Diagnosis of type 2 diabetes mellitus encompasses multiple pathophysiological and clinical situations. Type 2 diabetes mellitus is characterized by a long and changing natural history. Personal circumstances and preferences also condition the actual effectiveness and safety of drugs used. In recent decades, modern drugs have markedly expanded and improved therapeutic options. However, their effectiveness remains limited in clinical practice. The main objective of decreasing macrovascular complications is not fully proven. Adverse events, especially hypoglycemia and weight gain, are still frequent and decrease treatment adherence. The constant loss of endogenous islet cell reserve is the main determinant of the need for intensified therapies. Current treatments have failed to improve long-term beta cell mass/function. It is desirable to move forward to obtain new drugs that offer solutions sustainable in the long term. These drugs should be able to fit the individual circumstances and preferences of patients with diabetes mellitus.
Dentro del diagnóstico de diabetes tipo 2 se incluyen múltiples situaciones clínicas y fisiopatológicas. La diabetes tipo 2 se caracteriza por una larga y cambiante historia natural. Las circunstancias y preferencias personales condicionan asimismo la eficacia y seguridad real de los fármacos empleados. En las últimas décadas se han ampliado y mejorado notablemente las opciones terapéuticas, sin embargo su eficacia sigue siendo limitada en la práctica clínica. El objetivo principal de reducción de las complicaciones macrovasculares no está completamente probado. Los efectos adversos, especialmente hipoglucemia y aumento de peso, son todavía frecuentes y reducen la adhesión al tratamiento. La pérdida constante de reserva insular endógena es el principal determinante de la necesidad de intensificación del tratamiento. Los tratamientos actuales no han demostrado mejorar la masa/función de las células beta a largo plazo. Es deseable seguir avanzando para conseguir tratamientos farmacológicos que ofrezcan soluciones sostenibles a largo plazo y adaptables a las circunstancias individuales y preferencias de los pacientes con diabetes mellitus.
The term diabetes mellitus (DM) encompasses multiple diseases mainly characterized by inadequate control of carbohydrate metabolism. Most of these are chronic conditions in which impaired management of macronutrients and other associated events (inflammation, prothrombotic state...) may cause complications in any organ, although vascular complications are the most common and disabling. DM is one of the most significant causes of morbidity and mortality and is increasing worldwide.
This pathophysiological heterogeneity and complexity preclude a single and simple treatment. In addition to the indispensable and sustained approach using lifestyle measures, drug treatment is currently multifactorial almost since diagnosis.
The most common type of DM is type 2 DM (T2DM), which may account for approximately 90% of DM cases.1 However, it is obvious that this diagnosis includes phenotypes with highly different genetic basis, pathophysiology, and clinical behavior. Moreover, different measures are required depending on the point in the natural history of disease where each patient is. Finally, but no less important, individual characteristics have a substantial impact on the potential efficacy and safety of each therapeutic option.
In recent decades, very interesting new options and modifications of already known therapeutic classes (new insulins, sulfonylureas...) have been incorporated into drug treatment for DM, and drug classes with novel mechanisms of action have been introduced. This review will try and describe currently unmet objectives in the pharmacological approach to DM in an attempt to identify new challenges for DM treatment in the future.
Efficacy of current treatments for diabetesAntihyperglycemic efficacyIntensive treatment aimed at achieving a level of glycosylated hemoglobin A1c (HbA1c) less than 7% markedly decreases the incidence of microvascular disease in patients with T2DM.2 The efficacy of oral treatments for DM (measured as HbA1c decrease) is approximately 1% (Table 1).3 Although insulin therapy has traditionally been considered to have an unlimited hypoglycemic potency, the HbA1c goal is difficult to achieve in clinical practice with the current strategies and formulations. Even when intensive strategies for T2DM control are used, such as treatment with multiple basal-bolus insulin doses, HbA1c levels<7% are achieved in less than 60% of patients, as shown by the published meta-analyses.4,5
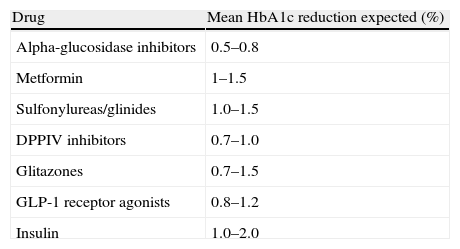
Hypoglycemic efficacy of drugs for type 2 diabetes mellitus.
| Drug | Mean HbA1c reduction expected (%) |
| Alpha-glucosidase inhibitors | 0.5–0.8 |
| Metformin | 1–1.5 |
| Sulfonylureas/glinides | 1.0–1.5 |
| DPPIV inhibitors | 0.7–1.0 |
| Glitazones | 0.7–1.5 |
| GLP-1 receptor agonists | 0.8–1.2 |
| Insulin | 1.0–2.0 |
HbA1c: glycosylated hemoglobin A1c; DPPIV: dipeptidyl peptidase IV; GLP-1: glucagon-like peptide 1.
Modified from Giugliano et al.4
The new GLP-1 receptor agonists (GLP-1ra) may achieve greater HbA1c reductions than some oral drugs (−0.97% [95% confidence interval −1.13 to −0.81%]),6 specially long-acting GLP1ra as compared to DPPIV inhibitors.7 In addition, they have associated advantages in terms of weight and blood pressure reduction. However, treatment is still limited by the occurrence of gastrointestinal untoward effects, cost, and lack of experience with long-term use.
Efficacy in overall control of cardiovascular risk factorsThe beneficial effect of intensive therapy on macrovascular disease is not no completely proven.8 Two meta-analyses of clinical studies assessing standard versus intensive treatment in cardiovascular (CV) risk reduction concluded that intensive therapy significantly decreased the risk of CV events, but not CV death or all-cause mortality.9,10
The most recent data confirm that we are far from achieving the control goals proposed in T2DM. The results of the National Health and Nutrition Examination Survey for the 1999–2010 period were published in 2013 in the New England Journal of Medicine.11 Although an improvement was seen in these years, 33.4–48.7% of patients did not meet the blood glucose (HbA1c), lipid (LDL cholesterol), weight, and blood pressure goals. In a study on data from 286.791 patients conducted in Spain (the Econtrol study), only 12.1% of adults with T2DM achieved the goals of HbA1c<7%, LDL cholesterol<100mg/dL, and blood pressure<130/80mmHg.12
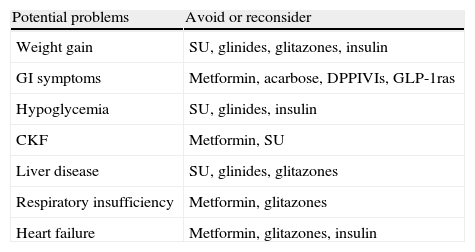
Adverse effects associated to current treatmentsTreatments used for T2DM are associated to common adverse effects which are potentially serious in some cases (Table 2). The most common adverse effects with standard therapies are hypoglycemia and weight increase.
Potential problems associated to drug treatment options.
| Potential problems | Avoid or reconsider |
| Weight gain | SU, glinides, glitazones, insulin |
| GI symptoms | Metformin, acarbose, DPPIVIs, GLP-1ras |
| Hypoglycemia | SU, glinides, insulin |
| CKF | Metformin, SU |
| Liver disease | SU, glinides, glitazones |
| Respiratory insufficiency | Metformin, glitazones |
| Heart failure | Metformin, glitazones, insulin |
GI: gastrointestinal; GLP-1ras: GLP-1 receptor agonists; CKF: chronic kidney failure; DPPIVIs: dipeptidyl peptidase IV inhibitors; SUs: sulfonylureas.
Modified from Rydén et al.13
Treatment intensification in T2DM (mainly intended to decrease CV risk) has been associated to weight increase with treatments used to date. An interesting post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes study reported the weight increases seen in the intensive treatment (+3kg) and standard treatment (+0.3) arms and suggested the potential associated factors.14 Although the analysis concluded that drug treatment alone would be responsible for 15% of the weight increase, raw data suggest significant differences depending on treatment used: patients on intensive treatment who did not use insulin or thiazolidinediones decreased weight by 2.9kg, but patients in this same arm who used both treatments showed weight increases ranging from 4.6 and 5.3kg.
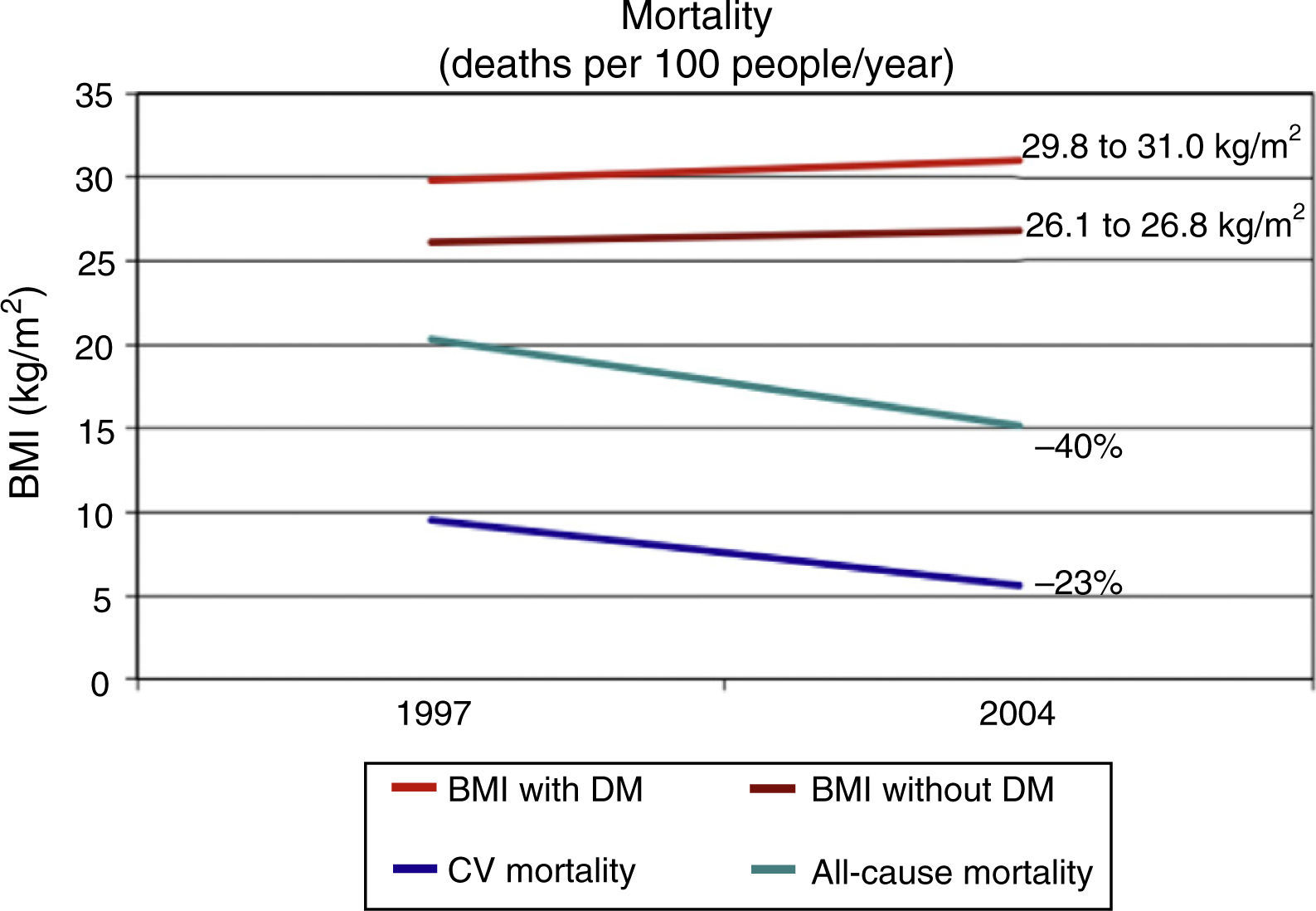
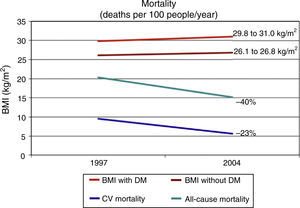
Presence of DM is associated to a decrease in life expectancy which had been estimated at 10 years for people diagnosed in middle age.15 Some epidemiological data suggest that the morbidity and mortality rates have clearly decreased in people with DM from developed countries. The National Health Interview Surveys analyzes every year data from 107,000 non-institutionalized North American citizens. A study reported by Gregg et al. used data from the National Health Interview Surveys corresponding to ≥242,383 adults aged 18 years from the 1997–2004 surveys, whose life status was followed up until December 2006 (10 years).16 In an analysis adjusted for multiple variables, CV mortality decreased by 40% and all-cause mortality by 23% from 1997 to 2004 in people with DM. However, this study again confirms the difficulty in reducing obesity, as the body mass index increased in that same period from 29.8 to 31.0kg/m2 in people with DM (Fig. 1).
Trends in all-cause and cardiovascular (CV) mortality and body mass index (BMI) in US adults (n=242,383) with (DM) and without diabetes (no DM) between 1997 and 2004.
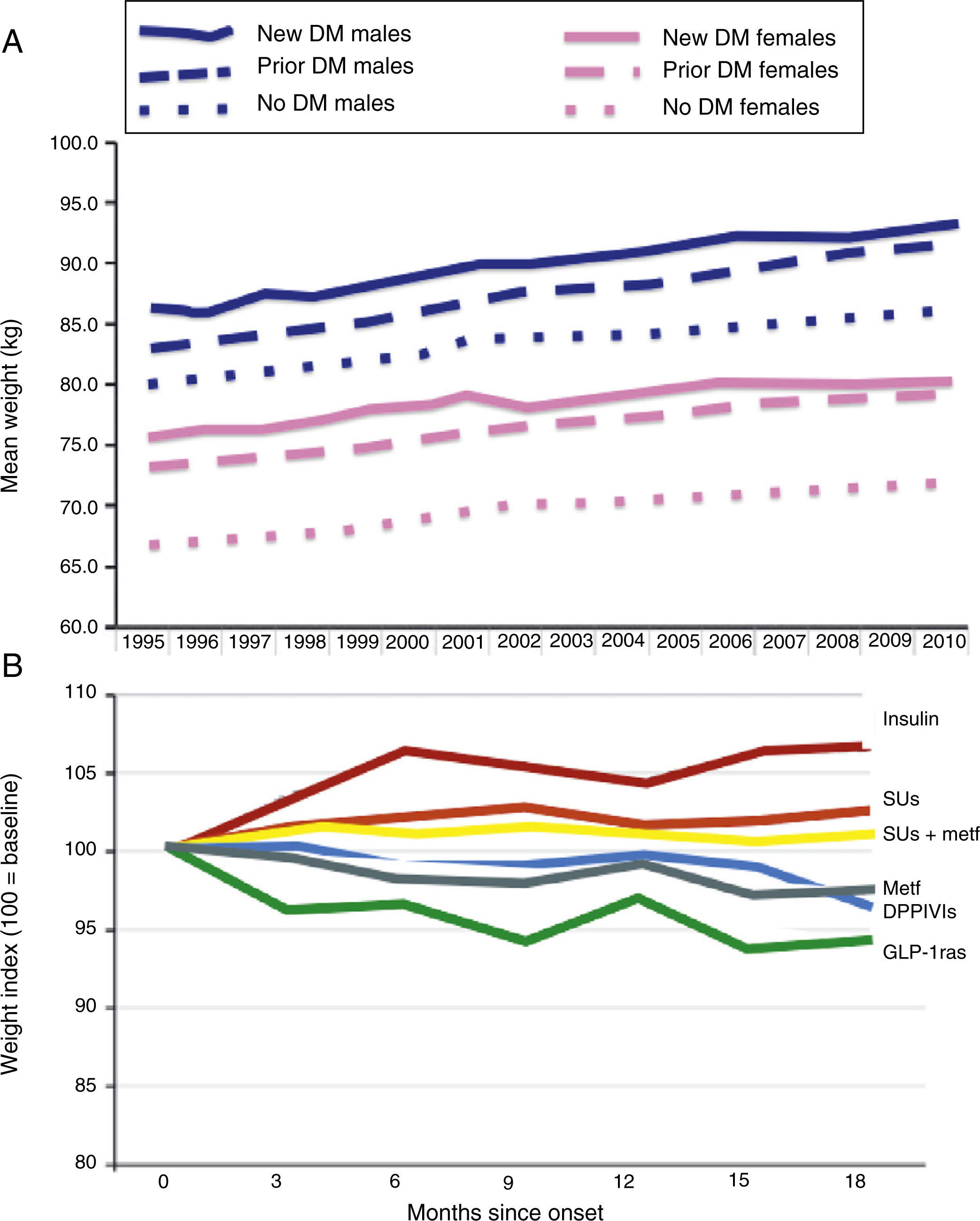
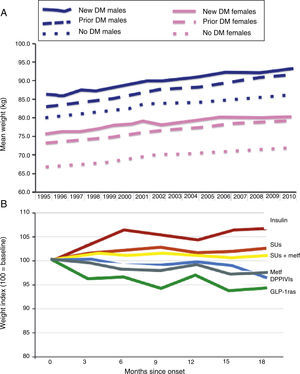
Epidemiological studies clearly show a constant weight increase in the population with T2DM. This secular trend appears to have decrease as the result of recent introduction of new incretin mimetic treatments including GLP-1ra and DPPIV inhibitors. Morgan et al. reviewed data collected at 350 primary care centers in the United Kingdom, corresponding to 10 million patients, 184,474 with DM (2010), in the 1995–2010 period.17 As shown in Fig. 2, use of these drugs appears to result in a lower trend to weight increase in these patients. Other future options (sodium-glucose co-transporter-2 inhibitors, etc.) should try such a reverse trend.
Secular trends in weight change in people with T2DM (A) and impact of alternative antihyperglycemic treatments (B). DPPIVIs: dipeptidyl peptidase IV inhibitors; GLP-1ras: glucagon-like type 1 peptide receptor agonists; SUs: sulfonylureas.
The most limiting adverse effect with use of drugs for DM is possibly hypoglycemia. Hypoglycemia is a significant reason for non-compliance and may increase mortality and CV and hospitalization risk.18,19 A recent study reviewed the drugs or therapeutic classes associated to urgent hospital admission in people over 65 years of age.20 Insulins and oral antidiabetic agents ranked second and fourth respectively by frequency of associated hospital admissions. Endocrine treatments accounted for 42.1% of all emergency admissions in this population, and hypoglycemia was the reason for admission in 94.6% in them.20
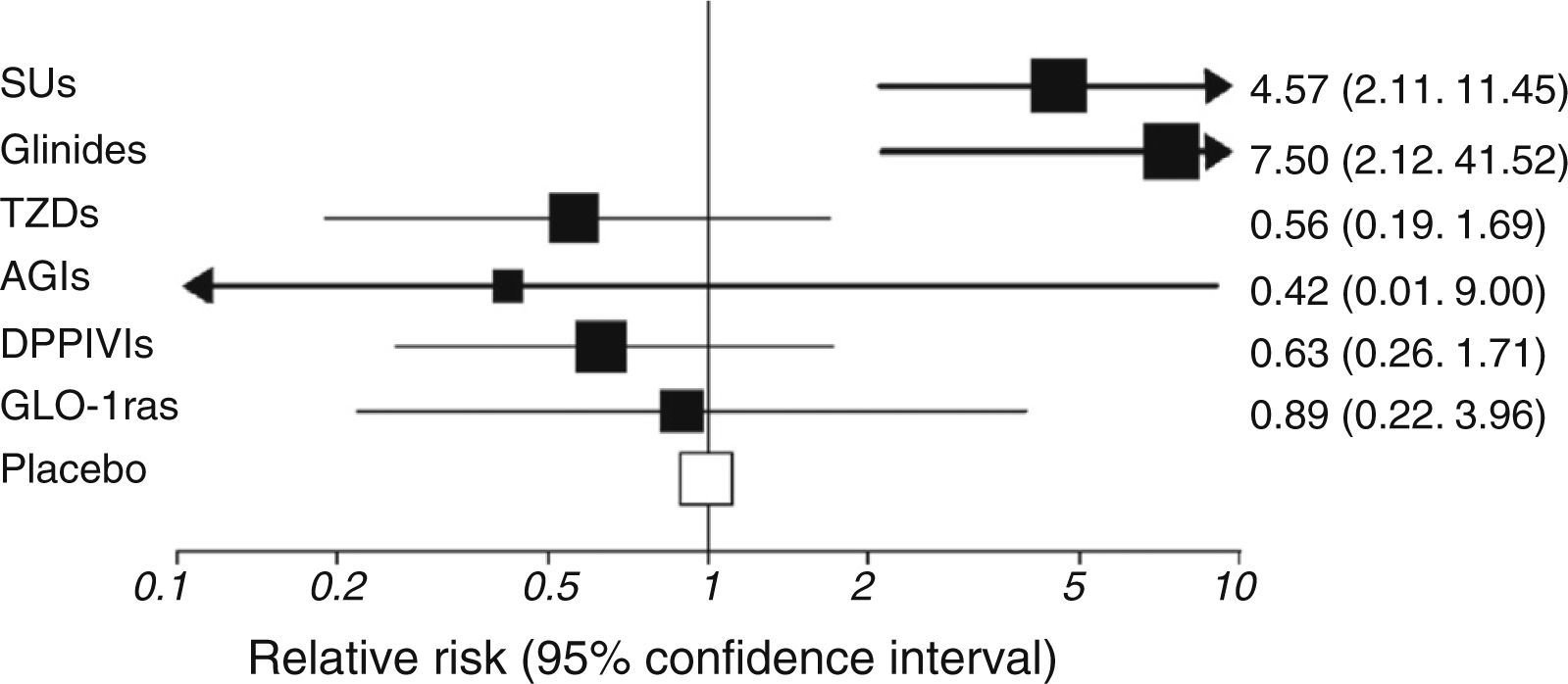
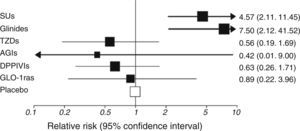
Risk of hypoglycemia is widely different depending on treatment used and its combinations (Fig. 3).21 Risk was lower with the most recent treatments (incretin mimetics, sodium-glucose cotransporter-2 inhibitors…), and it may be expected that molecules that minimize this major acute complication continue to be developed in the future.
Risk of hypoglycemia associated to different treatments for type 2 diabetes mellitus added to metformin. AGIs: alpha-glucosidase inhibitors; DPPIVIs: dipeptidyl peptidase IV inhibitors; GLP-1ras: glucagon-like type 1 peptide receptor agonists; SUs: sulfonylureas; TZDs: thiazolidinediones.
All current treatments are associated to potential adverse effects (Table 2). While most such effects are not severe, they are common and responsible for a drug significant discontinuation rate.
An example of this is the current drug of first choice, metformin. The rate of patients with gastrointestinal adverse effects reported varies widely (5–50%).22 Metformin discontinuation is more common in clinical practice than reported in the literature. Patients in whom metformin is contraindicated should be added to this discontinuation rate. Use of glitazones is not considered appropriate either in many of these patients because of the limitations and side effects of these drugs, which leaves a significant niche of patients with no specific insulin sensitizing treatment.
Sustainability of long-term efficacy: beta cell preservationLoss of adequate insulin secretion is the key pathophysiological event in DM onset and development.23 In people with T2DM, both qualitative (loss of the first phase of secretion in response to intake, loss of normal pulsatility,…) and quantitative (reduction in beta cell mass/endogenous islet cell reserve) changes occur.24 Decreased endogenous islet cell reserve marks progression in the natural history of T2DM and the constant need to increase drug treatment until exogenous insulin therapy is started.25 Duration of the effect of current non-insulin drugs is limited by loss of mass and function of pancreatic beta cells.26 Improvement in blood glucose control represents a relief for normal function of the remaining beta cell mass, but this is a transient effect.27 Several drug classes (glitazones, incretin mimetics) have shown in vitro results suggesting improvement in beta cell mass/function.28,29 This has not translated to date in evidence showing sustainability of their long-term efficacy (more than 3–5 years) in clinical use in humans, as required by a chronic disease such as T2DM.
Objectives not contemplated with current treatmentsOther facets of the complex prism of DM and insulin resistance are not specifically addressed by current treatments.
Inflammation/oxidative stressA two-way relationship exists between inflammation and insulin resistance-T2DM. A proinflammatory state may possibly be involved in the origin of T2DM, and T2DM also causes inflammation.30,31 Part of the CV risk associated to T2DM may be caused by oxidative inflammation.32 Insulin secretion by beta cells may also be threatened by inflammatory mechanisms mediating the known glucotoxicity and lipotoxicity events.27 Some drugs currently being developed act specifically on these targets and could provide benefits additional to purely antihyperglycemic benefits.33
Prothrombotic stateT2DM leads to a prothrombotic state that increases the risk of ischemic CV events,34 including stroke. Hypoglycemic treatment modifies risk of stroke comparatively less than risk of other major CV events such as myocardial infarction.35,36 This prothrombotic state not controlled by current antidiabetic therapies may usually contribute to a relative failure in reduction of macrovascular complications.
Individualized treatmentAlthough one of the main objectives of the most commonly used consensus treatment for T2DM (ADA-EASD 2012)37 is to promote individualized treatment, many professionals use rigid drug treatment algorithms.
Today, a long treatment period is needed to show drug inefficacy/intolerance. Advances in pharmacogenomics and other clinical data should allow for individual preselection of drugs to be used.
An ideal drug treatment should allow for selecting drugs:
- •
with actions aimed at the specific pathophysiological changes present in an individual at each moment of his/her natural history.
- •
with a dosage and mechanism of action adapted to patient preferences and lifestyle,
- •
predicted to have a high benefit/risk ratio as determined by the clinical and genetic profile of each patient.
There have been huge advances in treatment for T2DM in the past decades. Associated complications have been reduced, and life expectancy of people with T2DM has improved. However, current options for a disease such as this, with a high and increasing prevalence, continue to have significant efficacy and safety limitations. There are many underlying pathophysiological changes in T2DM, which has multiple clinical forms that are not always adequately addressed with current drugs. Continued progress is desirable to achieve drug treatments that provide long-term sustainable solutions that may de adapted to the individual circumstances and preferences of patients with DM.
Conflicts of interestF. Gomez-Peralta. Researcher: L, SA, NN, B; speaker/consultant: L, N, NN, AZ, BMS, E, MSD, GSK.
C. Abreu Padín. Researcher: L, SA, NN, B; speaker/consultant: A, L, N, NN, AZ, BMS, E, MSD.
Laboratories: AZ: Astra-Zeneca; B: Boehringer Ingelheim; BMS: Bristol-Myers Squibb; E: Esteve; GSK: Glaxo Smith Kline; L: Lilly; MSD: MSD; N: Novartis; NN: Novo Nordisk; SA: Sanofi-Aventis.
Please cite this article as: Gomez-Peralta F, Abreu Padín C. ¿Necesitamos nuevos tratamientos para la diabetes tipo 2? Endocrinol Nutr. 2014;61:323–328.