Mycoplasma genitalium (MG) is responsible for 10–25% of cases of non-gonococcal urethritis (NGU) in Europe.1 The rate of macrolide resistance in men who have sex with men (MSM) is currently estimated to be up to 35%,2 probably due to long-term use of azithromycin for empirical treatment of NGU. MG infection can cause various complications in both genders and can facilitate the transmission of human immunodeficiency virus (HIV).3 Targeted therapy and a careful study of sexual contact in cases of NGU is required to control this infection. The purpose of the following case report is to determine the best treatment for MG urethritis.
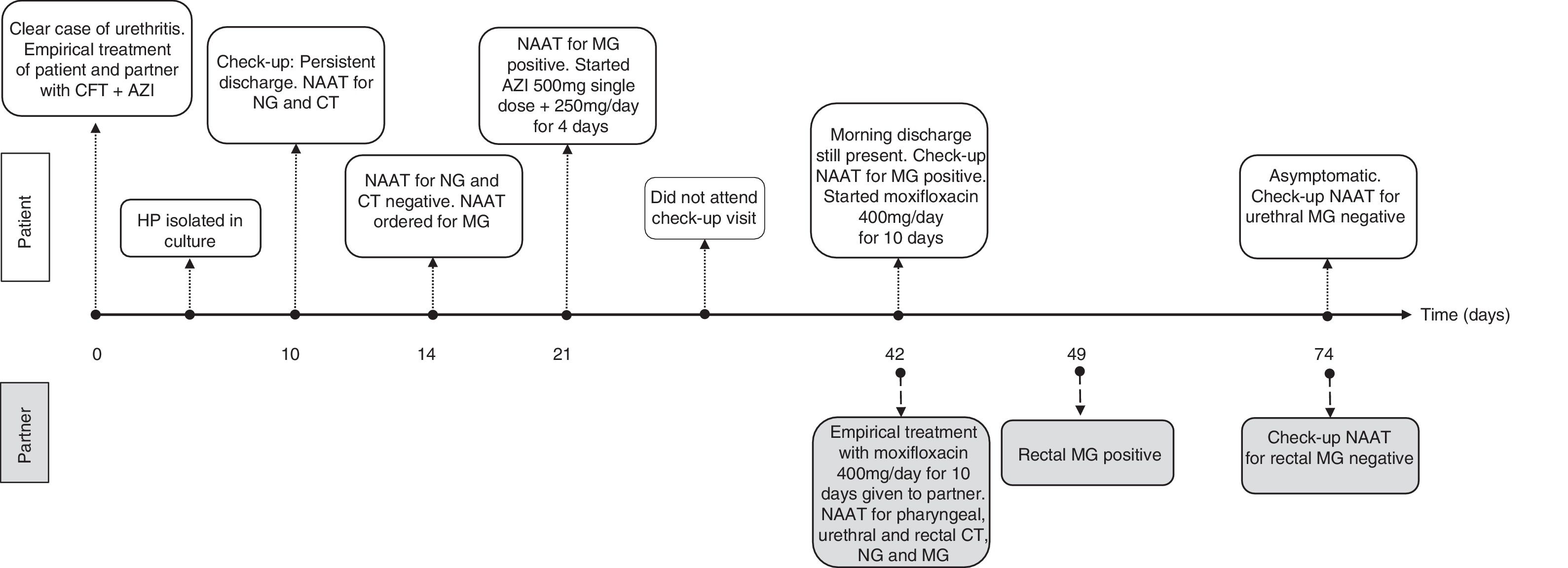
A 31-year-old male patient presented with HIV infection, which was adequately controlled with antiretroviral therapy. He reported experiencing symptoms of urethral discharge and dysuria for several weeks. He disclosed having had unprotected sex with his permanent partner within the last 3 months. Once urethral samples had been taken for culture of Neisseria gonorrhoeae (NG) and nucleic acid amplification testing (NAAT) for NG and Chlamydia trachomatis (CT) (Anyplex® CT/NG Real-time Detection kit [Seegene]), empirical treatment with ceftriaxone 1g IM and oral azithromycin 1g was prescribed for both the patient and his partner. It was recommended that unprotected sex be avoided until the results came back. Haemophilus parainfluenzae that was susceptible to the prescribed treatment was isolated from the microbiological culture, but all other tests were negative. Nevertheless, the patient continued to have discharge after 10 days and therefore the culture and dual NAAT were repeated, which were negative. NAAT for MG (RealCycler® Monotest MGTVUS [Progenie Molecular]) in urine was then requested, which gave a positive result. Given that a dose of 1g azithromycin may not have been enough to control the infection, a dose of 500mg oral azithromycin followed by 250mg/day for 4 days was prescribed, with no clinical improvement observed. Check-up NAAT showed that MG was still present in the urethra. Since macrolide-resistant MG infection was suspected, empirical treatment with moxifloxacin 400mg/day for 10 days was prescribed for both the patient and his partner. NAATs for pharyngeal, urethral and rectal MG were also conducted, with the latter proving positive. After completing treatment, the patient was asymptomatic and a test-of-cure performed at 3 weeks was negative for both. Fig. 1 shows the progress of the patient and his partner over time.
The European guideline on the management of NGU recommends performing NAAT for MG and for screening for macrolide resistance,4,5 despite the fact that these techniques are not available at many sites. At our unit, we test patients with NGU who do not respond to treatment and who have negative NG-CT NAAT results for MG, but we do not screen for macrolide resistance. The high rate of resistance of MG to this antibiotic group has resulted in single-dose azithromycin no longer being recommended for the empirical treatment of NGU in Europe.4 In patients who show no clinical or microbiological response to a 1g single dose of oral azithromycin, the guideline recommends assuming that the patient has a macrolide-resistant strain and the recommended regimen would be to treat directly with moxifloxacin,4 as shown in this case report.
The role of rectal MG as a reservoir and the need to treat asymptomatic rectal MG carriers have been controversial.6 A recent study in MSM with MG urethritis showed that up to 40% of sexual contacts undergoing a rectal exam were positive for MG.7 The presence of rectal MG is often asymptomatic and rectal MG load is higher among patients with symptomatic proctitis than among asymptomatic rectal MG carriers.8 However, the prevalence of oropharyngeal MG is very low and does not appear to constitute a major reservoir.9
The European guideline on the management of MG infections recommends examining the sexual contacts of those infected with MG and treating them in the case of positive test results.4,5 There are no cost-effectiveness studies on the treatment of the sexual contacts of patients with MG infection, although this does seem to be an effective tool for reducing incidence rate. There is also no evidence of aspects such as the natural history of the infection or preventable morbidity with treatment, and therefore screening asymptomatic patients for MG is not currently recommended.10 In high-risk populations, such as HIV-positive MSM with higher rates of asymptomatic rectal MG carriers,6 targeted screening could be more cost-effective.
To conclude, cases such as the one observed in our case report support the need for and the importance of screening the sexual partners of patients with MG infection and for treating rectal MG carriers in order to reduce the incidence of MG infections. Furthermore, macrolide resistance testing using molecular test methods at the same as screening would allow targeted treatment.
Please cite this article as: Riera-Monroig J, Fuertes I, Alsina M, Blanco JL. Relevancia del tratamiento de portadores rectales asintomáticos de Mycoplasma genitalium en hombres que tienen sexo con hombres. Enferm Infecc Microbiol Clin. 2019;37:544–545.