Ecchymosis and/or haematoma are the most common adverse events after subcutaneous administration of low molecular weight heparin. There is no strong recommendation as to the puncture site.
ObjectiveTo evaluate the adverse events, ecchymosis and/or haematoma after the administration of prophylactic subcutaneous enoxaparin in the abdomen vs the arm in the critically ill patient.
MethodologyA randomised, two-arm clinical trial (injection in the abdomen vs the arm), performed between July 2014 and January 2017, in an 18-bed, polyvalent intensive care unit. Patients receiving prophylactic enoxaparin, admitted >72h, with no liver or haematological disorders, a body mass index (BMI) >18.5, not pregnant, of legal age and with no skin lesions which would impede assessment were included. We excluded patients who died or who were transferred to another hospital before completing the evaluation. We gathered demographic and clinical variables, and the onset of ecchymosis and/or haematomas at the injection site after 12, 24, 48 and 72h. A descriptive analysis was undertaken, with group comparison and logistic regression. The study was approved by the ethics committee with the signed consent of patients/families.
Results301 cases (11 excluded): 149 were injected in the abdomen vs 141 in the arm. There were no significant differences in demographic and clinical variables, BMI, enoxaparin dose or antiplatelet administration [ecchymosis, abdomen vs arm, n (%): 66 (44) vs 72 (51), P=0.25] [haematoma abdomen vs arm, n (%): 9 (6) vs 14 (10), P=0.2]. Statistical significance was found in the size of the haematomas after 72h: [area of haematoma (mm2) abdomen vs arm, median (IQR): 2 (1–5.25) vs 20(5.25–156), P=0.027].
ConclusionsIn our patient cohort, prophylactic subcutaneous enoxaparin administered in the abdomen causes fewer haematomas after 72h, than when administered in the arm. The incidence rate of ecchymosis and haematoma was lower than the published incidence in critically ill patients, although patients receiving anti-platelet agents present a higher risk of injury. No relationship was observed in relation to BMI.
Los eventos adversos más frecuentes de la administración subcutánea de heparina de bajo peso molecular son la equimosis y/o el hematoma. No existe una fuerte recomendación sobre la zona de punción.
ObjetivoEvaluar los eventos adversos, equimosis y/o hematoma, tras administración de enoxaparina subcutánea profiláctica en abdomen vs. brazo, en pacientes críticos.
MetodologíaEnsayo clínico aleatorizado en dos ramas (inyección abdomen vs. brazo), entre julio de 2014 y enero de 2017, en una unidad de cuidados intensivos polivalente de 18 camas. Incluidos pacientes con enoxaparina profiláctica, ingreso >72h, sin hepatopatías o enfermedades hematológicas, con índice de masa corporal (IMC) >18,5, no embarazadas, mayores de edad y sin lesiones cutáneas que impidan la valoración. Excluidos fallecimientos o traslados de hospital antes de finalizar la valoración. Recogidas variables demográficas, clínicas y aparición de equimosis y/o hematoma en lugar de inyección a las 12, 24, 48 y 72h. Análisis descriptivo, comparación de grupos y regresión logística. Aprobado por la comité de ética, con consentimiento firmado de pacientes/familiares.
ResultadosUn total de 301 casos (11 excluidos): 149 en abdomen vs. 141 en brazo. Sin diferencias significativas en variables demográficas, clínicas, IMC, dosis de enoxaparina y administración de antiagregantes. Equimosis en el 48% de los pacientes y hematoma en el 8%, sin diferencias estadísticas abdomen vs. brazo [equimosis, abdomen vs. brazo, n(%): 66(44) vs. 72(51), p=0,25] [hematoma abdomen vs. brazo, n(%):9(6) vs. 14(10), p=0,2]. Se halla significación estadística en el tamaño del hematoma a las 72h: [área de hematoma (mm2) abdomen vs. brazo, mediana (RIC): 2(1-5,25) vs. 20(5,25-156), p=0,027].
ConclusionesEn nuestra cohorte de pacientes, la enoxaparina subcutánea profiláctica administrada en el abdomen produce menos hematomas, a las 72h, que administrada en el brazo. La tasa de incidencia de equimosis y hematomas es menor a la publicada en pacientes críticos, advirtiéndose que pacientes con antiagregantes presentan mayor riesgo de presentar lesiones, no observándose relación de su aparición con el IMC.
The subcutaneous administration of low molecular weight heparin at prophylactic doses is a habitual practice in intensive care units. Nevertheless, there is insufficient scientific evidence regarding the technique of administration and the appearance of ecchymosis and haematomas in critical patients. These adverse effects alter the bodily image and self-confidence of patients.
This study focuses on the problem by proposing an administration technique that is based on the best scientific evidence found, the norms of the drug manufacturer and the consensus of experts, as well as the results following its use.
Implications of the studyThis study relates the appearance of ecchymosis and/or haematomas in critical patients with the injection site, according to the described technique. Studies such as this one offer the theoretical bases for the development of evidence-based nursing techniques.
Since low molecular weight heparin (LMWH) was developed in 1980, its utility and frequency of use have been optimised. The origin compound1 has been replaced and it has come into widespread clinical use, in primary care as well as in specialised care.
Its advantages have led to extensive progress in its usage in comparison with unfractionated heparin, such as its high bioavailability and predictable pharmacokinetics.2 Although LMWHs contain anti-Xa, the main active ingredient that inhibits coagulation factor Xa, they are effective and safe without the need for monitoring.1,3,4 The doses uses for deep vein thrombosis prophylaxis do not significantly modify overall bleeding and coagulation times, and nor do they affect platelet aggregation or the binding of fibrogen to platelets.5 Nevertheless, the College of American Pathologists6 recommends monitoring patients in the case of extremely low weight or overweight, pregnancy, children and kidney failure.
Enoxaparin is used as an active treatment for thrombosis, unstable angina and myocardial infarction without the Q wave, among other pathologies.5,7 It is prescribed as a prophylaxis to prevent thromboembolism, deep vein thrombosis and the formation of coagulations during the haemodialysis of patients with chronic kidney failure, with one dose every 24h.8
As the medication has to be absorbed slowly, the route of choice for the administration of enoxaparin is subcutaneous. The medication is injected under the epidermis, between the fat and the connective tissue underlying the skin, where the blood flow is less and where absorption is therefore slower. Lack of knowledge of this technique may lead to the accidental administration of the medication in the muscle tissue, affecting absorption and harming the patient.9
The adverse effects of subcutaneously administering enoxaparin include haemorrhaging complications, severe haematomas of the abdominal wall, necrosis of the skin and subcutaneous tissue10 and, over the longer term, osteoporosis and thrombocytopenia.1,5,7 The appearance of ecchymosis and/or haematoma is the most frequent adverse event, with incidences that vary from 10% to 90%8,10–22 depending on the technique used. Although the appearance of bruising should not lead to a severe clinical situation, it may determine patient perception and cause anxiety, body image disorder and loss of trust in the nurse supplying care.16
There is little in the bibliography that contextualises the presence of ecchymosis and/or haematoma in critical patients,10,13–15,17 as the majority of studies are about post-surgical care in hospitalisation units.8,11,12,16,18–23
Enoxaparin is presented in single dose prefilled syringes8 with 0.2ml for every 2000 units of sodium enoxaparin and with a Hypak-type 29 calibre needle.24 This form of presentation hinders interference with the length or calibre of the needle,14 the size of the syringe18 or the volume injected, all of which may influence the appearance of haematomas or ecchymosis. Nevertheless, we are able to influence the injection technique, to evaluate its influence on the appearance of injuries.
Aguilera et al.8 report that in non-obese patients (cutaneous abdominal fold <4cm) the formation of the fold would function as a protective factor against the creation of a haematoma, while in obese patients (cutaneous abdominal fold >4cm) the formation of the fold may be a risk factor for causing a haematoma. Whether or not the fold is maintained during injection is studied by Vanbree et al.,13 who found not conclusive results.
Aspiration prior to injection is studied by several authors.10,12,13,15,22 Garrido et al.12 show that fewer and smaller haematomas are caused when the aspiration process is suppressed in the administration technique, while the study by Avşar and Kaşikçi22 found that the incidence and size of ecchymosis are also smaller when a technique without aspiration is used.
Respecting purging the air bubble before injection, Wooldridge and Jackson18 state in their study that the air should not be purged, and that it should therefore be included in the end of injection.
Several studies11,16,17,20,21,25 were found that investigate the relationship between injection administration time and the appearance of ecchymosis/haematoma. In their study, Akpinar and Celebioglu16 find that injecting the drug in 30s (s) or injecting it in 10s then waiting another 10s before withdrawing the needle reduces the size and number of ecchymosis in the administration zone. The studies by Zaybak and Khorshid11 and Chan20 observe a reduction in the incidence and size of ecchymosis when the injection lasts for 30s. Pourghaznein et al.17 administer the injection in 15s and wait for 5s before withdrawing the needle, thereby reducing the incidence of ecchymosis in their study. Palese et al.21 conclude that the injection time should last for more than 30s to reduce ecchymosis at the injection site.
In the majority of the studies reviewed an administration technique described as standard is used. Although some of the steps involved in this technique are based on consensus, they are not scientifically supported and authors vary widely in this respect. This is the case for aspects such as maintaining the fold during injection,4,5,7,10–12,14,20–22,26–28 creating the fold only for injection and without discriminating between obese and non-obese patients,18 not rubbing the injection site,4,7–9,13,14,16–19,21,23,26–28 injecting at an angle of 90°,7,9,11–14,16,18,19,21–23,26–28 injecting at an angle of 45° in cachexic patients27 and withdrawing the needle at the same angle in which it was inserted.13,14,16,18–21,26–28
Another aspect of the technique to be studied is the selection of the injection site. Traditionally the anterolateral and posterolateral abdominal waist has been considered to be ideal for administration, while trying not to inject heparin in an area of 5cm around the navel to avoid the navel veins.23,28 Several authors analyse the effects of injecting in the abdomen,4,5,7,8,10–13,16,18,20,22,23,26,28 but sometimes this is not possible due to wounds, scars or other injuries, so that another injection site has to be considered. Fahs and Kinney19 studied 101 medical-surgical patients distributed at random in three groups. Each group received heparin injections using the same technique in different locations (the abdomen, the rear of the arm and thigh) after which ecchymosis was measured at 48, 60 and 72h. They found no differences in the rate of ecchymosis, with an overall incidence of 30%.
The nursing practice of administering LMWH in the abdomen seems to be based more on tradition than it is on scientific evidence.19 Annersten and Willman29 reviewed subcutaneous injection technique and concluded that further studies were required, given that the existing ones were heterogeneous in their design and description of different aspects of the technique, so that the scientific basis for the use of the technique is weak.
The variability found in the bibliography that was reviewed led us to plan a study with the main aim of evaluating adverse events, ecchymosis and/or haematoma, following the administration of prophylactic subcutaneous enoxaparin in the abdomen vs The arm, using an injection technique based on the best available evidence, the manufacturer's instructions and the consensus of experts.
The secondary objectives were to determine the incidence of lesions, to observe their evolution over time and to evaluate whether the administration of anti-aggregants/fibrinolytics and the body mass index (BMI) influence the appearance or size of lesions.
MethodologyThis randomised, prospective and blind trial was undertaken in a multipurpose intensive care unit. It compares the prophylactic administration of enoxaparin in the abdomen vs the arm.
Given the characteristics of the study it was impossible to ensure that it was blind for the nurse who administered the drug. Due to this, it was kept blind only for the member of the research team who analysed the data.
The administration of enoxaparin followed the best evidence contained in the bibliography and the manufacturer's instructions. In the cases where we found no evidence in the literature the criteria of professional consensus were followed (Appendix 1).
Patients includedAll of the patients who were prescribed enoxaparin as a prophylaxis were included, if they were foreseen to stay at least 72h after inclusion in the study. The dose varied from 20 to 60mg, depending on body weight, every 24h, and the informed consent document was signed by the patient or their legal representative.
Patients were excluded if they had haematological pathology or hepatopathology diagnosed at admission, together with pregnant women, those aged under 19 years old, patients with major burns, those with a BMI≤18.50 and those with injuries (surgical wound, scars, ecchymosis/haematoma or drainages, etc.) in any of the zones selected for the study.
Excluded patientsAfter they had been included in the study, patients were excluded if they were moved outside the department or died before finalising the observation period (72h).
The patients who could not be monitored for any reason were also excluded (subsequent surgery in the injection zone or patients in prone decubitus, among others).
Sample sizeAccepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral comparison, it was estimated that 147 patients would be necessary in each group to detect a statistically significant difference between both proportions, which for group 1 was estimated to stand at 52% while for group 2 the corresponding figure was 35%. A rate of losses during follow-up of 10% was estimated.
RandomisationBoth arms of the study were randomised in blocks of 40, using a computer-generated list that was kept hidden in closed correlatively numbered envelopes. This randomisation was performed by the team member who subsequently carried out the analysis and did not take part in the selection of patients.
Study variablesThe independent variableThe injection site was assigned randomly (the anterolateral and posterolateral abdominal waist vs the rear face of the arm).
Dependent variablesThe appearance of ecchymosis and/or haematoma in the injection zone 12, 24, 48 and 72h after the administration of prophylactic subcutaneous enoxaparin, together with the size of the lesion.
Ecchymosis: colouration of the skin caused by the infiltration of blood in the subcutaneous tissues or the breakage of subcutaneous capillary vessels.10
Haematoma: an abnormal swelling or hardening caused by the accumulation of blood (a haematoma exists when there is hardening, regardless of whether or not there is colouration of the skin).10
Area of the lesion (mm2): maximum height by maximum width of the lesion.
Patient variablesDemographic variables (age, sex and BMI), Simplified Acute Physiologic Score-II (SAPS II) as the severity indicator, the dose of enoxaparin prescribed, the administration of antiaggregants and/or fibrinolytics.
ProcedureAt the moment patients were admitted and after confirming that they fulfilled the inclusion criteria and obtaining their signed consent, the injection site (the abdomen or arm) was selected at random. For the purposes of study follow-up only one injection per patient was considered.
The enoxaparin injection was carried out by one of the 12 nurses in the unit who formed the research team, using the technique described in Appendix 1.
The injection site was identified by a 5cm perimeter circle drawn with a waterproof pen for subsequent monitoring. Subsequent injections that were not monitored for this study were made at sites other than the one selected at random.
A nurse in the team evaluated the site for ecchymosis and/or haematoma at 12, 24, 48 and 72h after the injection. When a lesion was detected it was measured and its maximum height and width were measured and recorded using graph paper.
Statistical analysisBasic descriptive statistics were applied, expressing quantitative data as either an average (standard deviation) or median (interquartile range), and qualitative data were expressed as proportions.
Groups were compared using either the Student t-test for quantitative variables or non-parametric tests, as applicable, and the Fischer or chi-squared test was used for qualitative variables.
Analysis of the appearance of ecchymosis and/or haematoma was performed by means of a logistic regression in which individually significant variables were introduced. A lineal regression was performed to determine the correlation between the different variables and larger size of the lesions.
The SPSS Statistics 21.0 statistical package for Windows was used (SPSS Inc., Chicago IL, USA).
Ethical considerationsThis study was approved by the Clinical Ethics and Research Committee of the hospital, and informed consent was obtained in all cases, either from the patient or a direct family member.
The procedure followed obeyed the ethical norms of the relevant human experimentation committee, and it was also according to the stipulations of the World Medical Association and the Helsinki Declaration.30
The authors declare that they received no type of economic aid for this study, and that there is no conflict of economic or personal interest that may distort or influence them in their actions.
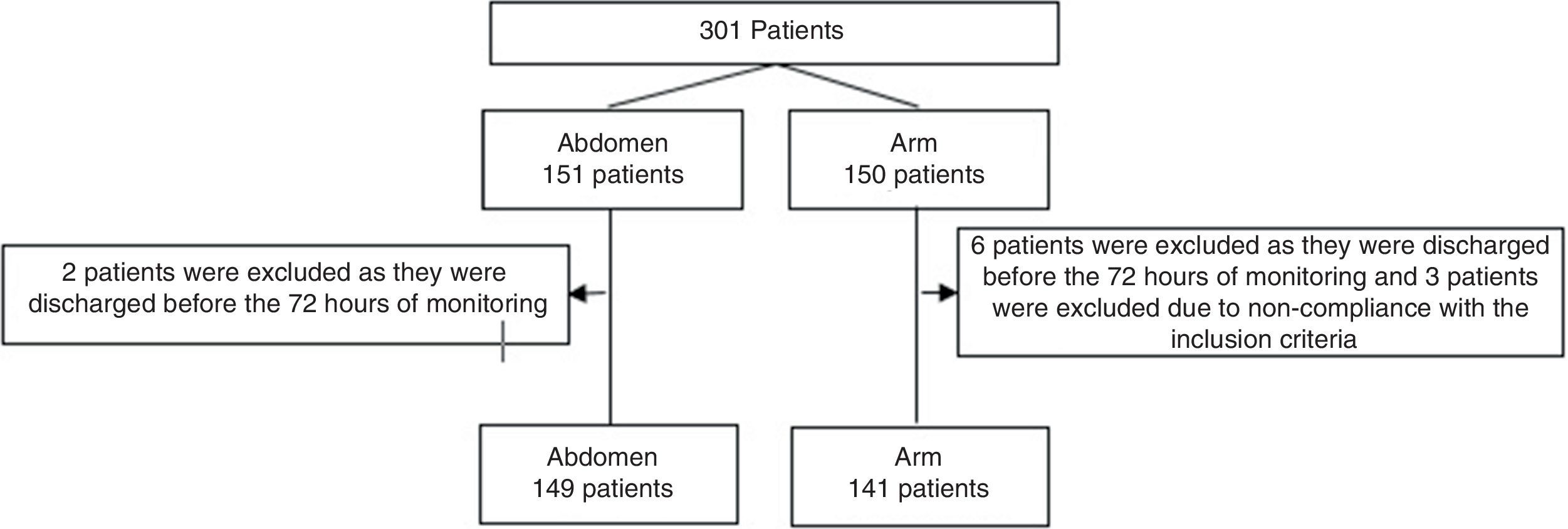
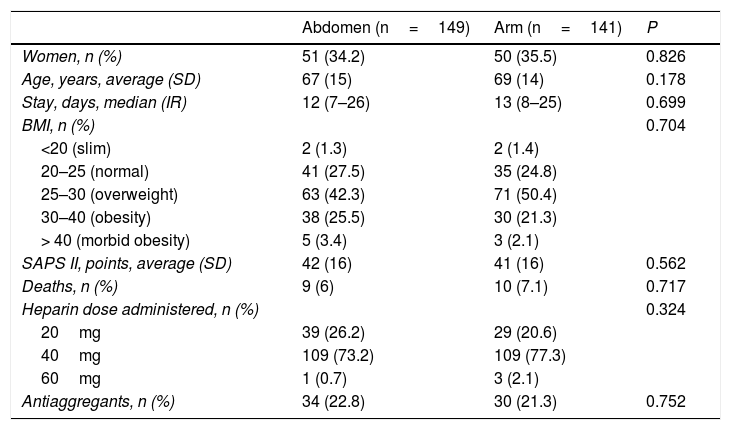
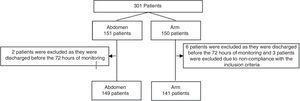
Results301 patients were monitored from July 2014 to January 2017. They were randomised with 151 injections in the abdomen and 150 in an arm. 11 patients were excluded (Fig. 1). No statistically significant differences were found between the groups. The enoxaparin dose administered the most often was 40mg (Table 1).
Patient descriptors.
| Abdomen (n=149) | Arm (n=141) | P | |
|---|---|---|---|
| Women, n (%) | 51 (34.2) | 50 (35.5) | 0.826 |
| Age, years, average (SD) | 67 (15) | 69 (14) | 0.178 |
| Stay, days, median (IR) | 12 (7–26) | 13 (8–25) | 0.699 |
| BMI, n (%) | 0.704 | ||
| <20 (slim) | 2 (1.3) | 2 (1.4) | |
| 20–25 (normal) | 41 (27.5) | 35 (24.8) | |
| 25–30 (overweight) | 63 (42.3) | 71 (50.4) | |
| 30–40 (obesity) | 38 (25.5) | 30 (21.3) | |
| > 40 (morbid obesity) | 5 (3.4) | 3 (2.1) | |
| SAPS II, points, average (SD) | 42 (16) | 41 (16) | 0.562 |
| Deaths, n (%) | 9 (6) | 10 (7.1) | 0.717 |
| Heparin dose administered, n (%) | 0.324 | ||
| 20mg | 39 (26.2) | 29 (20.6) | |
| 40mg | 109 (73.2) | 109 (77.3) | |
| 60mg | 1 (0.7) | 3 (2.1) | |
| Antiaggregants, n (%) | 34 (22.8) | 30 (21.3) | 0.752 |
SD: standard deviation; BMI: body mass index; IR: interquartile range; SAPS: Simplified Acute Physiologic Score-II.
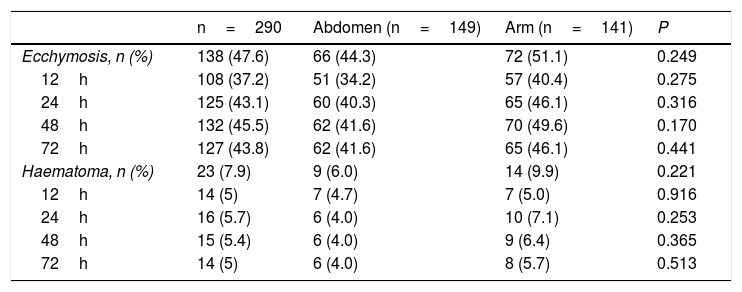
The overall incidence of the appearance of observed ecchymosis was 47.6%, while the corresponding figure for haematoma was 7.9%. We found a lower incidence of these lesions in the abdomen than we did in the arm, although this was not significant (ecchymosis 44.3% vs 51.1%, P=0.249; haematoma 6% vs 9.9%, P=0.221). The findings are similar at 12, 24, 48 and 72h, with a lower incidence in the abdomen, although this was not statistically significant (Table 2).
The overall incidence of ecchymosis/haematoma and abdomen vs arm at 12, 24, 48 and 72h after the administration of enoxaparin.
| n=290 | Abdomen (n=149) | Arm (n=141) | P | |
|---|---|---|---|---|
| Ecchymosis, n (%) | 138 (47.6) | 66 (44.3) | 72 (51.1) | 0.249 |
| 12h | 108 (37.2) | 51 (34.2) | 57 (40.4) | 0.275 |
| 24h | 125 (43.1) | 60 (40.3) | 65 (46.1) | 0.316 |
| 48h | 132 (45.5) | 62 (41.6) | 70 (49.6) | 0.170 |
| 72h | 127 (43.8) | 62 (41.6) | 65 (46.1) | 0.441 |
| Haematoma, n (%) | 23 (7.9) | 9 (6.0) | 14 (9.9) | 0.221 |
| 12h | 14 (5) | 7 (4.7) | 7 (5.0) | 0.916 |
| 24h | 16 (5.7) | 6 (4.0) | 10 (7.1) | 0.253 |
| 48h | 15 (5.4) | 6 (4.0) | 9 (6.4) | 0.365 |
| 72h | 14 (5) | 6 (4.0) | 8 (5.7) | 0.513 |
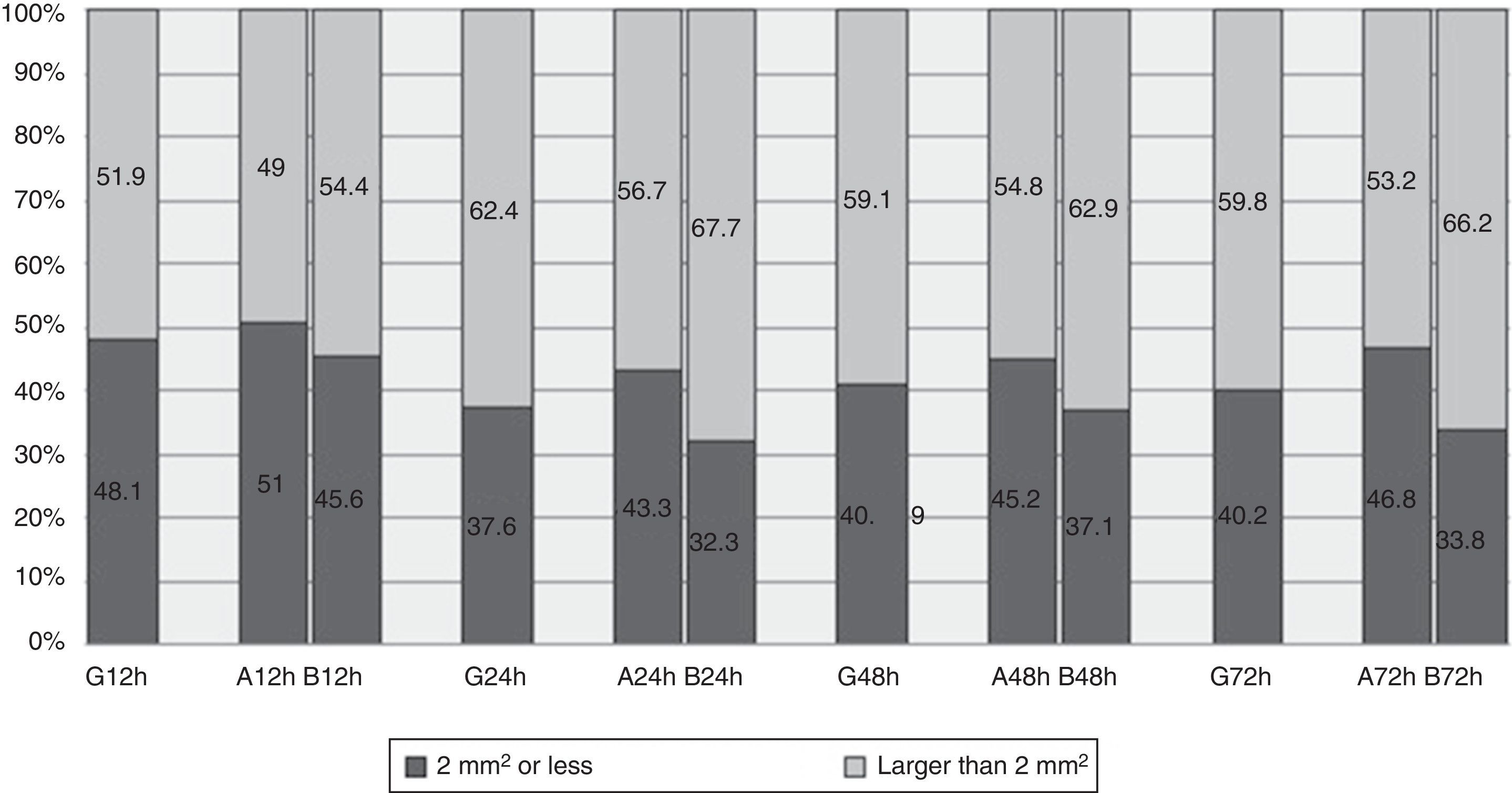
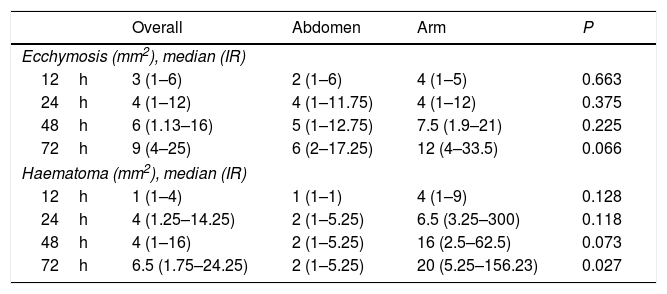
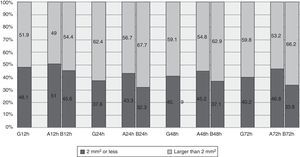
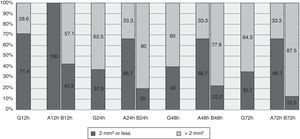
The median area of all the cases of ecchymosis observed runs from 3mm2 at 12h after the administration of enoxaparin and 9mm2 after 72h (Table 3). Nevertheless, 48.1% of cases had an area of 2mm2 or less 12h after administration, and this percentage remained at around 40% at 24, 48 and 72h (Fig. 2).
The overall incidence of ecchymosis/haematoma and abdomen vs arm at 12, 24, 48 and 72h after the administration of enoxaparin.
| Overall | Abdomen | Arm | P | |
|---|---|---|---|---|
| Ecchymosis (mm2), median (IR) | ||||
| 12h | 3 (1–6) | 2 (1–6) | 4 (1–5) | 0.663 |
| 24h | 4 (1–12) | 4 (1–11.75) | 4 (1–12) | 0.375 |
| 48h | 6 (1.13–16) | 5 (1–12.75) | 7.5 (1.9–21) | 0.225 |
| 72h | 9 (4–25) | 6 (2–17.25) | 12 (4–33.5) | 0.066 |
| Haematoma (mm2), median (IR) | ||||
| 12h | 1 (1–4) | 1 (1–1) | 4 (1–9) | 0.128 |
| 24h | 4 (1.25–14.25) | 2 (1–5.25) | 6.5 (3.25–300) | 0.118 |
| 48h | 4 (1–16) | 2 (1–5.25) | 16 (2.5–62.5) | 0.073 |
| 72h | 6.5 (1.75–24.25) | 2 (1–5.25) | 20 (5.25–156.23) | 0.027 |
IR: interquartile range.
If we observe evolution over time between the groups, the lesion is found to be practically equal at 24h. It starts to differentiate at 48h, and at 72h the area observed on the arm duplicated the one on the abdomen (Table 3). Although differences were found between the zones, these did not reach statistical significance.
Comparison between the groups shows a higher percentage of lesions measuring 2mm2 or less in the abdomen vs the arm, and at 12h this was 51% vs 45.6%. This difference increases over time, and percentages could be seen to stand at around 45% in the abdomen and 34% in the arm (Fig. 2).
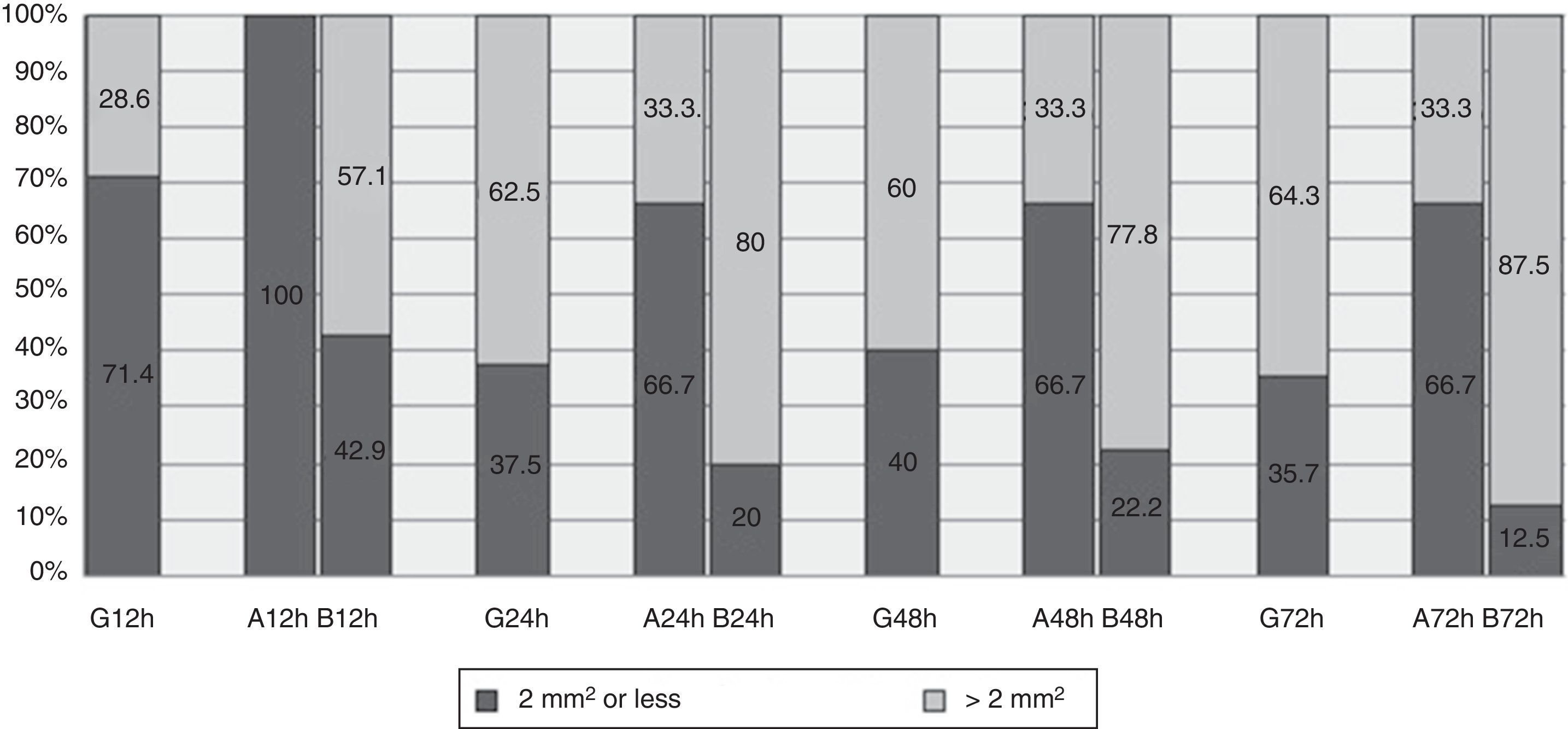
HaematomaThe maximum area covered by any of the haematomas in general was 6.5mm2 at 72h (Table 3). At 12h 71.4% of the haematomas observed had a median area of 2mm2 or less, and this figure remained at around 37% in the following measurements (Fig. 3).
Although the area on the abdomen remains stable over time, the area on the arm increases significantly, reaching up to 20mm2 at 72h (Table 3). Fig. 3 shows how at 12h all of the haematomas on the abdomen had an area of 2mm2 or less, while this percentage fell to 66.7% in the other measurements. 42.9% of the haematomas located on the arm at 12h were found to have an area of 2mm2 or less, at 72h the corresponding figure was 12.5%.
No patient had been prescribed fibrinolytics, and the prescription of antiaggregants was similar in both groups (22.8% abdomen vs 21.3% arm; P=0.752).
In a logistical regression model containing variables associated with the appearance of ecchymosis, age, BMI and the administration of antiaggregants, the patients receiving antiaggregants were found to be at twice the risk of developing ecchymosis (OR: 2.177; CI 95%: 1.231–3.851; P=0.008). The other variables were not significant.
We obtained the same result in the analysis of the appearance of haematomas. In this case the patients receiving antiaggregants were at 4 times more risk than the patients who were not receiving them (OR: 4.329; CI 95%: 1.755–10.679; P=0.001).
The size of the ecchymosis and haematoma at 72h also had a significant lineal correlation with the administration of antiaggregants (ecchymosis P=0.009; haematoma P=0.023).
DiscussionIn our cohort of patients we observed that ecchymosis as well as haematomas appeared more often and were larger on the arm than was the case on the abdomen. The overall incidence was greater in the appearance of ecchymosis than it was for haematomas. The patients receiving antiaggregants were at greater risk of developing these lesions, and BMI was not associated with the appearance of these lesions.
The high level of heterogeneity found in the studies that evaluate the administration of prophylactic enoxaparin makes it complicated to compare and extrapolate our results. This variability is basically due to patient type, as few studies have been undertaken on critical patients, the injection technique, the use of a syringe preloaded with the drug or otherwise and the injection site, while they also do not discriminate between ecchymosis and haematoma.
Although it is true that the aim of our study was not to evaluate administration technique, our results may indicate that the technique used did influence them. However, it would be necessary to undertake a study of this specific aspect due to the diversity found in the bibliography.
Our results may be compared with those of studies undertaken in the context of critical patients,10,13–15,17 three of the latter were carried out in the 1980s.13–15 Brenner et al.15 compared two injection techniques without preloaded syringes, and considering haematoma to be “decolouration” they observed lesions in 50% of cases after injection of the drug. Coley et al.,14 without preloaded syringes and without differentiating between ecchymosis and haematoma, observed lesions in 14% of cases after the administration of LMWH. However, they did not take into consideration lesions smaller than 1mm2. Vanbree et al.13 evaluated the appearance of ecchymosis in patients subjected to cardiac surgery and with LMWH administered every 12h. They compared three injection techniques, without finding differences between them and with a measurable incidence of ecchymosis of 56%.
The studies by Gómez et al.10 and Pourghaznein et al.17 are more recent. Gómez et al.10 evaluated the incidence of haematoma in the abdominal wall following the administration of the drug to patients in a cardiac intensive care unit. They compared four different administration techniques that included preloaded and non-preloaded syringes and aspiration/no aspiration. They obtained an overall result of 26.3% ecchymosis and 52% haematomas. In the cohort that was the most similar to our methodology, with preloaded enoxaparin and without aspiration, the results were 15% ecchymosis and 38% haematomas. In our study this prevalence was reversed, as we found ecchymosis in 49% of cases and 8% haematomas, although the sizes were strikingly smaller, even when the worst results of our study are considered. Gómez et al.10 describe an area of ecchymosis of 2.61cm2, compared to the 9mm2 observed in our study. The same tendency was found in the size of haematoma (11.11cm2 vs 20mm2).
Pourghaznein et al.,17 observed the appearance of ecchymosis using found different methods of administering LMWH every 12h in the abdomen and thighs, measuring the lesion after 48h. Of the four methods used in this study, the most similar to ours (10s injection and 5s waiting time before withdrawing the needle) was method B—which included injection during 10s and a waiting time of 10s before withdrawing the needle—and method C—injection lasting for 15s, with a waiting time of 5s. when both methods were applied on the abdomen they obtained a percentage of ecchymosis of 71% for method B and 53% for C. Both of these figures are higher than ours, which for the abdomen was 46%.
Respecting the areas of ecchymosis, for method B they obtained an average of 38mm2 (0–49) and for method C they obtained an average of 6mm2 (0–14). These values cannot be extrapolated as the calculation includes lesion-free injections (“zero” values).
Lastly, we have not found any paper that considers the administration of antiaggregants in critical patients as a risk factor for the appearance of ecchymosis and/or haematoma.
ConclusionsBased on the observations in our cohort of patients we are able to state that prophylactic subcutaneous enoxaparin administered in the abdomen produces fewer haematomas after 72h than doses administered in the arm.
We obtained a lower incidence of ecchymosis and haematomas than those published for other critical patients using the technique proposed in our study.
The patients who were concomitantly administered antiaggregants are at greater risk of lesions; there is no relationship between the appearance of lesions and BMI, while lesions are smaller on the abdomen than they are on the arm.
Based on these findings, it would be recommendable to administer subcutaneous doses of prophylactic enoxaparin in the abdomen rather than the arm of critical patients.
Conflict of interestsThe authors have no conflict of interests to declare.
- -
The patient must be lying down at the moment of the injection.
- -
Correct hand hygiene and wearing clean gloves.
- -
Disinfection of the assigned injection site with 70° alcohol.
- -
Remove the cap of the needle preloaded with enoxaparin.
- -
Do not extract the air bubble from the preloaded syringe.
- -
A drop may appear at the end of the needle: if this happens the drop has to be eliminated before administering the injection. To do this it is necessary to softly knock the syringe with a finger, always with the needle pointing downwards, until the drop separates.
- -
Take a fold of skin between the thumb and forefinger; this must be done with the non-dominant hand, and the fold has to be broad and without pressure in the injection site zone,7 keeping it so during the whole injection.
- -
Insert the needle completely at an angle of 90°.
- -
Do not aspirate before injecting.
- -
Inject the drug during 10s and keeping the needle completely inserted after the injection for 5s more.
- -
Withdraw the needle in the same direction as entry.
- -
Relax the skin fold.
- -
Lightly press the injection site for 2–3s without massaging or rubbing.
- -
Point the syringe downwards away from yourself and other people, then firmly press the plunger to activate the safety system. The protective sheath will automatically cover the needle and an audible click will confirm activation of the safety system.
- -
Deposit the syringe in a suitable container.
- -
Mark the injection site with a waterproof pen around a 5cm perimeter.
Please cite this article as: Jareño-Collado R, Sánchez-Sánchez MM, Fraile-Gamo MP, García-Crespo N, Barba-Aragón S, Bermejo-García H, et al. Formación de equimosis y/o hematoma tras la administración profiláctica de enoxaparina subcutánea en abdomen o brazo en pacientes críticos. Enferm Intensiva. 2018;29:4–13.