Parenteral nutrition-associated liver disease (PNALD) is a particularly important problem in patients who need this type of nutritional support for a long time. Prevalence of the condition is highly variable depending on the series, and its clinical presentation is different in adults and children. The etiology of PNALD is not well defined, and participation of several factors at the same time has been suggested. When a bilirubin level >2mg/dl is detected for a long time, other causes of liver disease should be ruled out and risk factors should be minimized.
The composition of lipid emulsions used in parenteral nutrition is one of the factors related to PNALD. This article reviews the different types of lipid emulsions and the potential benefits of emulsions enriched with omega-3 fatty acids.
La afectación de la función hepática asociada con la nutrición parenteral es un problema especialmente importante en los pacientes que precisan este tipo de soporte nutricional durante un tiempo prolongado. La prevalencia es muy variable según las series, y se presenta clínicamente de forma distinta en adultos y en niños. Su etiología no está bien definida y se contempla la participación de varios factores al mismo tiempo. Cuando se detecta un nivel de bilirrubina > 2mg/dl durante un período prolongado se deben descartar otras causas de hepatopatía y minimizar los factores de riesgo.
La composición de las emulsiones lipídicas empleadas en la nutrición parenteral es uno de los factores relacionados con la alteración de la función hepática. En este artículo se revisan los distintos tipos de emulsiones lipídicas, así como los posibles beneficios de las fórmulas enriquecidas con ácidos grasos omega-3.
The first safe lipid emulsion, still on the market, was developed in 1961 and consisted of soybean oil and egg lecithin. From that date, lipid emulsions became a feature of parenteral nutrition (PN). One decade later, these emulsions were combined with all the other nutrients to obtain the well known “all-in-one” admixtures currently used.
The latest generation of these emulsions includes formulas enriched with fish oil, based on omega-3 fatty acids (W3FA), and with a lower concentration of omega-6 fatty acids (W6FA), the main component of the original emulsions.
The traditional management of PN-associated liver disease (PNALD) includes the cyclic administration of PN for as short a time as possible, the early detection and treatment of catheter sepsis, the start of enteral nutrition as early as possible, the administration of ursodeoxycholic acid to decrease bile viscosity and antibiotics to prevent bacterial overgrowth, and an adequate provision of macronutrients and macronutrients in PN.
Data in the literature suggest that nutritional supplementation with fish oil may modify the inflammatory response, and some authors have reported positive results with the use of lipid emulsions with a high W3FA content in the treatment of PNALD in both children and adults.
Liver changes associated with parenteral nutritionThe administration of PN has been associated with liver changes such as steatosis, steatohepatitis, fibrosis, cirrhosis, and biliary changes such as cholestasis, cholelithiasis, and cholecystitis.1,2 These changes may occur in 25–100% of adult patients who receive PN.3 In Spanish studies, the prevalence of such changes has been reported as 30% in critically ill patients with PN, and as 36% in patients on home PN.4 If liver involvement progresses, it may lead to cirrhosis and liver and bowel transplant may be required.
Diagnosis is mainly based on bilirubin and liver enzyme levels, although the cut-off points for these markers may change depending on the hospital or in different studies.5
Moreover, the correlation between changes in these laboratory tests and histopathological findings in liver biopsies is low.2 The etiology of changes has not been fully elucidated, and is thought to be multifactorial.1,6 Changes have been related to factors associated with both PN itself, such as therapy duration, calorie overload, carbohydrate overload, amino acid overload or deficiency, carnitine, choline, or taurine deficiency, excess manganese and copper administration, and continuous infusion, and to patient-associated factors such as underlying disease, sepsis, intestinal bacterial overgrowth, the presence of short bowel syndrome, a lack of enteral stimulation, or hyperinsulinism.3,6
As regards lipids in PN, four factors have been related to liver dysfunction:
- -
Lipid dose: both a deficiency and an excess provision of essential fatty acids may cause liver damage. A lipid dose >1g/kg/day is associated with a marked increase in liver changes and a 2–5 times greater risk of experiencing some severe change in the liver,3,6 and has been included in current European guidelines as the value that should not be exceeded.7 At least 2–4% of total calories should be administered as lineoleic acid to prevent deficiency. The ratio of calories provided by carbohydrates and lipids also appears to be important, and should not exceed 60:40 in home patients according to various recommendations.7 The mechanism by which toxicity related to lipid dose occurs is not clear, but it could be changes in liver macrophages, phospholipid elimination in bile, or a blockade of liver capacity to mobilize lipids.6
- -
Lipid composition: lipid emulsions based on soybean oil, with high W6FA contents, have been associated with greater liver toxicity, particularly when they are used over a long period of time.3,6,7 To decrease this toxicity, the alternatives proposed have included lipid emulsions with lower W6FA contents, such as those consisting of mixtures of medium (MCT) and long-chain triglycerides (LCT), those based on olive oil, those containing W3FA,3,6,8 or even the provision of W3FA as the only lipid.9
- -
Phytosterolemia: lipid emulsions are mostly of plant origin and contain substantial amounts of phytosterols.3,6,10 These phytosterols have been related to liver toxicity in PN because they are considered to be able to alter cholesterol and bile acid production and to contribute to cholestasis.3,10
- -
α-Tocoferol: this may act as a potent antioxidant in cases of steatosis, protecting the liver from oxidative damage. W3FA-enriched formulas have greater amounts of α-tocoferol than those derived from soybean.
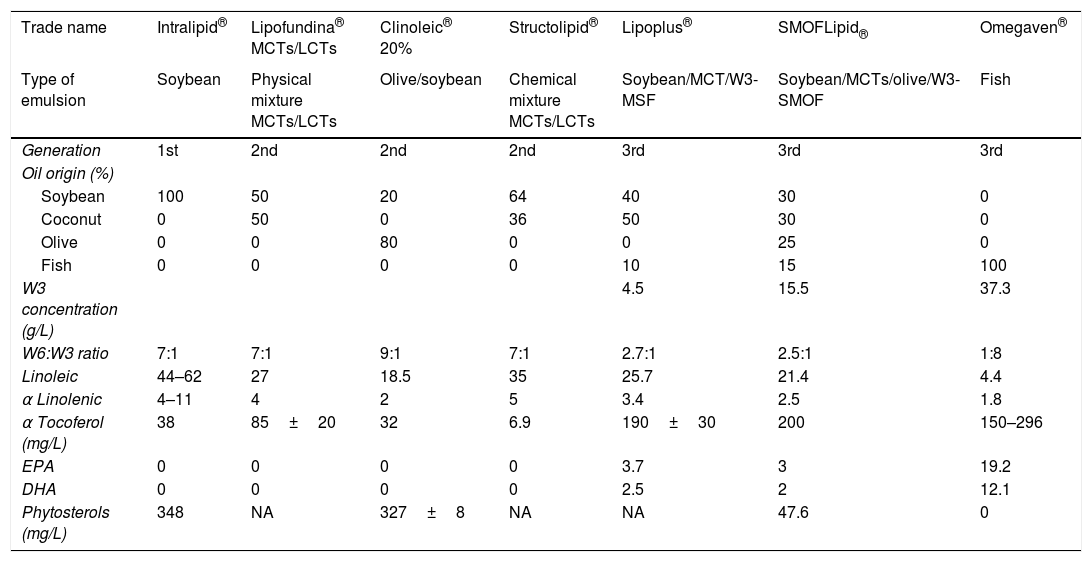
To date, there have been three generations of intravenous lipid emulsions.11 The fatty acid profile of these emulsions has evolved with our increased understanding of their metabolism and their importance in the regulation of processes triggered by aggression and immunity (Table 1).
Characteristics of lipid emulsions.
| Trade name | Intralipid® | Lipofundina® MCTs/LCTs | Clinoleic® 20% | Structolipid® | Lipoplus® | SMOFLipid® | Omegaven® |
|---|---|---|---|---|---|---|---|
| Type of emulsion | Soybean | Physical mixture MCTs/LCTs | Olive/soybean | Chemical mixture MCTs/LCTs | Soybean/MCT/W3-MSF | Soybean/MCTs/olive/W3-SMOF | Fish |
| Generation | 1st | 2nd | 2nd | 2nd | 3rd | 3rd | 3rd |
| Oil origin (%) | |||||||
| Soybean | 100 | 50 | 20 | 64 | 40 | 30 | 0 |
| Coconut | 0 | 50 | 0 | 36 | 50 | 30 | 0 |
| Olive | 0 | 0 | 80 | 0 | 0 | 25 | 0 |
| Fish | 0 | 0 | 0 | 0 | 10 | 15 | 100 |
| W3 concentration (g/L) | 4.5 | 15.5 | 37.3 | ||||
| W6:W3 ratio | 7:1 | 7:1 | 9:1 | 7:1 | 2.7:1 | 2.5:1 | 1:8 |
| Linoleic | 44–62 | 27 | 18.5 | 35 | 25.7 | 21.4 | 4.4 |
| α Linolenic | 4–11 | 4 | 2 | 5 | 3.4 | 2.5 | 1.8 |
| α Tocoferol (mg/L) | 38 | 85±20 | 32 | 6.9 | 190±30 | 200 | 150–296 |
| EPA | 0 | 0 | 0 | 0 | 3.7 | 3 | 19.2 |
| DHA | 0 | 0 | 0 | 0 | 2.5 | 2 | 12.1 |
| Phytosterols (mg/L) | 348 | NA | 327±8 | NA | NA | 47.6 | 0 |
DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; LCTs: long-chain triglycerides; MCTs: medium-chain triglycerides; MSF: MCTs/soybean/fish; SMOF: soybean/MCTs/olive/fish.
These were the first lipid emulsions to be marketed (1961). Derived from soybean oil, they contain long-chain triglycerides ([LCTs] 16–18 carbon atoms) only. They were originally intended to provide essential fatty acids to prevent their deficiency, and were subsequently accepted as a concentrated source of energy.11 They contain a high proportion of W6FA, approximately 50–60%, mainly as linoleic acid, an essential fatty acid. Cumulative observations of the adverse effects derived from the high proportion of W6FA in these initial lipid emulsions indicated the need to develop new emulsions having a much lower content of this type of fat.
The second generation (developed to decrease the provision of W6FA in the first generation products; a mixture with medium- and long-chain triglycerides)Emulsions containing 50% of MCTs have been in clinical use since 1984. MCTs have 6–12 carbon atoms and are mainly found in coconut oil. They oxidate easily and enter mitochondria without the need for carnitine. MCTs are therefore more rapidly available for tissues than LCT emulsions and are considered immunologically neutral. As they are saturated fatty acids, they are resistant to lipid peroxidation,12 do not participate in eicosanoid synthesis,8 are not stored as triglycerides, and have a ketogenic effect.8,13,14
Lipid emulsions based on a mixture of soybean oil and medium-chain triglycerides (Lipofundina®)Chronologically, this emulsion appeared after the first-generation emulsions. It is a mixture of 50% soybean oil and 50% coconut or palm oil that contains a very high proportion of MCTs.10
Lipid emulsions based on soybean and olive oil (Clinoleic®)Another solution for decreasing W6FA content was to base the emulsion on olive oil (80%), with EFA provision being completed with soybean oil (20%). This emulsion contains oleic acid as the main component, a monounsaturated acid of the omega-9 family (W9FA), and has some benefits such as resistance to lipid peroxidation, an effect that is considered neutral and poorly toxic on the immune system, and a decreased generation of pro-inflammatory cell mediators.8,12,13 It has also been postulated that some phenolic compounds contained in olive oil could provide additional advantages as being antioxidant and anti-inflammatory.15
Lipid emulsions based on a chemical mixture of soybean oil and medium-chain triglycerides. Structured lipids (Structolipid®)These are emulsions consisting of synthetic lipids obtained through the hydrolysis of soybean and coconut oils, equivalent to 64% of soybean oil and 34% of coconut oil, and subsequent random reesterification. The result is a fat that contains LCTs and MCTs in the same glycerol and in a resulting equimolecular proportion.8,12,16 It appears that fewer changes occur in the liver17 and immune system12 as compared with physical mixture or soybean emulsions.
The third generationThis refers to the incorporation of W3FA into emulsions of a prior generation, decreasing W6FA provision, to positively modulate inflammation and immunosuppression. This generation is clearly aimed at achieving a pharmaconutrient effect.
W3FA doses >0.05g/kg/day have been reported to provide benefits, with optimal doses of 0.14–0.30g/kg/day.18 However, further studies confirming these results are needed.
These emulsions are considered to be safe, and no significant adverse effects have been reported.8,13,19 They have even improved or reversed the toxicity of PN for the liver.9
Two emulsions containing fish oil are currently being marketed in Spain:
A lipid emulsion based on soybean oil, medium-chain triglycerides, and fish oil: MSF emulsion (Lipoplus®)This is an emulsion based on the second generation of the physical mixture of soybean oil and MCTs in which part of the soybean oil is replaced by fish oil to achieve final concentrations of 40%, 50%, and 10% respectively. This emulsion has been found to increase contents in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in plasma phospholipids and erythrocyte membranes, and to the modulated production of pro-inflammatory leukotrienes19 with no negative impact on gas exchange or hemodynamics.
A lipid emulsion based on soybean oil, medium-chain triglycerides, olive oil, and fish oil: SMOF emulsion (SMOFlipid®)This is based on a physical mixture of soybean oil, MCTs, and olive and fish oils at 30%, 30%, 25%, and 15% respectively. This is a new concept of emulsion that combines the conditions of an “ideal” emulsion in the light of our current knowledge, with a balanced and more physiological fatty acid composition, and a ω-6/ω-3 ratio within the optimum range.8,19 This emulsion is metabolized 40% faster than soybean oil-based emulsions.
Increased plasma levels of W3FA, together with decreased W6FA levels, reduce levels of pro-inflammatory mediators, have a neutral impact on inflammatory and immune variables, and have been shown in several studies to decrease both hospital stay and mortality and the number of days of mechanical ventilation in critically ill patients.8,12,13,20
Omegaven® (not marketed in Spain)This is a 100% ultra-refined fish oil emulsion available on the market since 1998. It is recommended that it be administered together with other lipid emulsions at a maximum dose of 1–2mL/kg. The first case in which this product was used alone in a patient with soybean allergy to prevent EFA deficiency was reported in 2005.21
Emulsions of omega-3 fatty acids alone and the improvement of parenteral nutrition-associated liver diseaseSeveral articles reporting PNALD improvement with the use of Omegaven® (as a supplement of another lipid emulsion or as a single formula) in both children22–25 and adults have been published in recent years.26–30 In adult patients, liver function improvement has been seen in a short period (4–8 weeks), but no data are available beyond 16 weeks of follow-up.29
The patient reported by Jurewitsch et al.26 initially received a mixture of soybean emulsion together with Omegaven® (0.25g/kg/day) for four weeks. In view of the stabilization of bilirubin levels, Omegaven® alone was continued at the same dose as maintenance therapy, resulting in the normalization of bilirubin levels at 16 weeks, decreased hepatomegaly, and the disappearance of cholestasis in a biopsy performed five months after treatment start.
Xu et al.27 reported a series of 15 adults (9 females and 6 males) in which an emulsion of soybean oil and MCTs was replaced by Omegaven® (0.15–2g/kg/day, maximum 10g/day) for two months. Eighty percent of the subjects experienced a normalization of esterified bilirubin levels within four weeks, as well as decreased cholestasis and inflammation at biopsies.
Burns et al.28 replaced an emulsion based on soybean oil only by Omegaven® (1g/kg 5 days weekly) in a patient, resulting in laboratory data improvement at five weeks and a normalization of bilirubin levels at eight weeks. These results persisted at 29 weeks.
Venecourt et al.29 reported a patient receiving an emulsion of soybean and olive oil whose bilirubin levels decreased (at 8 weeks) and whose transaminase levels normalized (at 10 weeks) after switching to Omegaven® (1g/kg 6 days weekly).
The most recent series, reported by Pastor-Clerigues et al.,30 provided data on the anti-inflammatory and antifibrotic effect of Omegaven® administered to two patients for four months (1g/kg 5 days weekly). Liver function impairment recurred on the replacement of this emulsion by a mixture of soybean oil, MCTs, and olive and fish oils.
ConclusionsPatients diagnosed with PNALD have a high mortality rate as a result of liver cirrhosis/failure if PN discontinuation or liver and bowel transplant is not possible. An understanding of all the mechanisms involved in the development of this disease and the availability of effective and safe treatments are therefore especially important. In recent years there has been an increasing interest in treatment and prevention using W3FA-enriched lipid formulas. Although the exact mechanism is unknown, it could lie in a decreased hepatic apoptosis due to the effect of EPA and DHA contained in these types of formulas, along with an essential anti-inflammatory role, and to decreased W6FA provision.
Elsewhere31 in this issue of the journal we report the case of a patient who was treated with W3FA alone for 50 weeks, the longest treatment with Omegaven® reported in Spain. We believe that this case may lead to a new approach to the treatment of patients with PNALD. The improvement in the laboratory results was important, but the change in portal flow direction detected in the imaging tests was even more significant as indirect evidence of portal hypertension improvement. No deficiencies in essential fatty acids were found during the follow-up period.
Larger series and longer follow-up periods will provide more information about the ideal proportions of W3FA and W6FA and the dose that should be given in PN to cover nutritional requirements and prevent hepatic complications.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Zugasti Murillo A, Petrina Jáuregui E, Elizondo Armendáriz J. Hepatopatía asociada a nutrición parenteral y emulsiones lipídicas. Endocrinol Nutr. 2015;62:285–289.