Infections in cirrhotic patients caused by multidrug-resistant bacteria are currently increasing and are associated with greater morbidity and mortality.
ObjectivesTo assess the epidemiology, risk factors and prognoses of infections caused by multidrug-resistant bacterial infections in cirrhotic patients.
Patients and methodsRetrospective study on patients with liver cirrhosis who developed an infection during hospitalisations between July 2014 and August 2016 at our centre (Hospital Universitari i Politècnic La Fe, Valencia, Spain).
ResultsUrinary tract infection (30.2%) and spontaneous bacterial peritonitis (22.1%) were the most common infections. A total of 102 microbiological isolates were analysed: 50% in community-acquired infections, 36% in isolates associated with healthcare infections and 14% in nosocomial infections. Escherichia coli was the main aetiology (29.4%). The overall multiresistance rate was 28.4%. The univariate analysis showed that infection caused by multidrug-resistant bacteria (28.4%) was associated with nosocomial infection compared to those associated with healthcare (OR 5.46; 95% CI: 1.22–24.43; p=.039) and healthcare-associated infections (compared to community-acquired infections, OR 3.39; 95% CI: 1.09–10.54; p=.048), use of antibiotics (OR 4.37; 95% CI: 1.59–11.99; p=.005), hospital admission in the previous 90 days (OR 3.18; 95% CI: 1.19–8.47; p=.018), active cancer (OR 2.93; 95% CI: 1.08–7.99; p=.038), and use of prophylactic norfloxacin (OR 3; 95% CI: 1.02–8.79; p=.012). Moreover, it was associated with a higher rate of sepsis (OR 3.13; 95% CI: 1.18–8.32; p=.025). The failure of initial treatment was related to greater development of acute renal failure (p<.001), sepsis (p=.012), septic shock (p=.002), ICU admission (p<.001) and mortality (p<.001).
ConclusionThe rate of multidrug-resistant bacteria infections in our centre is comparable to that of other European centres with similar characteristics. The results obtained make it recommendable to implement the antibiotic treatment guidelines in current clinical practice guidelines, limiting the use of carbapenems to nosocomial infections and healthcare-associated infections with other risk factors of multidrug resistance or signs of severe sepsis. Early and adequate empirical treatment correlates with a better prognosis.
Las infecciones por bacterias multirresistentes en pacientes cirróticos se encuentran en aumento y se asocian a una mayor morbimortalidad.
ObjetivosEstudiar la epidemiología y los factores de riesgo y pronósticos de las infecciones por gérmenes multirresistentes en pacientes cirróticos.
Pacientes y métodosEstudio retrospectivo en el que se analizaron a pacientes con cirrosis hepática que presentaron una infección al ingreso o durante la hospitalización entre julio del 2014 y agosto del 2016 en el Hospital Universitario y Politécnico La Fe (Valencia, España).
ResultadosLa infección urinaria (30,2%) y la peritonitis bacteriana espontánea (22,1%) fueron las infecciones más frecuentes. Se analizaron 102 aislamientos microbiológicos: el 50% en infecciones comunitarias, el 36% en asociadas a los cuidados de la salud y el 14% en nosocomiales. Escherichia coli fue el germen más frecuentemente aislado (29,4%). La tasa de multirresistencia fue del 28,4%. El análisis univariante mostró que la infección por gérmenes multirresistentes (28,4%) se asoció a infección nosocomial respecto a las asociadas a los cuidados de la salud (OR 5,46; IC del 95%: 1,22–24,43; p=0,039) y asociada a los cuidados de la salud (respecto a las comunitarias OR 3,39; IC del 95%: 1,09-10,54; p=0,048), uso de antibióticos (OR 4,37; IC del 95%: 1,59-11,99; p=0,005) e ingreso hospitalario en los últimos 90 días (OR 3,18; IC del 95%: 1,19-8,47; p=0,018), neoplasia activa (OR 2,93; IC del 95%: 1,08-7,99; p=0,038) y toma de norfloxacino profiláctico (OR 3; IC del 95%: 1,02-8,79; p=0,012). Además, se asoció a mayor frecuencia de sepsis (OR 3,13; IC del 95% 1,18-8,32; p=0,025). El fracaso del tratamiento inicial se relacionó con mayor desarrollo de insuficiencia renal aguda (p<0,001), sepsis (p=0,012), shock séptico (p=0,002), ingreso en UCI (p<0,001) y mortalidad (p<0,001).
ConclusiónLa tasa de infecciones por gérmenes multirresistentes en nuestro centro es comparable con la de otros centros europeos de características similares. Los resultados obtenidos hacen recomendable la adopción de las pautas de tratamiento antibiótico contempladas en las guías de práctica clínica actuales, limitando el uso de carbapenemes a las infecciones nosocomiales y a las asociadas a los cuidados de salud con otros factores de riesgo de multirresistencia o con signos de gravedad. Un tratamiento empírico adecuado de forma precoz se correlaciona con un mejor pronóstico.
Bacterial infections in patients with cirrhosis are one of the most common causes of acute decompensation and acute-on-chronic liver failure.1,2 Its prevalence upon admission or during a hospital stay is 25–40%, almost five times higher than in the general population.3–5 What is more, complications and mortality are higher than in patients without cirrhosis,6 with a greater tendency to develop sepsis and septic shock, particularly in intensive care patients.7
This can be attributed to the onset of various immune system abnormalities that have recently been grouped together under the umbrella term “cirrhosis-associated immune dysfunction” (CAID),8 which affect the innate and adaptive response. This all entails an increased risk of infection, as well as an abnormal immune response that increases predisposition to systemic inflammatory response syndrome (SIRS) above the predisposition of the general population.9,10
The most common infections are spontaneous bacterial peritonitis (SBP) and urinary tract infections (UTIs). Classic aetiological agents include (Escherichia coli, Klebsiella pneumoniae, etc.) and other Gram-negative bacilli, which is why third-generation cephalosporins (TGCs) have traditionally been considered the empirical treatment of choice.
However, increased resistance to cephalosporins,11,12 the rise of infections by Gram-positive bacteria (Enterococcus spp., Staphylococcus, etc.) and multidrug-resistant bacteria3,5,11,13,14 have necessitated a paradigm shift in the treatment of infected cirrhotic patients.
The prevalence of multidrug-resistant (MDR) bacteria varies considerably from country to country and even between one hospital and another. There is agreement among the most recent publications5,11,13,15 that the three pillars that should guide the choice of empirical antibiotic therapy are the local epidemiology, the severity of infection and any possible risk factors for developing MDR bacterial infection.
The primary objective of this study was to evaluate the prevalence of MDR bacterial infections in our tertiary hospital in Spain. The secondary objectives were to analyse the risk factors and complications associated with the onset of MDR bacterial infections, and the correlation between prognosis and the effectiveness of the empirical antibiotic therapy.
Patients and methodsStudy designThis descriptive, retrospective, observational study enrolled patients over the age of 18 years with a confirmed diagnosis of liver cirrhosis and at least one proven microbiological isolate during their hospital stay (either upon admission or during hospitalisation), admitted to Hospital Universitario y Politécnico La Fe in Valencia (Spain) between 1 July 2014 and 31 August 2016. Solid-organ transplant recipients or patients who had received haematopoietic tissue, as well as patients infected with human immunodeficiency virus (HIV) or any other type of immunodeficiency were excluded.
Patient data were collected from diagnosis codes on the discharge reports of the electronic medical record and were cross-referenced with the information obtained from the microbiology laboratory.
Study variablesThe following variables were analysed:
- –
Microbiological data: germ, type of bacteria (Gram-positive or negative), multidrug-resistance criteria.
- –
Risk factors: gender, age, cirrhosis aetiology, chronic kidney failure, diabetes mellitus, active cancer, active alcoholism, severity criteria upon admission (creatinine, bilirubin, International Normalised Ratio [INR], Model for End-stage Liver Disease [MELD] score), focus of infection, site of acquisition, hospitalisation in the last 90 days, antibiotics taken in the last 90 days, antibiotic prophylaxis with norfloxacin, treatment with rifaximin, effectiveness of empirical treatment, stay in the Intensive Care Unit (ICU) and isolation of MDR bacteria in the last six months.
- –
Type of infection: SBP, UTI, pneumonia, spontaneous bacteraemia in patients with cirrhosis, cellulitis, endocarditis, spontaneous bacterial empyema and catheter sepsis.
- –
Measurement of clinical impact: admission to the ICU and tracheal intubation, development of acute kidney failure, sepsis, septic shock and mortality.
During the study period, patients with community-acquired SBP or UTI received treatment with TGCs. In SBP with a poor clinical course, treatment was empirically switched to piperacillin–tazobactam or carbapenems. Linezolid was normally used as a third-line treatment in combination with one of the aforementioned drugs. Hospital-acquired SBP or UTI were treated with piperacillin–tazobactam or carbapenems in the first line, adding linezolid in the event of a lack of response.
Community-acquired pneumonia was treated with TGCs in combination with azithromycin or with levofloxacin. Hospital-acquired pneumonia was generally treated with carbapenems and levofloxacin.
Patients with community-acquired cellulitis received treatment with amoxicillin/clavulanic acid, while those with hospital acquired cellulitis received piperacillin–tazobactam. Our hospital did not have a defined antibiotic therapy policy for healthcare-associated infections.
Multidrug-resistance criteriaBacteria resistant to three or more families of antibiotics were deemed to be multidrug-resistant. Intrinsic resistance to different groups of antimicrobials was not taken into account for this definition.16
The isolated bacteria considered to be multidrug-resistant were: extended-spectrum beta-lactamase (ESBL), AmpC beta-lactamase or carbapenemase producing enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis), Pseudomonas aeruginosa, vancomycin-sensitive Enterococcus faecium, Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus (MRSA) and Stenotrophomonas maltophilia.
Focus of infection classificationSBP: defined as the finding of more than 250 polymorphonuclear leukocytes (PMN) per mm3 in ascitic fluid, irrespective of the culture results, without a secondary cause. The infection was considered resolved when the PMN count fell below 250 per mm3.
UTI: defined as urinary symptoms accompanied by pathological urinary sediment (more than 10 leukocytes per field) or a positive urine culture. The infection was considered resolved if the UTI clinically improved or following a negative urine culture that was previously positive.
Pneumonia: signs and symptoms suggestive of respiratory infection accompanied by compatible findings in imaging tests. Pneumonia that showed clinical improvement together with the disappearance of the radiological findings was considered to be resolved.
Others: spontaneous bacteraemia in patients with cirrhosis, cellulitis, endocarditis, spontaneous pleural empyema and catheter sepsis, defined according to traditional criteria.
Classification of infection by site of acquisition17Community-acquired infections: infections present at admission or onset within the first 48 hours of admission.
Healthcare-associated infections: infections with onset within the first 48 hours of admission in patients who had recently been admitted to hospital (at least 48 hours in the last 90 days), treated at a haemodialysis centre in the last 30 days or long-term residents of a care home or who require special medical care.
Hospital-acquired infections: infections that manifest at least 48 hours after admission.
SIRS, sepsis and septic shock18SIRS: if two or more of the following criteria are met:
- –
Body temperature >38°C or <36°C.
- –
Tachycardia >90 beats per minute
- –
Tachypnoea >20 breaths per minute or hypocapnia <32mmHg.
- –
Leukocytes >12,000/μl or <4000/μl or immature forms >10%.
Sepsis: defined as SIRS caused by an underlying infection.
Septic shock: sepsis associated with circulatory failure (systolic blood pressure below 90mmHg or a fall of more than 40mmHg from baseline, or average blood pressure below 60mmHg after appropriate fluid resuscitation) not explainable by other causes.
Acute kidney failure19Increased serum creatinine ≥0.3mg/dl in 48 hours or >50% increase in baseline creatinine in the last seven days.
Effectiveness of empirical treatmentAn empirical treatment failure is defined as switching the initial antibiotic for another. The following distinctions were made:
Failure due to resistance: if the antibiogram showed resistance to the first antibiotic prescribed. It was also assessed whether resistance was intrinsic (specific to the members of the species) or acquired (variable between different strains).
Lack of initial response: if the medication was switched due to a poor clinical course or blood panel despite the subsequent antibiogram showing sensitivity to the initial antibiotic.
The addition of another antibiotic was only considered to be a treatment failure if the antibiogram showed resistance to the initial antibiotic prescribed.
Data analysisA descriptive analysis of the baseline characteristics of the population and the other study variables was performed. The risk factors associated with the onset of MDR bacterial infections and their clinical impact were also evaluated. A bivariate analysis using Student's t test, the Mann–Whitney U test and ANOVA for quantitative variables, and the Pearson's chi-squared test for qualitative variables, was performed to establish the relationship between each individual variable and outcome. Results with a p-value less than 0.05 were considered statistically significant. The software SPSS Statistics 23.0® was used for data collection and analysis, performed by the Biomedical Research Network Centre in the field of Liver and Gastrointestinal Diseases (CIBEREHD), Instituto de Salud Carlos III, Madrid, Spain.
Ethical considerationsThe study protocol adhered to the ethical principles of the 1975 Declaration of Helsinki. The study design did not require written informed consent. The identity of the patients was protected and anonymised at all times, encoded by alphanumeric code during the data collection phase. All files were digitised and password-protected, thereby prohibiting access to non-study personnel and complying with the confidentiality regulations. The study was approved by the Independent Ethics Committee of the Hospital Universitario y Politécnico La Fe's Instituto de Investigación Sanitaria [Health Research Institute] (register number: 2017/0210).
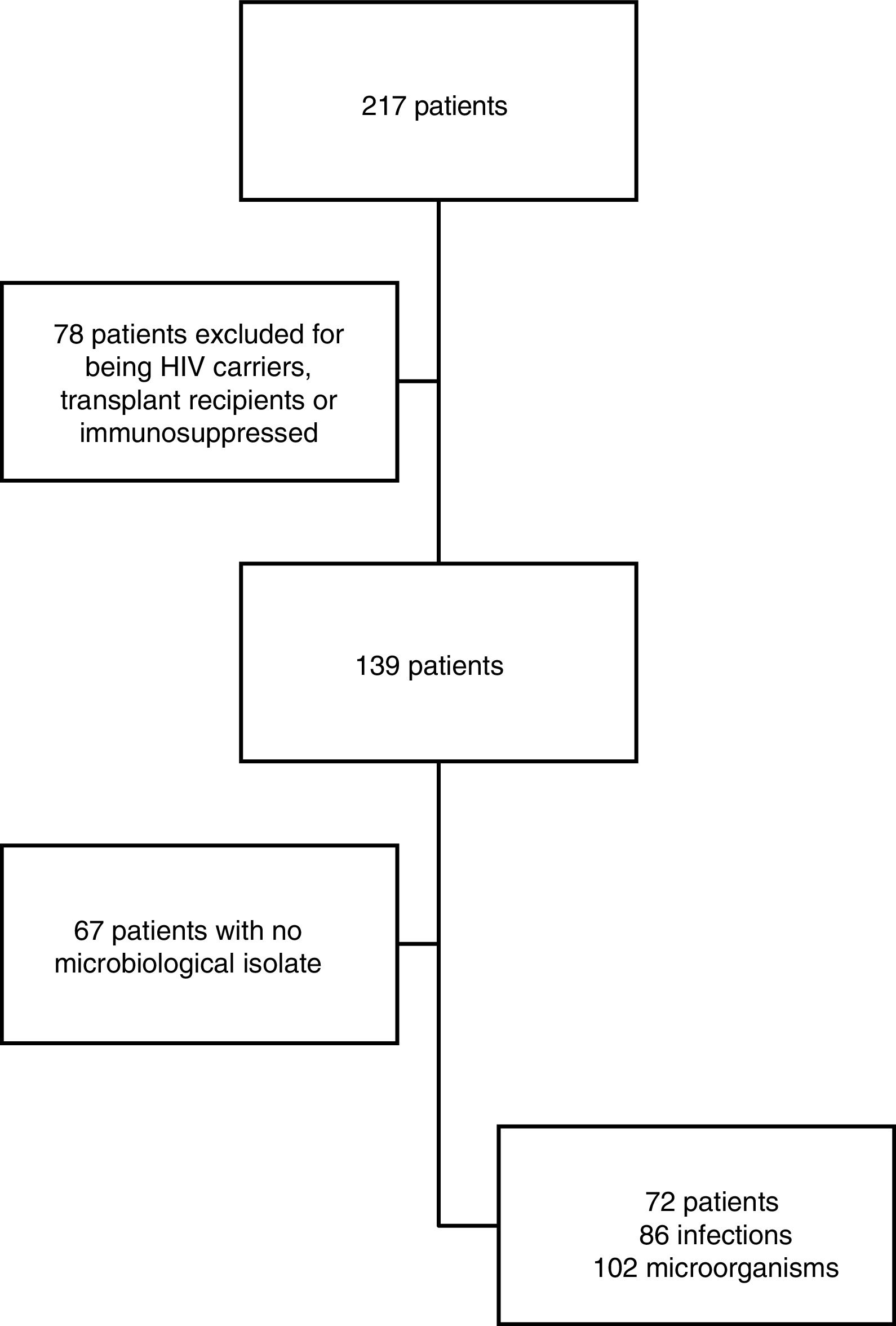
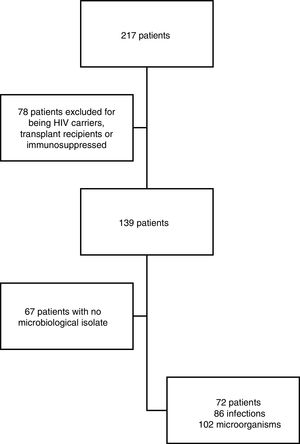
ResultsStudy populationDuring the study period (1 July 2014 to 31 August 2016), 217 patients were initially included, 78 of whom were excluded due to exhibiting some form of immunosuppression and 67 due to a lack of a positive culture. Ultimately, 72 patients were analysed, isolating 102 bacteria in 86 infections (Fig. 1). Every infection identified during hospitalisation was considered to be a separate episode.
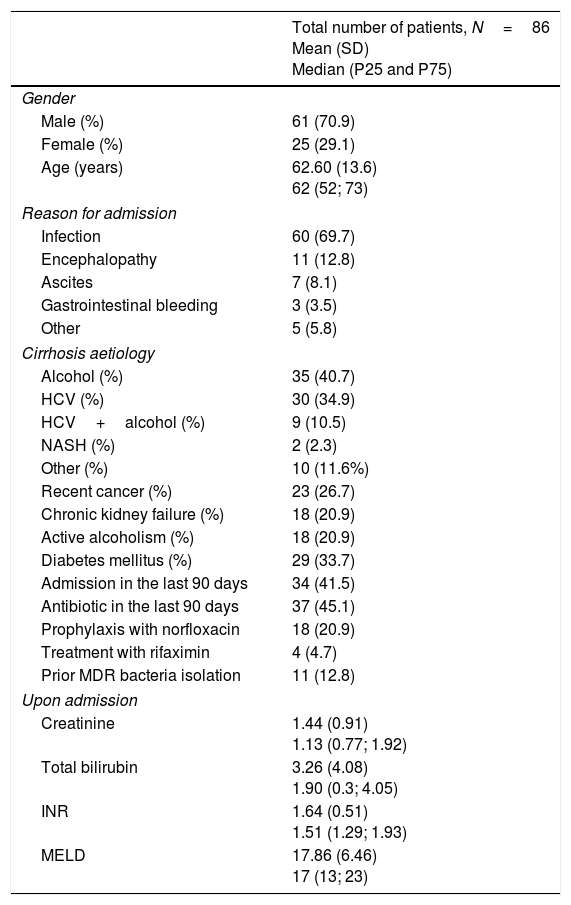
Baseline characteristicsData were obtained from 86 cases of infection (Table 1), 70.9% of which were from men (61 in total). The median age was 62 years, with a range of 63 years (26–89 years). The most common cause of cirrhosis was alcoholism, followed by hepatitis C virus (HCV), which individually or collectively affected 74 patients (86.1%).
Baseline characteristics of the population.
| Total number of patients, N=86 Mean (SD) Median (P25 and P75) | |
|---|---|
| Gender | |
| Male (%) | 61 (70.9) |
| Female (%) | 25 (29.1) |
| Age (years) | 62.60 (13.6) 62 (52; 73) |
| Reason for admission | |
| Infection | 60 (69.7) |
| Encephalopathy | 11 (12.8) |
| Ascites | 7 (8.1) |
| Gastrointestinal bleeding | 3 (3.5) |
| Other | 5 (5.8) |
| Cirrhosis aetiology | |
| Alcohol (%) | 35 (40.7) |
| HCV (%) | 30 (34.9) |
| HCV+alcohol (%) | 9 (10.5) |
| NASH (%) | 2 (2.3) |
| Other (%) | 10 (11.6%) |
| Recent cancer (%) | 23 (26.7) |
| Chronic kidney failure (%) | 18 (20.9) |
| Active alcoholism (%) | 18 (20.9) |
| Diabetes mellitus (%) | 29 (33.7) |
| Admission in the last 90 days | 34 (41.5) |
| Antibiotic in the last 90 days | 37 (45.1) |
| Prophylaxis with norfloxacin | 18 (20.9) |
| Treatment with rifaximin | 4 (4.7) |
| Prior MDR bacteria isolation | 11 (12.8) |
| Upon admission | |
| Creatinine | 1.44 (0.91) 1.13 (0.77; 1.92) |
| Total bilirubin | 3.26 (4.08) 1.90 (0.3; 4.05) |
| INR | 1.64 (0.51) 1.51 (1.29; 1.93) |
| MELD | 17.86 (6.46) 17 (13; 23) |
HCV: hepatitis C virus; INR: International Normalised Ratio; MELD: Model End-stage Liver Disease; NASH: nonalcoholic steatohepatitis.
41.5% of patients had been admitted within the last 90 days and 45.1% (37 patients) had taken an antibiotic (including norfloxacin for SBP prophylaxis in 18 cases) during the previous 90 days.
Upon admission, the mean creatinine level was 1.44 (SD=0.91) and the mean INR was 1.64 (SD=0.51), median bilirubin was 1.90 (P25=0.3 and P75=4.05) and the MELD score was 17 (P25=13 and P75=23).
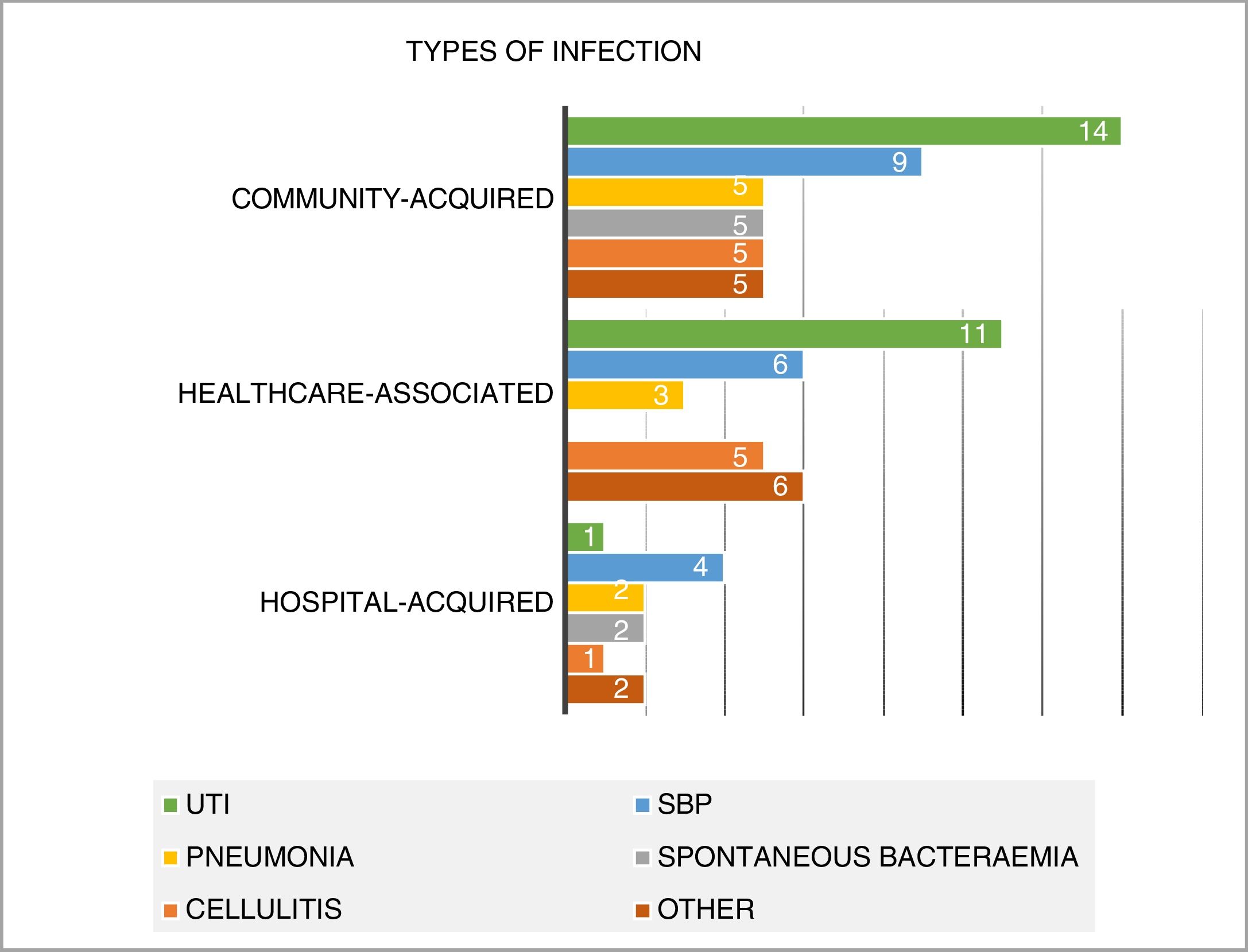
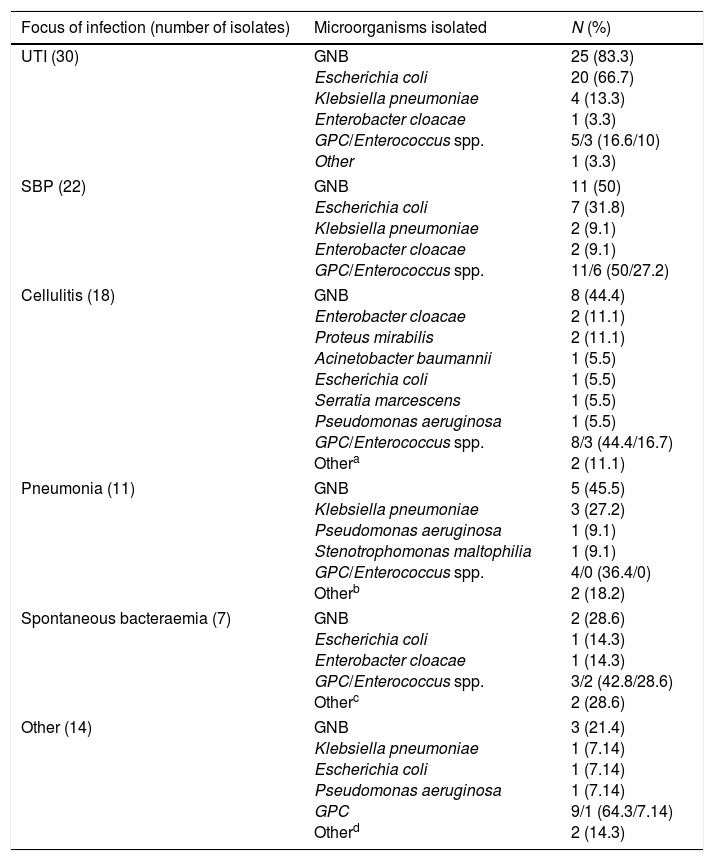
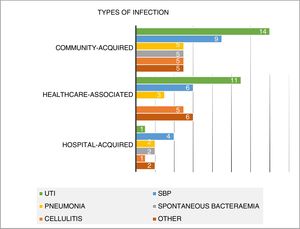
Analysis by focus of infection (Fig. 2)Overall, urinary tract infection was the most common focus of infection (26 cases; 30.2% of the total sample). This was followed by SBP (19 cases; 22.1%), cellulitis (11 cases; 12.8%), pneumonia (10 cases; 11.6%) and spontaneous bacteraemia (seven cases; 8.1%). There were 13 less common infections (endocarditis, catheter sepsis, Clostridium difficile colitis, meningitis, cholangitis and pleural empyema) accounting for 15.2% of the total.
A more detailed analysis of the microbiological isolates by focus of infection is provided in Table 2.
Microbiological isolates by focus on infection.
| Focus of infection (number of isolates) | Microorganisms isolated | N (%) |
|---|---|---|
| UTI (30) | GNB Escherichia coli Klebsiella pneumoniae Enterobacter cloacae GPC/Enterococcus spp. Other | 25 (83.3) 20 (66.7) 4 (13.3) 1 (3.3) 5/3 (16.6/10) 1 (3.3) |
| SBP (22) | GNB Escherichia coli Klebsiella pneumoniae Enterobacter cloacae GPC/Enterococcus spp. | 11 (50) 7 (31.8) 2 (9.1) 2 (9.1) 11/6 (50/27.2) |
| Cellulitis (18) | GNB Enterobacter cloacae Proteus mirabilis Acinetobacter baumannii Escherichia coli Serratia marcescens Pseudomonas aeruginosa GPC/Enterococcus spp. Othera | 8 (44.4) 2 (11.1) 2 (11.1) 1 (5.5) 1 (5.5) 1 (5.5) 1 (5.5) 8/3 (44.4/16.7) 2 (11.1) |
| Pneumonia (11) | GNB Klebsiella pneumoniae Pseudomonas aeruginosa Stenotrophomonas maltophilia GPC/Enterococcus spp. Otherb | 5 (45.5) 3 (27.2) 1 (9.1) 1 (9.1) 4/0 (36.4/0) 2 (18.2) |
| Spontaneous bacteraemia (7) | GNB Escherichia coli Enterobacter cloacae GPC/Enterococcus spp. Otherc | 2 (28.6) 1 (14.3) 1 (14.3) 3/2 (42.8/28.6) 2 (28.6) |
| Other (14) | GNB Klebsiella pneumoniae Escherichia coli Pseudomonas aeruginosa GPC Otherd | 3 (21.4) 1 (7.14) 1 (7.14) 1 (7.14) 9/1 (64.3/7.14) 2 (14.3) |
In the right-hand column, the detection percentage of each bacterium compared to the total number of isolates at each focus is expressed in brackets.
GNB: Gram-negative bacilli; GPC: Gram-positive cocci.
Half of the infections were community-acquired (43 cases in total). 36% were considered to be healthcare-associated infections (31 cases) and 12 hospital-acquired (14%). UTI was the most common focus (32.6%), followed by SBP (20.9%). This trend was also seen in healthcare-associated infections.
With regards to the hospital setting, SBP was the predominant infection (33.3% of all hospital-acquired infections), with only one case of hospital-acquired UTI with a valid microbiological isolate identified (Fig. 2).
Microbiological isolatesIsolates were obtained from 26 blood cultures (30.2%), 24 urine cultures (27.9%), 16 ascitic fluid cultures (18.6%), nine exudates (10.5%), six bronchoalveolar lavage or sputum samples (7%), two faecal serological tests (2.4%), one serological blood test (1.2%), one antigenuria (1.2%) and one pleural fluid sample (1.2%).
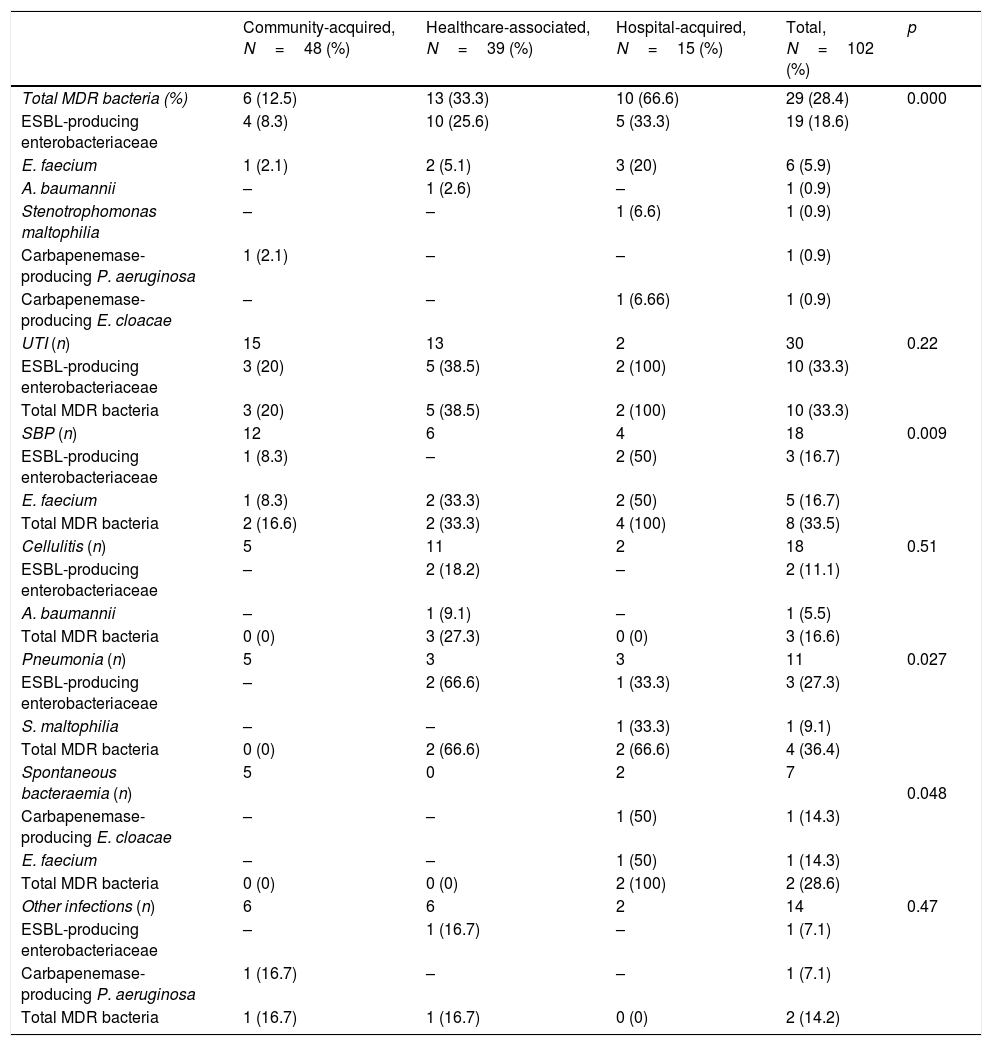
Of the 102 isolated bacteria (Table 3), 48 were community-acquired (47%), 39 were healthcare-associated (38.2%) and 15 were hospital-acquired (14.7%).
Prevalence of multidrug-resistant bacteria by type of infection and site of acquisition.
| Community-acquired, N=48 (%) | Healthcare-associated, N=39 (%) | Hospital-acquired, N=15 (%) | Total, N=102 (%) | p | |
|---|---|---|---|---|---|
| Total MDR bacteria (%) | 6 (12.5) | 13 (33.3) | 10 (66.6) | 29 (28.4) | 0.000 |
| ESBL-producing enterobacteriaceae | 4 (8.3) | 10 (25.6) | 5 (33.3) | 19 (18.6) | |
| E. faecium | 1 (2.1) | 2 (5.1) | 3 (20) | 6 (5.9) | |
| A. baumannii | – | 1 (2.6) | – | 1 (0.9) | |
| Stenotrophomonas maltophilia | – | – | 1 (6.6) | 1 (0.9) | |
| Carbapenemase-producing P. aeruginosa | 1 (2.1) | – | – | 1 (0.9) | |
| Carbapenemase-producing E. cloacae | – | – | 1 (6.66) | 1 (0.9) | |
| UTI (n) | 15 | 13 | 2 | 30 | 0.22 |
| ESBL-producing enterobacteriaceae | 3 (20) | 5 (38.5) | 2 (100) | 10 (33.3) | |
| Total MDR bacteria | 3 (20) | 5 (38.5) | 2 (100) | 10 (33.3) | |
| SBP (n) | 12 | 6 | 4 | 18 | 0.009 |
| ESBL-producing enterobacteriaceae | 1 (8.3) | – | 2 (50) | 3 (16.7) | |
| E. faecium | 1 (8.3) | 2 (33.3) | 2 (50) | 5 (16.7) | |
| Total MDR bacteria | 2 (16.6) | 2 (33.3) | 4 (100) | 8 (33.5) | |
| Cellulitis (n) | 5 | 11 | 2 | 18 | 0.51 |
| ESBL-producing enterobacteriaceae | – | 2 (18.2) | – | 2 (11.1) | |
| A. baumannii | – | 1 (9.1) | – | 1 (5.5) | |
| Total MDR bacteria | 0 (0) | 3 (27.3) | 0 (0) | 3 (16.6) | |
| Pneumonia (n) | 5 | 3 | 3 | 11 | 0.027 |
| ESBL-producing enterobacteriaceae | – | 2 (66.6) | 1 (33.3) | 3 (27.3) | |
| S. maltophilia | – | – | 1 (33.3) | 1 (9.1) | |
| Total MDR bacteria | 0 (0) | 2 (66.6) | 2 (66.6) | 4 (36.4) | |
| Spontaneous bacteraemia (n) | 5 | 0 | 2 | 7 | 0.048 |
| Carbapenemase-producing E. cloacae | – | – | 1 (50) | 1 (14.3) | |
| E. faecium | – | – | 1 (50) | 1 (14.3) | |
| Total MDR bacteria | 0 (0) | 0 (0) | 2 (100) | 2 (28.6) | |
| Other infections (n) | 6 | 6 | 2 | 14 | 0.47 |
| ESBL-producing enterobacteriaceae | – | 1 (16.7) | – | 1 (7.1) | |
| Carbapenemase-producing P. aeruginosa | 1 (16.7) | – | – | 1 (7.1) | |
| Total MDR bacteria | 1 (16.7) | 1 (16.7) | 0 (0) | 2 (14.2) |
The figures in brackets indicate the proportion of MDR bacteria compared to the total number of microbiological isolates by focus of infection and site of acquisition.
Fifty-seven (55.8%) were Gram-negative bacteria and 45 (44.2%) were Gram-positive. The Enterobacteriaceae family accounted for the most common cause of infection (49 microorganisms). E. coli (30 cases; 29.4%), K. pneumoniae (10 cases; 9.8%), S. aureus (10 cases; 9.8%), E. faecalis (9 cases; 8.8%), E. faecium (6 cases; 5.8%) and S. pneumoniae (6 cases; 5.8%) were the most commonly isolated bacteria overall.
In total, 29 bacteria (28.4%) met the multidrug-resistance criteria, giving rise to the onset of 26 different infections. The most prevalent were extended-spectrum beta-lactamase-producing enterobacteriaceae and Enterococcus faecium, which were detected on 19 (18.6%) and six (5.9%) occasions, respectively. Two carbapenemase-producing bacteria were also isolated (Enterobacter cloacae and Pseudomonas aeruginosa). Other MDR bacteria, such as Acinetobacter baumannii and Stenotrophomonas maltophilia, were isolated on one occasion. No cases of methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus (VRE) were isolated.
Most MDR bacteria were isolated in hospital-acquired and healthcare-associated infections (10 and 13 cases, respectively; p<0.001). Overall, MDR bacteria were isolated in 66.6% of hospital-acquired infections, 33.3% of healthcare-associated infections and 12.5% of community-acquired infections.
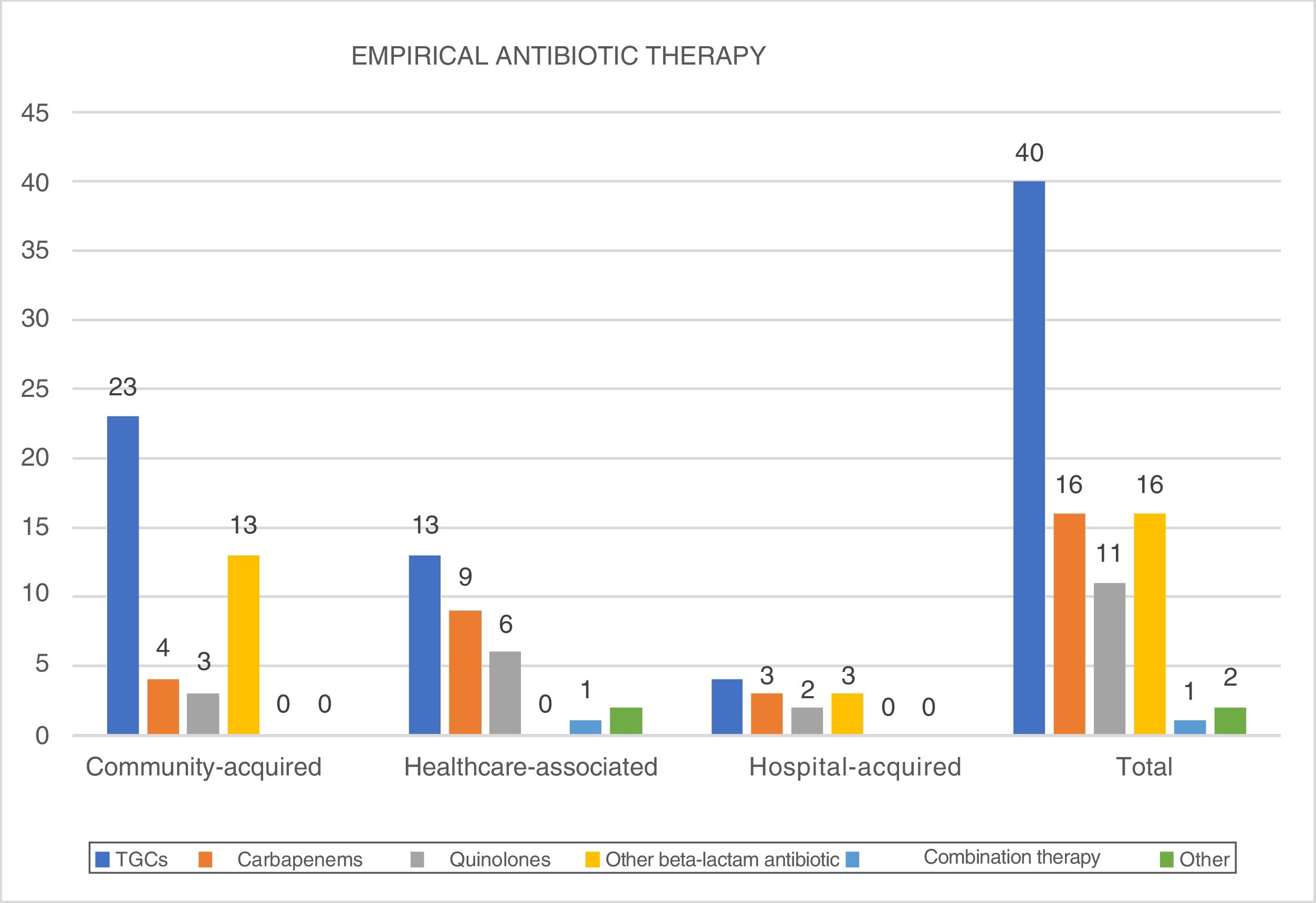
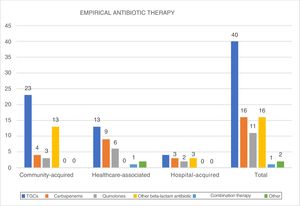
Empirical antibiotic therapy (Fig. 3)TGCs were administered as the treatment of choice in 46.5% of patients, followed by carbapenems and other beta-lactam antibiotics (18.6% of patients). Carbapenems were particularly used in hospital-acquired infections (25%) and healthcare-associated infections (29%).
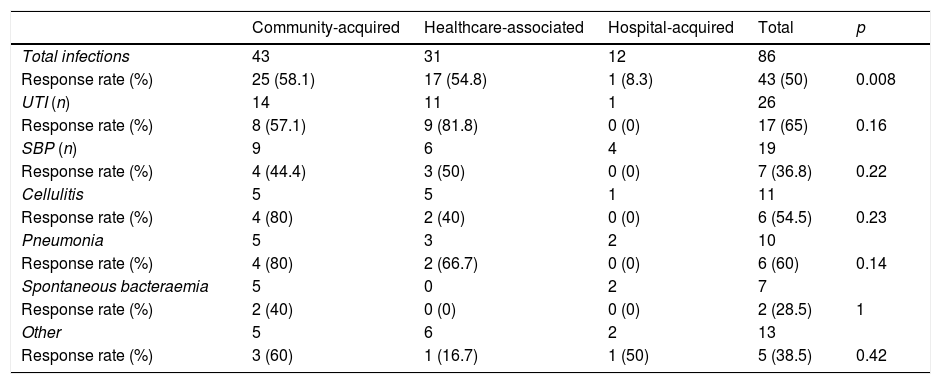
Effectiveness of empirical treatmentOverall, the chosen empirical antibiotic therapy was effective in 50% of cases (Table 4), particularly in community acquired infections (58.1%) and healthcare-acquired infections (54.8%), but was markedly less effective in hospital-acquired infections (8.3%; p=0.008).
Effectiveness of empirical antibiotic therapy by focus and site of acquisition.
| Community-acquired | Healthcare-associated | Hospital-acquired | Total | p | |
|---|---|---|---|---|---|
| Total infections | 43 | 31 | 12 | 86 | |
| Response rate (%) | 25 (58.1) | 17 (54.8) | 1 (8.3) | 43 (50) | 0.008 |
| UTI (n) | 14 | 11 | 1 | 26 | |
| Response rate (%) | 8 (57.1) | 9 (81.8) | 0 (0) | 17 (65) | 0.16 |
| SBP (n) | 9 | 6 | 4 | 19 | |
| Response rate (%) | 4 (44.4) | 3 (50) | 0 (0) | 7 (36.8) | 0.22 |
| Cellulitis | 5 | 5 | 1 | 11 | |
| Response rate (%) | 4 (80) | 2 (40) | 0 (0) | 6 (54.5) | 0.23 |
| Pneumonia | 5 | 3 | 2 | 10 | |
| Response rate (%) | 4 (80) | 2 (66.7) | 0 (0) | 6 (60) | 0.14 |
| Spontaneous bacteraemia | 5 | 0 | 2 | 7 | |
| Response rate (%) | 2 (40) | 0 (0) | 0 (0) | 2 (28.5) | 1 |
| Other | 5 | 6 | 2 | 13 | |
| Response rate (%) | 3 (60) | 1 (16.7) | 1 (50) | 5 (38.5) | 0.42 |
The most common cause of treatment failure in community-acquired infections was a lack of initial response (10 out of 18 cases), despite a subsequent antibiogram showing sensitivity to the prescribed antibiotic. Four bacteria (22.2%) were resistant (two ESBL-producing enterobacteriaceae, one Listeria monocytogenes and one coagulase-negative Staphylococcus) and a further four were intrinsically resistant (three Enterococcus faecalis and one Listeria monocytogenes) to the empirical treatment.
With regards to healthcare-associated infections, resistance was detected in six of the 14 cases (42.9%), lack of initial treatment response in a further six (42.9%) and intrinsic resistance in two cases (14.3%). Resistance to the prescribed treatment was the cause of treatment failure in eight of the 11 cases (72.7%) of hospital-acquired infection. Seven of those eight cases (87.5%) were caused by MDR bacteria.
Clinical impactThe onset of sepsis was significantly higher in MDR bacterial infections (OR 3.13; 95% CI: 1.18–8.32; p=0.025). Although the mortality rate and prevalence of septic shock was higher in this group, it did not reach statistical significance (p=0.55 and p=0.28, respectively).
Failure of the empirical antibiotic therapy was associated with higher mortality (OR 7.05; 95% CI: 1.86–26.75; p=0.000); acute kidney failure at any time during the hospital stay (OR 2.72; 95% CI: 1.45–5.13; p=0.000); admission to the ICU (OR 3.53; 95% CI: 1.23–10.11; p=0.003); sepsis (OR 2.024; 95% CI: 1.13–3.63; p=0.012) and septic shock (OR 4.69; 95% CI: 1.26–17.39; p=0.002).
Risk factorsThe bivariate analysis revealed significant differences in the following variables: hospital-acquired infection (versus community-acquired: OR 18.5; 95% CI: 3.87–88.54; p=0.000, and versus healthcare-associated infections: OR 5.46; 95% CI: 1.22–24.43; p=0.039); healthcare-associated infection (versus community-acquired: OR 3.39; 95% CI: 1.09–10.54; p=0.048); antibiotic use in the last 90 days, both with prophylaxis with norfloxacin (OR 4.37; 95% CI: 1.59–11.99; p=0.005), and without (OR 3.62; 95% CI: 1.35–9.67; p=0.01); hospital stay in excess of 48 hours in the last 90 days (OR 3.18; 95% CI: 1.19–8.47; p=0.018); active cancer (OR 2.93; 95% CI: 1.08–7.99; p=0.038) and use of norfloxacin as SBP prophylaxis (OR 3; 95% CI: 1.02–8.79; p=0.012). In contrast, use of rifaximin was not associated with an increased risk of MDR bacterial infection (OR 2.42; 95% CI: 0.32–18.16; p=0.58).
Although not reaching statistical significance, the proportion of MDR bacterial infections was much higher in patients who had recently been admitted to the ICU (OR 3.45; 95% CI: 0.71–16.7; p=0.19) and in those patients in whom any MDR bacteria had been isolated in the last six months (OR 2.14; 95% CI: 0.59–7.78; p=0.29). No significant differences were found in the following variables: gender (39.1% versus 25.4%; p=0.21); active alcoholism (8.7% versus 25.4%; p=0.09); diabetes (34.8% versus 33.3%; p=0.90) or chronic kidney failure (17.4% versus 22.4% p=0.62).
DiscussionThe prevalence of multidrug-resistant bacterial infections is increasing, particularly in patients with liver cirrhosis. Knowledge of the local epidemiology is essential in order to prescribe the appropriate empirical treatment.5,11,13,15
The multidrug resistance rate in our study (28.4% of all infections) is comparable with the rate published in a recent European multicentre study,20 which found an overall MDR rate of 31%, with 14% of infections caused by ESBL-producing bacteria and 7% by Enterococcus faecium. However, a detailed analysis reveals very important differences For example: in Italy, multidrug resistance rates of up to 50% have been published,21 while other tertiary hospitals in Spain have reported similar rates to ours (32.6% at Hospital Universitario Gregorio Marañón and 28% at Hospital Clínic de Barcelona4,22), but with very different proportions of ESBL-producing bacteria (7–5–8.7% at Hospital Clínic (depending on the series), 8–9% at Hospital Universitario de Bellvitge12 and 25.2% at Hospital Gregorio Marañón22).
These data add further weight to the importance of knowing the local epidemiology in order to adapt the empirical antibiotic therapy regimens to suit the prevalence of the different MDR bacteria.
Risk factorsIn our study, the presence of MDR bacterial infections was associated with the hospital setting, the use of antibiotics or hospital admission in the last 90 days, the existence of active cancer and the use of norfloxacin as SBP prophylaxis, which is consistent with the results published in the scientific literature.3,11,22 The association between active cancer and risk of MDR bacterial infection in cirrhotic patients had never been studied to date, although given the limitations of this study, the findings cannot yet be extrapolated to daily clinical practice. No association between recent ICU admission and prior MDR bacteria isolation was found.
Clinical impactThe results of the study point to a need to amend the empirical antibiotic therapy regimens used at our hospital, especially those prescribed for hospital-acquired and healthcare-associated infections. Given the high prevalence of ESBL-producing bacteria, use of carbapenems as the first-line treatment of hospital-acquired infections should be standardised. Furthermore, and in line with the results of a recent study,23 coverage against Gram-positive bacteria in hospital-acquired SBP should also be standardised, as the proportion of cases caused by Enterococcus spp. is very high. The implementation of these changes should result in the improved prognosis of cirrhotic patients admitted to our hospital, although a comparative study would be needed to confirm this hypothesis.
Despite the fact that the multidrug resistance rate in healthcare-associated infections is very similar to that of other studies, the response to empirical treatment is very similar to that for community-acquired infections (Table 4). This casts some doubt over whether it is necessary to implement the recommendations of current clinical practice guidelines, which endorse the use of carbapenems as first-line treatment in this group of patients. It must also be considered that use of these antibiotics may give rise to increased resistance, in addition to the high economic cost it would entail. The only study conducted to date that evaluates the economic impact of this strategy24 found that broad-spectrum antibiotics represent a cost saving in patients with healthcare-associated infections as it reduces morbidity and mortality and length of hospital stay.
In light of the results obtained, it would therefore seem reasonable to reserve carbapenems for patients with added multidrug-resistance risk factors as well as for patients who exhibit serious signs and symptoms, as early administration of an appropriate empirical treatment is associated with a better prognosis.
The immediate futureThe restricted use of antibiotics in the general population has proven to be an effective measure for reducing rates of antimicrobial resistance. An example of such success comes from a recent single-centre study conducted in the Netherlands,25 a country with a very restrictive antibiotics policy. The results showed that the MDR rate and the cephalosporin resistance rate in a population of cirrhotic patients with SBP were similar in two cohorts compared 10 years apart. Campaigns raising awareness of responsible antibiotic prescription practices represent one aspect that needs to be improved in order to control the rate of multidrug-resistant bacterial infections.
The widespread use of broad-spectrum antibiotics is giving rise to, and will continue to give rise to the emergence of new resistances, which will further hinder the management of these infections.11,26 Early antibiotic de-escalation (or step-down therapy) is essential in order to minimise the repercussions of their use. It is vital that cultures that provide early microbiological results are taken correctly in order to implement antibiotic de-escalation safely and prevent the generation of resistance.5,11 Epidemiological surveillance by taking periodic cultures, which is widely used in the ICU, is being implemented more and more on hospital wards as it effectively establishes early isolation measures if multidrug-resistant bacteria are detected and enables the antibiotic therapy to be adjusted in the event of infection.27
SBP prophylaxis is another important aspect. The effectiveness of primary prophylaxis with norfloxacin in patients with early-stage cirrhosis is much disputed.28,29 It is associated with multidrug-resistance3 and its efficacy in secondary prophylaxis after an initial episode of Gram-positive or MDR SBP is unknown. That is why it is important to conduct further studies that seek to limit norfloxacin's indications, as well as to look for treatment alternatives.29 One such alternative could be rifaximin, a non-absorbable antibiotic with a negligible resistance rate.30,31 Although the results are disputable,32,33 consensus for its protective role in SBP seems to be growing.34 Large-scale clinical trials are required to assess its actual relevance.
Our study has a series of methodological limitations. Its retrospective design prevented certain clinical data of interest from being obtained, particularly concerning prior use of antibiotics. The lack of diagnosis codes in the discharge reports may have reduced the sample size of the population studied, therefore also limiting the results obtained. In addition, it was a single-centre study, which means that the results obtained cannot be extrapolated to other hospitals without knowing the local bacterial epidemiology of each centre.
ConclusionsThe multidrug-resistant bacterial infection rate at our hospital is comparable with that of other similar European hospitals. The results obtained support the adoption of the antibiotic therapy regimens recommended by current clinical practice guidelines, limiting the use of carbapenems to hospital-acquired infections and to healthcare-associated infections with other multidrug-resistance risk factors or with serious signs or symptoms. The early administration of an appropriate empirical treatment is associated with improved prognosis.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Béjar-Serrano S, del Pozo P, Fernández-de la Varga M, Benlloch S. Infecciones por bacterias multirresistentes en pacientes cirróticos en un hospital terciario. Gastroenterol Hepatol. 2019;42:228–238.