Appearance of a thyroid nodule has become a daily occurrence in clinical practice. Adequate thyroid nodule assessment requires several diagnostic tests and multiple medical appointments, which results in a substantial delay in diagnosis. Implementation of a high-resolution thyroid nodule clinic largely avoids these drawbacks by condensing in a single appointment all tests required for adequate evaluation of thyroid nodule. This paper reviews the diagnostic and functional structure of a high-resolution thyroid nodule clinic.
La aparición de un nódulo tiroideo se ha convertido en un hecho cotidiano en la práctica clínica diaria. Habitualmente, la adecuada evaluación del nódulo tiroideo requiere la realización de diversas pruebas diagnósticas y múltiples citas médicas, con la consiguiente demora diagnóstica. La instauración de una consulta de alta resolución de nódulo tiroideo evita en gran medida estos inconvenientes, condensando en una única cita todas las pruebas necesarias para la correcta evaluación del nódulo tiroideo. En este trabajo revisamos cuál debe ser la estructura diagnóstica y funcional de una consulta de alta resolución de nódulo tiroideo.
Thyroid nodules are increasingly being found in clinical practice, particularly because of widespread use of imaging tests and increasing awareness of health care proffessionals.1,2
The primary objective when assessing thyroid nodule disease (TND) should be to differentiate benign thyroid nodules from those with thyroid cancer. Three main elements are usually required to adequately perform such differentiation: (a) endocrinological assessment; (b) thyroid ultrasound examination; and (c) thyroid cytology.3
This diagnostic process involves a significant number of medical visits, a long delay, and a significant inconvenience for patients (loss of working hours, economic damage, and anxiety due to delay in diagnosis).
In current medicine, maximizing efficiency and cost-effectiveness of any diagnostic strategy should be paramount. Various national groups, aware of the importance of this aspect, have promoted implementation of high-resolution thyroid nodule clinics in standard clinical practice. The purpose of such clinics is to perform all examinations needed for adequate diagnosis of TND in a single medical act, thus maximizing diagnostic efficiency.4–6
This article, which is the result of the cumulative experience of three large national groups, discusses the diagnostic and functional structure of a high-resolution thyroid nodule clinic.
The clinical problem: thyroid noduleA thyroid nodule is defined as a discrete lesion within the thyroid gland which differentiates from the surrounding thyroid parenchyma on radiographic images.1
Although a thyroid nodule may be due to various thyroid and extrathyroid conditions, the most common benign etiology is usually a colloid nodule, while a finding of malignancy is mainly due to the presence of a papillary carcinoma.7
Thyroid nodule is a very common condition in daily clinical practice. Its prevalence increases linearly with age, radiation exposure, and iodine deficiency. Thyroid nodule is 10 times more prevalent in females as compared to males.8 Nodules are found by palpation in 4–8% of adults, disclosed by ultrasound examination in 20–67% of patients,1 and found in 50% of necropsies.9,10
In a patient with TND, clinical management should be aimed at ruling out malignancy, occurring in 5–15% of thyroid nodules.11 When malignancy exists in a given nodule, early diagnosis increases the chance that thyroid cancer is limited to the gland and, depending on size that the condition may be resolved with surgery alone. Early diagnosis of thyroid nodule is also of paramount importance for patients, who experience substantial anxiety and cancerophobia until cytological diagnosis is made. A delay in thyroid nodule diagnosis increases the chance of extrathyroid tumor extension and more aggressive and expensive surgery, as well as the need for ablation of thyroid remnants with radioiodine, with the resultant impact on patient quality of life and associates costs.6
High-resolution thyroid nodule clinicThe role of a high-resolution thyroid nodule clinicPublic health systems currently face the challenge of providing adequate care to the population with limited resources. These resources are even more reduced at times of economic restraints. The strategies available in this situation includes: (a) to reduce services or benefits; (b) to maintain benefits decreasing their quality; (c) to improve internal efficiency (do more with the same resources, stop doing what gives no added value); and (d) any combination of the above.12
It is therefore not surprising that at a time such as this, when sustainability of the different national health systems has become a priority, improving diagnostic efficiency is one of the most advisable options.13
A perfect example of diagnostic yield and efficiency are such high-resolution clinics, based on simultaneous cooperation of various specialists and on performance of multiple diagnostic tests. High-resolution clinics have been shown to be superior to standard clinics in different diseases (diabetes, hepatocellular carcinoma, heart failure, thyroid cancer) both in terms of clinical outcomes and economic efficiency.14–17
TNE is undoubtedly becoming a significant healthcare burden for national health systems because of its high prevalence and the need for long-term follow-up. This statement is supported by the following data: if the estimated annual incidence of thyroid nodules is 0.1% and the estimated Spanish population is 46,116,779,18 more than 46,000 new thyroid nodules may expected to occur annually in Spain and to require study to rule out malignancy in them.
Therefore, availability of a diagnostic strategy for TND that is able to precisely and efficiently differentiate malignant from benign nodules, to minimize iatrogeny inherent in any diagnostic process, and to avoid unnecessary and potentially harmful surgery should be an indispensable requirement in TND management.19
Implementation of high-resolution thyroid nodule clinics is an adequate response to this need. The spirit of such clinics is to unify in the same physical space and time frame integral thyroid nodule care, performing on the same day and setting the endocrinological assessment, thyroid ultrasound examination, and fine needle aspiration (FNA). Integration of the different diagnostic tools and team work in the thyroid nodule clinic allow for seeing a higher number of patients per year, require less diagnostic tests, and shorten diagnostic and treatment times. Because of this, implementation of thyroid nodule clinics is gradually increasing.4–6
To date, three national groups have reported their experience with single act thyroid nodule clinics, whose organization and structure are discussed in the following section.
Review of reported experiencesTofé-Povedano et al. were the first to report their results in 2009.4 Three endocrinologists and two pathologists participate in this model of high-resolution thyroid nodule clinic. An endocrinologist performs thyroid ultrasound examination (using a 6–13MHz probe) and FNA (using winged 18–22G needles and 10cc syringes). Local anesthesia is not used for puncture, and 2–3 punctures are done per nodule studied, with no in situ verification.
Sebastián-Ochoa et al. reported in 2011 their clinical experience at the high-resolution thyroid nodule clinic.5 This second model of clinic has a different organization and involves participation of one endocrinologist, three radiologists, and two pathologists. Initial evaluation is done by the endocrinologist, while a radiologist performs thyroid ultrasound examination (using a 6–13MHz probe), and a pathologist performs the puncture (guided by the radiologist) using a 21G needle attached to a plastic 20mL syringe. Thyroid puncture is performed without local anesthesia, and a sample is taken from each nodule. In situ verification is not done.
Finally, Castells et al. reported in 2012 their results in a monographic thyroid nodule clinic.6 An endocrinologist and a pathologist are involved in this clinic model. The endocrinologist performs thyroid ultrasound examination using a 6–11MHz probe and FNA using a 23–25G needle attached to a disposable 10mL syringe. The number of punctures depends on nodule characteristics, and prior anesthesia is not used either. In this clinic model, cytology is assessed in situ.
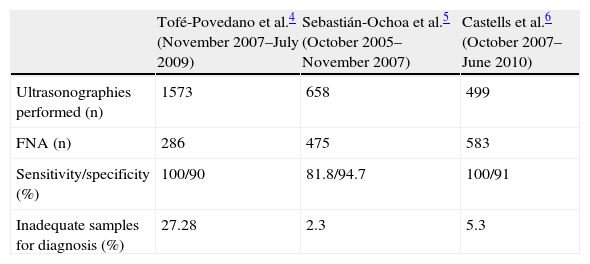
Table 1 shows the results achieved in each clinic.
Comparison of results in different high-resolution thyroid nodule clinics.
| Tofé-Povedano et al.4 (November 2007–July 2009) | Sebastián-Ochoa et al.5 (October 2005–November 2007) | Castells et al.6 (October 2007–June 2010) | |
| Ultrasonographies performed (n) | 1573 | 658 | 499 |
| FNA (n) | 286 | 475 | 583 |
| Sensitivity/specificity (%) | 100/90 | 81.8/94.7 | 100/91 |
| Inadequate samples for diagnosis (%) | 27.28 | 2.3 | 5.3 |
FNA, fine needle aspiration.
As seen in the different experiences reported. There is no single functional organization possible or single adequate model of high-resolution thyroid nodule clinic, but each endocrinology unit has adapted to its specific functional situation and has organized the thyroid nodule clinic as efficiently as possible.
Thus, ultrasonography and FNA are performed by endocrinologists in two of the above examples. This diagnostic approach is cost-effective because the endocrinologist, based on all required elements (clinical data, thyroid function tests, and ultrasonographic results), decides the most adequate therapeutic approach.20,21 This option also gives greater autonomy to physicians and has been shown to be cost-effective for reducing waiting times.4,6 It should be noted that an indispensable requirement for successful results in this model of clinic is adequate theoretical and practical training in thyroid ultrasound examination, which is obtained by performing at least 100–125 supervised examinations (of which 70% should be diagnostic and 30% should be ultrasound-guided FNA).20 Thus, thyroid puncture results are clearly related to the learning curve and prior experience in FNA, as shown by the Tofé-Povedano et al. study, where the number of punctures adequate for diagnosis increased in parallel to the experience gained, and was also greater in endocrinologists previously trained in thyroid FNA.4
Another possible model of single act thyroid nodule clinic is based on a diagnostic network coordinating different services (endocrinology–radiography–pathology), where initial evaluation would be done by the endocrinologist, subsequent ultrasonography by the radiologist, and final FNA by the pathologist, all of them on the same day and physical setting. This option, which is only valid, requires adequate coordination and relationship between the departments involved, which is not always simple.5
The discussed models of high-resolution thyroid nodule clinic are not the only ones possible, and there are probably other feasible alternative models. The essential thing, in fact, is that each medical department implements the model that best adapts to its daily clinical practice and to qualification of its professionals. It should also be reminded that when a thyroid nodule is created, it is critical to assess the results achieved to identify any weaknesses and determine potential points for improvement, in order to achieve a more effective and efficient diagnostic structure.
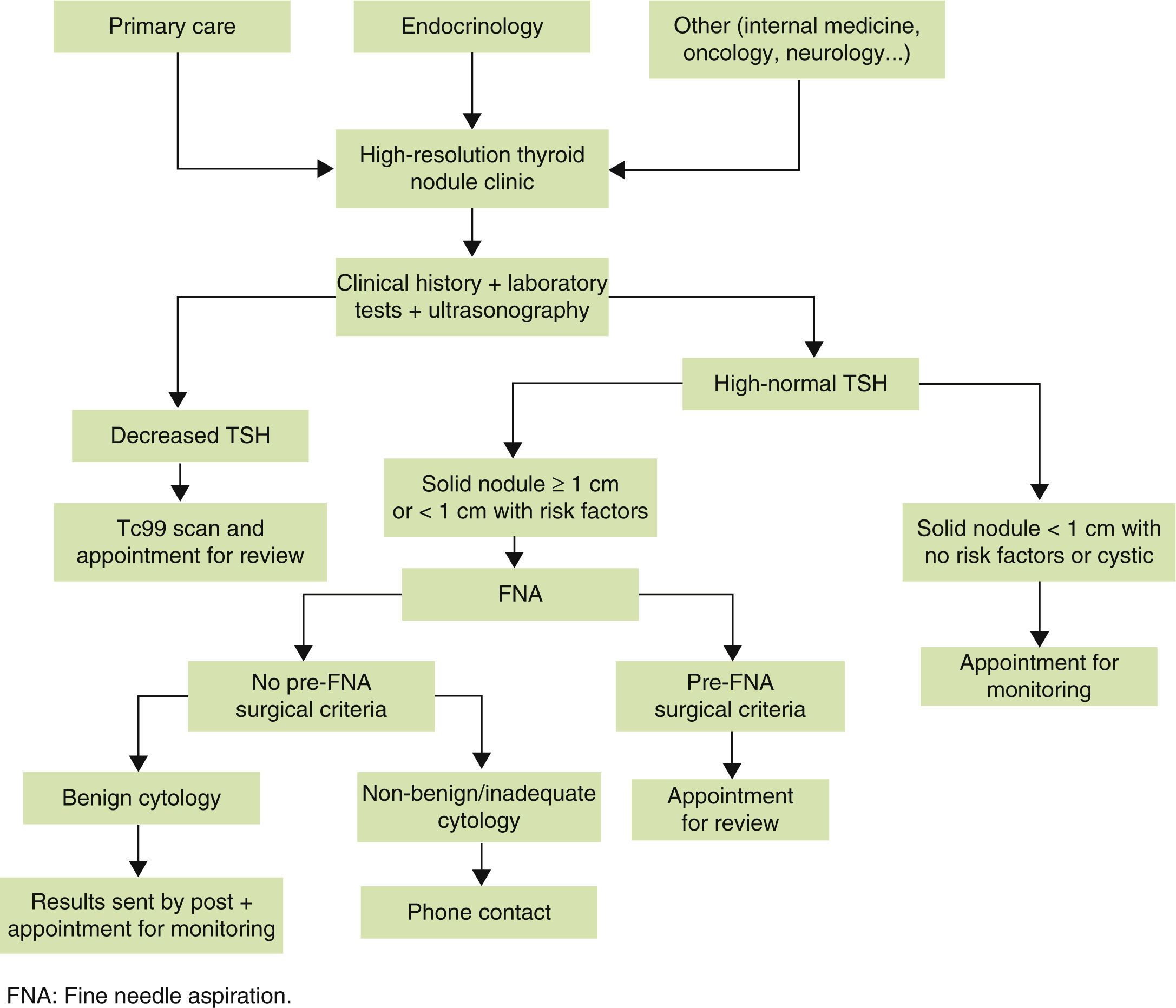
Referral criteria and functional organizationThe main referral criterion at the high-resolution thyroid nodule clinic should be the suspicion, clinical finding (by examination), or radiographic detection (using tests other than thyroid ultrasonography) of a thyroid nodule. Since the primary objective of a high-resolution thyroid nodule clinic is fast and efficient study of TND, thyroid nodules that will most benefit from study at such clinic should be selected. We therefore think that nodules first detected and never examined using ultrasonography and FNA should preferentially be referred to the high-resolution thyroid nodule clinic, while the benefit achieved in already known and/or previously studied nodules will not be so significant.
An indispensable requirement for adequate referral to the high-resolution thyroid nodule clinic should be that recent thyroid function tests should be available. Measurement of thyroid-stimulating hormone (TSH) is indispensable, while measurement of free thyroxine (FT4) and anti-peroxidase antibody (TPO Ab) is highly recommended.22
The high-resolution thyroid nodule clinic should be open to any physician, provided the referral criteria are adequately met. The greatest proportion of patients will of course come from the endocrinology department and primary care, but patients will also be referred from other specialties (internal medicine, neurology, oncology…). It should be noted that for adequate referral to the high-resolution clinic, prior dissemination of the objectives of the clinic and the referral criteria is essential.
Although the high-resolution thyroid nodule clinic is organized and designed as a single act clinic, this does not mean that the cytological result is reported on the same day of the medical visit. In fact, in our clinic model, patients receive the cytological results at a later time. While rapid staining procedures (Diff-Quik) are available for air-dried cytological smears which allow for diagnosis in less than 10min, these are not precise enough to adequately visualize nuclear grooves (the distinctive cytological feature of papillary thyroid carcinoma) or “salt and pepper” nuclear chromatin (cytological feature typical of medullary carcinoma). In these situations, use of the Papanicolaou or Romanowsky stains is preferred because they are more precise and have lower false negative rates, although they have as disadvantage that they do not allow for fast cytological diagnosis as Diff-Quik.23
Similarly, delayed cytological results should not represent any disadvantage because patients, informed about the procedure, are sent the cytological results (available in a week) by post, together with recommendations to be followed and the next appointment, if needed (to avoid a new visit, travels, and loss of time).
As an exception to the general rule, there are some situations requiring control visits:
- 1.
Patients with TSH in the lower limit or decreased, in whom thyroid scan is performed to assess nodule function and the need for FNA si decided based on results.24
- 2.
Patients with surgical criteria before puncture (based on size, associated symptoms, etc.), who are appointed after cytological results are available (because these may influence surgical approach) and referred for surgery.
- 3.
Patients whose cytological result requires surgery. These patients are appointed for a visit to collect the results at which they are explained the results obtained, any possible questions are answered, and they are referred for surgery.
Fig. 1 schematically shows the structure of the high-resolution thyroid nodule clinic.
Finally, it should be emphasized that a high-resolution thyroid nodule clinic should be the ideal place to gradually introduce the most recent molecular and radiographic procedures related to TND. This includes measurement of some tumor markers which allow for detecting malignancy in a given nodule25,26 and performance of state-of-the-art radiographic techniques, such as elastography.27
Monitoring of thyroid nodule disease at a thyroid nodule clinicIt is recommended that nodules initially studied at a high-resolution thyroid nodule clinic continue to be monitored at it, because of its diagnostic capacity, although after initial diagnosis patients may optionally be referred to be monitored by the general endocrinology clinic. As multiple factors may influence this decision (professionals involved, availability of appointment, waiting time in the different clinics), each department involved should make the decision best adapted to its needs.
The criteria for thyroid nodule monitoring by a high-resolution thyroid nodule clinic do not actually differ from those recommended by the different consensuses reported.28,29 This is not surprising, because the strength of high-resolution clinics is not to introduce novelties in monitoring, but to be faster and more efficient in diagnosis. Thus, it is usually recommended that any thyroid nodule with initial benign cytology is re-evaluated at 6–18 months. Two options are available depending on results.
If nodule growth is found (defined as growth greater than 50% in nodule volume or at least 20% in two nodule dimensions), repeat FNA should be performed. If benign cytology is reported, repeat ultrasonographic assessment is recommended in 6–18 months.
If nodule size remains stable (growth less than 50% in nodule volume or less than 20% in two nodule dimensions), no repeat FNA is needed, and time to the next ultrasonographic control may be increased to 2–3 years, provided no clinical changes justifying prior re-evaluation occur during this time period.
Despite these general recommendations, the control interval may be increased to 2–3 years for nodules with no significant changes in two consecutive controls.
On the other hand, good clinical judgment and common sense of each physician should be an indispensable component in TND work-up and, as occur in other diseases, it is critical to consider factors such as patient age, underlying diseases, and life expectancy. These considerations are important to avoid as much as possible unnecessary tests that will not provide clinical benefits and will only cause discomfort and futile concerns.
ConclusionImplementation of a high-resolution thyroid nodule clinic has been shown to increase diagnostic yield and efficiency, and to decrease diagnostic delay and patient discomfort. Implementation of high-resolution thyroid nodule clinics should therefore be a sine qua non requirement to achieve the highest standards in medical care for TND and should be intensively promoted. Clinics should be adapted to the organizational and functional possibilities of each care department.
Conflicts of interestThe authors declare that they have no conflicts of interest related to this paper.
FundingJosé Carlos Fernández-García has been awarded by “Río Hortega” grant by Instituto de Salud Carlos III, Madrid (CM12/00059).
Please cite this article as: Fernández-García JC, Mancha-Doblas I, Ortega-Jiménez MV, Ruiz-Escalante JF, Castells-Fusté I, Tofé-Povedano S, et al. Estructura diagnóstica y funcional de una consulta de alta resolución de nódulo tiroideo. Endocrinol Nutr. 2014;61:329–334.