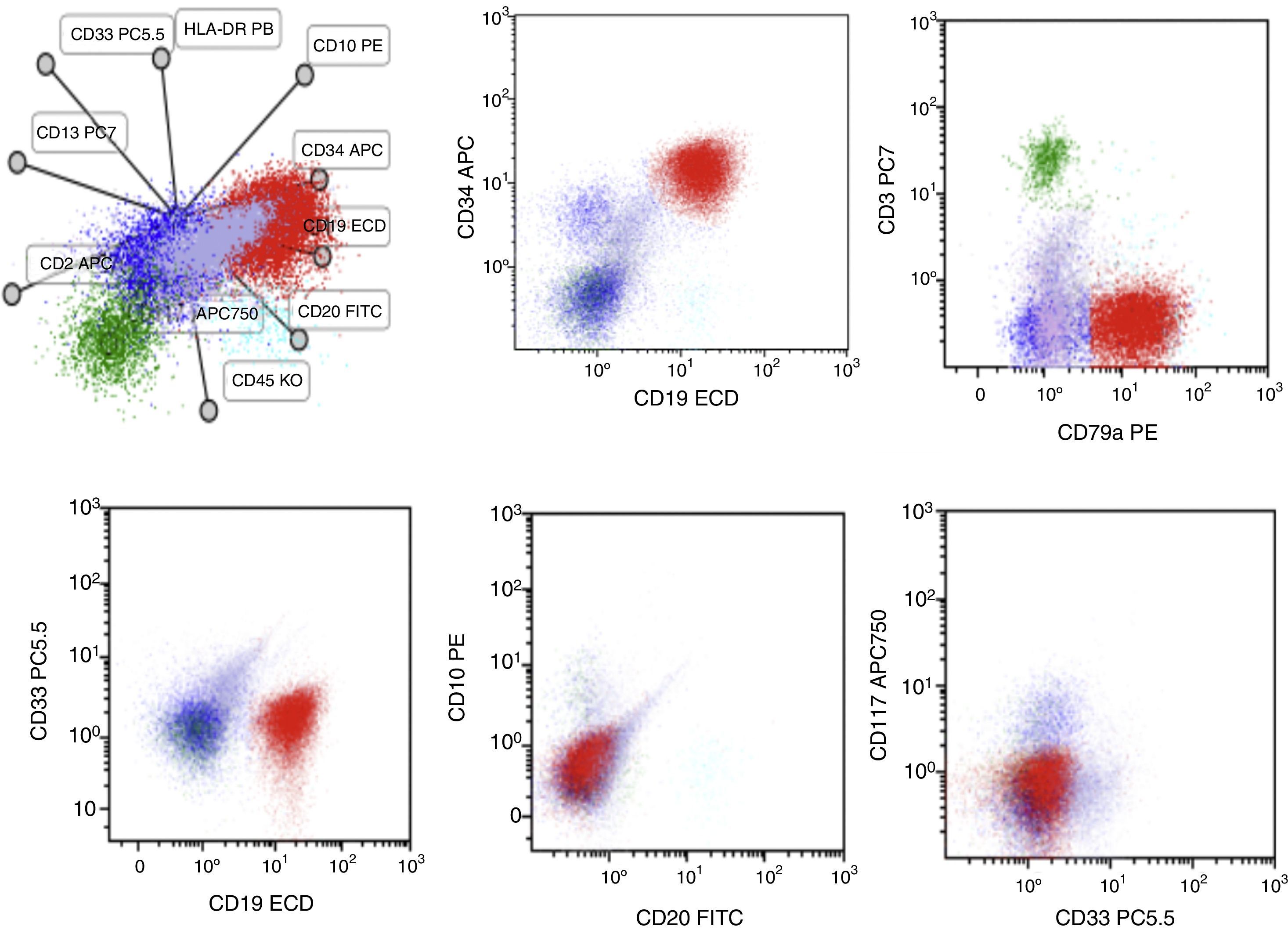
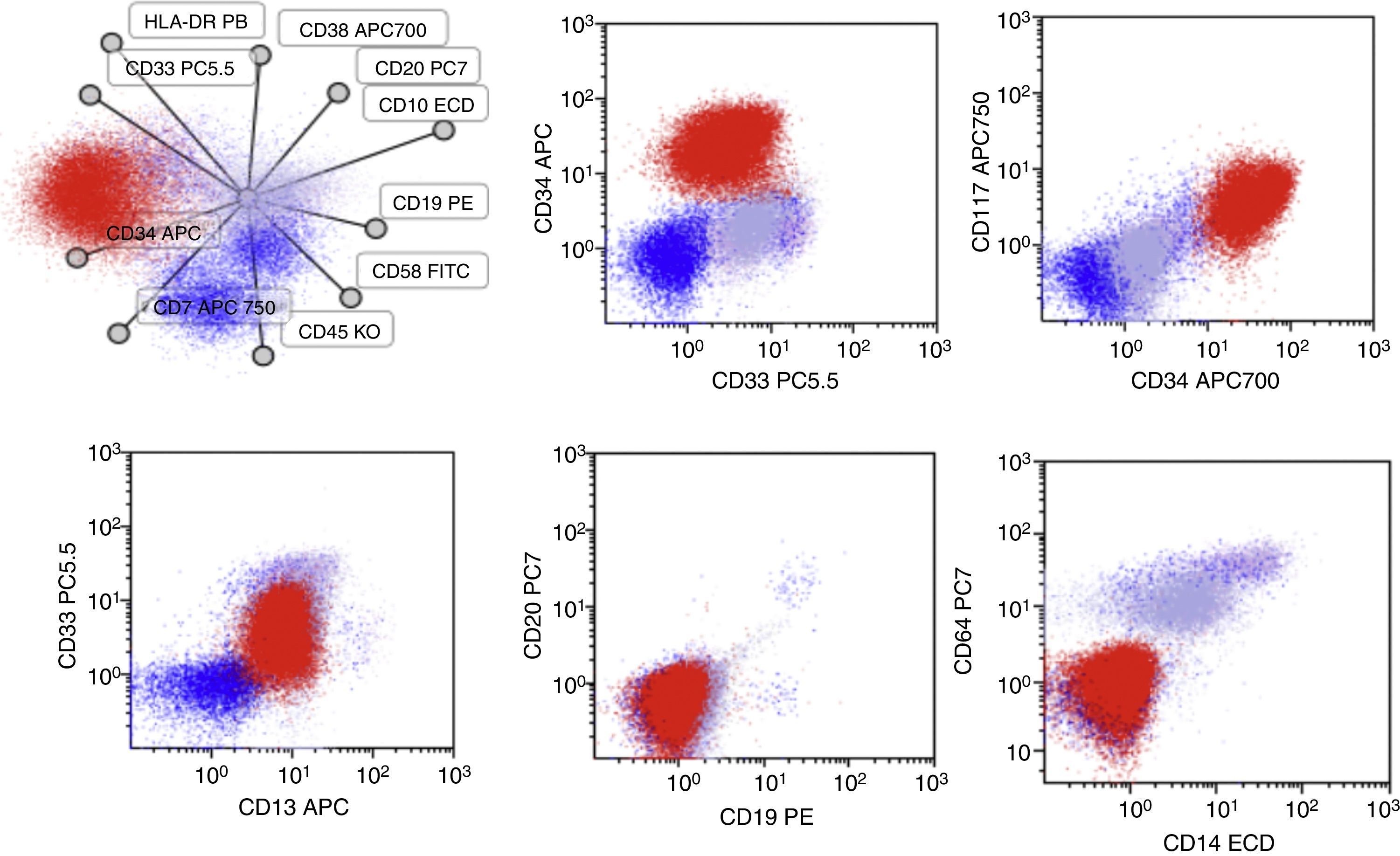
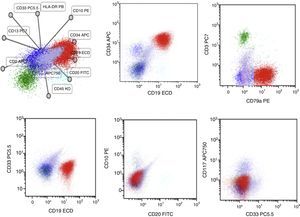
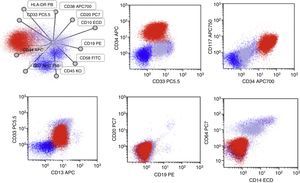
Case presentationA 60-year-old male was seen due to pancytopenia, weight loss and weakness. Initial laboratory work-up was performed. Bone and marrow aspirate flow cytometric analysis disclosed pre-B lymphoblastic acute leukemia BCR ABL (−), 46 XY, hyperdiploid, CD20(−), CD 10 (−), CD19 (+), CD33 (−), CD34 (+), CD38 (+), CD79a (+), TdT (+), IgS(−), CD45 (+/−), HLA-DR (+), MLL (−), FLT3 (−), TEL AML (−). He was treated with a pediatric-inspired TOTAL XI schedule. Sixty days afterward, induction blasts appeared in the peripheral blood, but immunophenotyping was not conclusive for MRD+ status. One week later, he presented blasts in the peripheral blood compatible with acute myeloid leukemia. CD7 (+−), CD13 (+), CD14 (−), CD15(−), CD33(+), CD34(+), CD38(+), CD45(+−), CD64(−), CD117(+), HLA-DR heterogenous. BCR-ABL, PML-RAR alfa, and FLT-3 were repeated in peripheral blood when AML developed and was negative. The patient started subcutaneous cytarabine and was alive 90 days after initial diagnosis with active AML leukemia.
ConclusionThere is a small number of reports of lineage conversion in the literature, probably because immunophenotyping is performed at diagnosis without a follow up.
Approximately 5000 new cases of acute lymphoblastic leukemia (ALL) are diagnosed each year in the United States, more than half of these in children. ALL represents the most common pediatric malignancy, accounting for at least 25% of childhood cancer. The peak prevalence of ALL is between the ages of 2 and 9 years. There is a slight male predominance, and Caucasians have a twofold increased risk compared to African Americans. Lineage switch accounts for approximately 6–9% of relapsed cases, and is more often observed in childhood patients, for whom an appropriate standard treatment is not available.1
We report a case of an adult with lineage switch from acute lymphoblastic leukemia to acute myeloid leukemia (AML).
Case reportA 60-year-old male was seen at our Hematology Center due to pancytopenia, weight loss and weakness. Upon physical examination, he was pale, referred asthenia, bone pain, and hepatosplenomegaly. The rest of the examination showed no other abnormalities. Initial laboratory work was performed. Bone and marrow aspirate flow cytometric analysis disclosed pre-B lymphoblastic acute leukemia BCR ABL (−), 46 XY, hyperdiploid, CD20(−), CD 10 (−), CD19 (+), CD33 (−), CD34 (+), CD38 (+), CD79a (+), TdT (+), IgS(−), CD45 (+/−), HLA−DR (+), MLL (−), FLT3 (−) and TEL AML (−) (Fig. 1). Cerebrospinal fluid cytology showed no blasts. He was treated with a modification of the pediatric-inspired TOTAL XI schedule. During the induction phase, he presented significant myelotoxicity and significant adverse reactions from the chemotherapy and early response to the treatment. During this phase he developed an anal abscess that required urgent surgery. The dose of the chemotherapy was reduced to 50% but toxicity persisted. Sixty days after starting induction to remission treatment, blasts appeared in the peripheral blood, but the immunophenotype was not conclusive for an MRD positive status. The minimal residual disease in the bone marrow revealed: CD2 (−), CD3 (−), CD3c (−), CD4(−), CD5(−), CD7 (+−), CD8(−), CD34 (+), CD38 (+), CD45 (+−), HLA DR (+−). He continued the induction chemotherapy. He developed acute appendicitis that had to be removed in the operating room.
One week later, he presented blasts in the peripheral blood compatible with acute myeloid leukemia. CD7 (+−), CD13 (+), CD14 (−), CD15 (−), CD33 (+), CD34 (+), CD38 (+), CD45 (+−), CD64 (−), CD117 (+), and HLA-DR heterogenous (Fig. 2). Molecular markers such as BCR-ABL, PML-RAR alfa, and FLT-3 were repeated in peripheral blood when AML developed, and were negative. The patient started subcutaneous cytarabine and he is currently alive, 90 days after the initial diagnosis, with active AML leukemia.
Salient features of the immunophenotype at 2016-November-25. Blasts (Red) now co-express CD13/CD33/CD34 and CD117 while they do not express the CD19 antigen. In this test, a expression of CD10 and CD20 was not detected, neither was the expression of myeloid antigens as CD14, CD15 and CD64.
Conversions of the leukemic cell lineage (lymphoid or myeloid) during the course of the disease have only rarely been reported in the literature. Usually, the phenotype of the blasts of patients with relapsed acute leukemia (AL) adheres to the original lineage, despite minor changes in some markers. However, complete lineage switches do occur, and it is relevant to determine whether the blast cells arise from the same leukemic clone or whether they should be considered as a therapy-related malignancy.2 Therapy-related leukemias generally take place after 2–5 years, during the follow-up of de novo AL, and are most frequently of myeloid lineage, having a poor prognosis.3 These leukemias can be diagnosed by establishing a clearly distinct cytogenetic partner from the original disease.4
There are several hypotheses for explaining a true lineage switch within a leukemic clone, but the actual mechanism involved remains unclear.5 Recent studies suggest that lineage commitment of plastic hematopoietic progenitors may be multidirectional and reversible upon specific signals provided by both intrinsic and environmental cues.6 Aberrant functions of specific fusion genes and surrounding microenvironmental cues might guide leukemia phenotype conversion through modulation of plasticity within the leukemia's initiating cells. Moreover, clinical features could play important roles in establishing environmental scenarios proper for cell conversion events.6
Another possible explanation could be the “selection” of a pre-existent chemotherapy-insensitive minor population of cells of a different lineage among the predominant population of leukemic blasts at diagnosis (biphenotypic or bilineal leukemias) with the consequent emergence of the resistant “sub-clone” expressing a different repertory of antigens.7 Another possibility might be the reprogramming of a malignant pluripotent stem cell, or rather, taking into account the most frequently involved cell types in these conversions, of a common bipotent B-myeloid precursor.8,9 Finally, the myeloid form could develop from an already-committed B-cell progenitor, either directly through trans-differentiation or indirectly through dedifferentiation and redifferentiation.10
The correct diagnosis of these infrequent lineage conversion cases depends on the documentation that the same cytogenetic or molecular alterations remain despite the phenotypic changes, to distinguish them from secondary leukemias.2,4 There are few reports of lineage conversion cases in the literature, probably because immunophenotyping is generally performed at diagnosis but not repeated along with the treatment, and thus, its rate might be underestimated.
Rossi, et al. found a significant correlation between the presence of MLL fusion genes and the development of conversion. The occurrence of lineage switches with high frequency in MLL-positive AL compared to other leukemias is thus another distinctive trait that also suggests that MLL-AL originates from an immature precursor.11 This fact, together with the different biological characteristics, clinical features, responses to treatment and outcomes, support the proposal that this AL subtype should be considered as a different entity.12 Moreover, blasts from infant MLL-positive forms of ALL typically express a unique marker profile: CD10-negative with co-expression of myeloid antigens (CDw65 and CD15), also suggesting that they are derived from a precursor cell with a shared Bcp lympho-myeloid differentiation potential.13
The fact that the conversion took place between the B lymphoid and myeloid lineages emphasizes the idea that the target of malignant transformation is a common B-myeloid precursor, already reported as a minor subpopulation among normal bone marrow cells.9,14,15
The mostly single-case pediatric reports in the literature also support this notion, describing switches from Bcp to myelo-monocytic lineages.5,7,10,16–24 Conversions from AML (or bilineal) to ALL are even more rare,25–30 and also involve mostly Bcp and myelo-monocytic lineages.
However, there are also a few communications involving T and myeloid lineages in adults.31–33 A recent report by Gerr et al.24 about ambiguous lineage leukemias in children included eight cases of lineage conversion. However, these cases were not described in detail. The authors pointed out that seven switched from lymphoid Bcp to myeloid, while one switched from a T to a myeloid lineage. They also pointed out that three of them disclosed trisomy 8 and only one case had alterations involving the MLL gene.24
Several hypotheses have been proposed for explaining lineage conversions. Gene profiling studies of samples from cases of MLL positive AL revealed patterns halfway between AML and ALL, also consistent with early hematopoietic progenitors.12 In addition, as stated above, the presence of early bipotential B-macrophage progenitors in normal bone marrow has been demonstrated, raising the possibility that the lineage switching event could be a consequence of the leukemogenic mutation targeting this early bi-potential progenitor cell.8,9,34 In addition, regarding cellular ontogeny, the idea that lineage fate decisions are binary and irreversible is becoming increasingly questionable. Indeed, it has been also reported that mature B cell to macrophage conversion occurs under normal circumstances.15,35,36 It could also be that, being that the target is a stem cell (irrespective of the cell lineage), the cellular environment,37 growth factors, or an inherent instability of the leukemic clone might allow lineage inter-conversions.38 It has been recently demonstrated that MLL fusion proteins can also induce leukemia in already committed progenitors by activation of stem cell programs.39 Finally, the therapy induced “selection” of a minor subset from a bilineal population of blasts already present at diagnosis is also another possible mechanism. Indeed, the hypothesis of selection of a minor pre-existing resistant subset has been demonstrated even for relapses within the same lineage in pediatric leukemia.40
Treatment recommendations for lineage switch leukemia have been proposed in a recent publication: these patients should be initially treated according to phenotype, and then the therapy should be changed to the respective other treatment following the lineage conversion.24
Even when chemotherapy is administered based on immunophenotype findings, this strategy has not overcome the dismal prognosis of these rare entities.
Conflict of interestThe authors declare that they have no conflicts of interest