This work reports the experience with use of continuous subcutaneous insulin infusion (CSII) in 112 type 1 diabetic patients followed up for 7 years and previously treated with multiple daily insulin injections (MDII).
Material and methodsA retrospective, observational study in 112 patients with diabetes mellitus treated with CSII from 2005 to 2012, previously treated with MDII and receiving individualized diabetic education with a specific protocol. Variables analyzed included: prevalence of the different indications of pump treatment; mean annual HbA1c and fructosamine values before and after CSII treatment; and hypoglycemia frequency and symptoms.
ResultsThe most common reason for pump treatment was brittle diabetes (74.1%), followed by frequent or severe hypoglycemia or hypoglycemia unawareness (44.6%). Other indications were irregular food intake times for professional reasons (20.2%), dawn phenomenon (15.7%), pregnancy (12.3%), requirement of very low insulin doses (8.9%), and gestational diabetes (0.9%). HbA1c decreased between 0.6% and 0.9%, and fructosamine between 5.1% and 12.26%. Nine percent of patients experienced hypoglycemia weekly, 24% every two weeks, and 48% monthly. No hypoglycemia occurred in 19% of the patients. Only 10% had neuroglycopenic symptoms. Hypoglycemia unawareness was found in 21%. Hypoglycemia was more common at treatment start, and its frequency rapidly decreased thereafter.
ConclusionCSII therapy provides a better glycemic control than MDII treatment. Specific patient training and fine adjustment of insulin infusion doses are required to prevent hypoglycemic episodes, which are the most common complications, mainly at the start of treatment.
En este trabajo se aporta la experiencia adquirida con el tratamiento con bombas de infusión subcutánea continua de insulina (ISCI) en 112 pacientes con diabetes mellitus a lo largo de 7 años, que previamente venían siendo tratados con múltiples dosis de insulina bolo-basal.
Material y métodosEstudio retrospectivo observacional de 112 pacientes con diabetes mellitus, tratados antes con pauta de insulina bolo-basal y luego con ISCI, desde de 2005 al 2012 que recibieron educación diabética individualizada con un protocolo específico. Se estudiaron las siguientes variables: frecuencia porcentual de las distintas indicaciones autorizadas para aplicar este tratamiento; valor medio anual de HbA1c y de fructosamina el año anterior a la instauración del tratamiento con la bomba de insulina y en los 7 años siguientes de seguimiento; frecuencia y sintomatología de las hipoglucemias.
ResultadosLa causa más común de indicación fue la diabetes inestable (74,1%), seguida de hipoglucemias graves, frecuentes o inadvertidas (44,6%). Otras indicaciones fueron: horarios de ingesta variables o imprevisibles por razones profesionales (20,2%), fenómeno del alba (15,7%), gestación (12,3%), requerimiento de dosis muy bajas de insulina (8,9%) y diabetes gestacional (0,9%). La HbA1c descendió entre 0,6% y 0,9%, en tanto que la fructosamina lo hizo entre 5,1% y 12,2%. El 9% de pacientes presentaron hipoglucemias semanales, el 24% cada dos semanas y en el 48% fueron mensuales; el 19% no presentaron hipoglucemias. Sólo el 10% presentaron síntomas neuroglucopénicos. En el 21% fueron asintomáticas. Las hipoglucemias fueron más frecuentes al comienzo del tratamiento, disminuyendo rápidamente poco tiempo después.
ConclusiónLa terapia con ISCI proporciona una mejoría del control glucémico en comparación con tratamiento de múltiples inyecciones. Requiere adiestramiento específico del paciente y ajustes de la dosificación de insulina para prevenir las hipoglucemias, que son la complicaciones más frecuentes, sobre todo al comienzo del tratamiento.
Under normal conditions, pancreatic ß cells achieve an adequate glucose balance through continuous insulin secretion throughout the day (baseline secretion) and acute insulin secretion in response to meals (prandial secretion). When beta cells are destroyed (due to an autoimmune cause in type 1 diabetes mellitus or with the progression of type 2 diabetes mellitus) insulin therapy is given to try and replace their function. Good blood glucose control by intensive insulin therapy may decrease the incidence or progression of short- and long-term metadiabetic complications.1
The best current strategy in insulin therapy for getting as close as possible to physiological blood glucose profiles is the so-called “basal-bolus” regimen.2,3 The basal-bolus regimen based on multiple subcutaneous doses (MDI) consists of the daily administration of one or more doses of long-acting insulin, in order to maintain normal basal or fasting blood glucose, and preprandial boluses of fast-acting insulin to control postprandial hypoglycemia. The disadvantages of this treatment include the need for multiple subcutaneous insulin injections daily, which has an impact on patient quality of life and may increase the rate of hypoglycemia.1,4
By contrast, continuous subcutaneous insulin infusion (CSII) systems provide greater flexibility in terms of insulin dosage and the infusion rate, and also do away with daily injections of multiple doses, but are more expensive to use and require specific training and diabetic education.5–7 Failures may also occur in the working of disposable pumps or catheters.7,8
Some studies have been conducted in recent years to check the safety and efficacy of treatment with CSII. Most of them have shown an improvement in blood glucose control, especially in the first months of treatment, as well as a decrease in the number of severe hypoglycemic episodes.7 The weight increase seen in early studies9 was not confirmed subsequently.10
On the other hand, technical advances in infusion systems have allowed for their more extensive use in the diabetic population. The current availability of multiple basal profiles and different bolus options is very helpful.10 Thus, the use of CSII allows for greater flexibility in insulin provision and for a more physiological profile, and also improves quality of life, because intake may be delayed, omitted and/or varied in its contents. Exercise intensity and timing may also be modified without compromising glucose control.6,8 CSII improves control of the “dawn phenomenon” because it may modify the basal insulin dose overnight,10 and also decreases the risk of both nocturnal hypoglycemia and unaware and severe hypoglycemia.6,11,12 Some studies, however, found no significant differences regarding severe hypoglycemia.13,14
The increasing use of continuous infusion pumps has made possible a greater understanding of the advantages and disadvantages of this therapeutic approach, enabling us to improve metabolic control in patients and to improve, or at least delay, metadiabetic complications without compromising quality of life.13 However, publications on this issue report small series, and some variables, such as serum ructosamine, have barely been analyzed.
This study therefore reports the experience accumulated by our research team from treatment over 7 years with continuous insulin infusion pumps of a large series of patients with type 1 diabetes who were previously treated with multiple basal-bolus insulin doses. The experience provided will contribute to our greater knowledge of the benefits of continuous subcutaneous infusion systems, to improvements in insulin dosing algorithms, and to preventing as much as possible the potential clinical complications and failures in infusion that may occur.
Material and methodsThis was a retrospective, observational study conducted in 112 patients with diabetes mellitus from the department of endocrinology and nutrition treated with CSII between 2005 and 2012 at Hospital Clínico Universitario de Salamanca and who had previously been treated with multiple basal-bolus insulin doses.
The indications for the use of the pump approved by the Department of Health of the Castile and León government are: unstable type 1 diabetes mellitus (DM) with wide blood glucose fluctuations, severe common or unaware hypoglycemia, dawn phenomenon, HbA1c>7%, type 1 DM requiring very low insulin doses, gestational DM, documented or planned pregnancy, intake times variable or difficult to predict for occupational reasons, and pancreas transplant patients. At least one of the criteria should be met to authorize the use of the pump, but the same patient may meet several criteria.
The selection criteria, all of which should also be met for authorization, include a highly motivated and collaborating patient who can be reasonably expected to comply with clinical protocols, has received diabetes education and adequate training in using the insulin pump, has performed at least four blood glucose self-measurements daily over the previous two months, and has adequate family support.
As an initial step in the protocol, once the start of CSII treatment has been accepted, patients have to be adequately trained at the diabetes education unit regarding the basic features of the treatment, understanding of the CSII system, the acquisition of skills for correctly setting up the system and on what should be done in the event of an emergency or loss of the CSII system, change of catheter, algorithms to modify baseline dosage and boluses for adequate blood glucose control, quantification and exchange of carbohydrates and their relationship to insulin doses and physical activity, information regarding the prevention and treatment of acute complications, the adaptation of nutritional requirements, and how to act in special situations (travel, intercurrent diseases, physical exercise). If these requirements have not been met by the end of the program, the patient's candidature for CSII is delayed or even cancelled.
Once training is completed, treatment with the insulin pump is started, and is then frequently reviewed to adjust basal infusion doses and insulin boluses based on the carbohydrate content of meals. Subcutaneous blood glucose monitoring often helps to optimize insulin dosage and prevent unaware hypoglycemia. The following infusion systems have been used: Accu-Check (Roche), Animas (Novalab), and Paradigm (Medtronic).
The following variables were included in our study: percent frequency of the different indications authorized to administer this treatment; mean annual values of glycosylated hemoglobin (HbA1c) in blood (as percentage) and fructosamine in serum (in μmol/L) in the year prior to the start of treatment with the insulin pump and in the following seven years of follow-up; and hypoglycemia rate and symptoms. Data on hypoglycemic episodes were collected from the documented records after a retrospective review of the clinical histories. Each patient was always seen by the same endocrinologist, who used standardized clinical criteria shared by all the physicians in the department.
Data on ketoacidotic decompensations and complications at the catheter insertion site were retrospectively collected from the clinical history records.
HbA1c values were measured using high pressure liquid chromatography (HPLC) (normal reference values, 4–6%), and serum fructosamine levels (normal range, 205–285μmol/L) were tested using the nitroblue tetrazolium colorimetric method.
Data collected were statistically analyzed using GraphPad Prism software (GraphPad Software Inc., USA). A Student's t test and a parametric Mann–Whitney test were respectively used for the statistical analysis of differences in normally and non-normally distributed results. A value of p<0.05 was considered statistically significant.
ResultsPatient age was 8.3±3.5 years (mean and standard deviation). Indications for CSII therapy by percentage included: unstable type 1 diabetes mellitus with wide glycemic deviation (74.1% of patients); severe, frequent, or unaware hypoglycemia (44.6%); dawn phenomenon (15.7%); glycosylated hemoglobin higher than 7% (65.1%); diabetes requiring very low insulin doses (8.9%); gestational diabetes (1.1%); current or planned pregnancy (12.3%); and intake times variable or difficult to predict for occupational reasons (20.2%). The sum of percentages is greater than 100% because different patients had more than one of the approved indications (e.g., unstable diabetes with wide glycemic fluctuations combined with frequent and unaware hypoglycemia and dawn phenomenon).
The cumulative numbers of patients monitored during the seven-year period was 19 patients over the seven years, 33 patients over six years, 38 patients over five years, 52 patients over four years, 74 patients over three years, 90 patients over two years, and 112 patients over one year. Patients were followed up every 3–6 months, and the follow-up was more frequent at the start of implantation until glycemic stabilization was achieved.
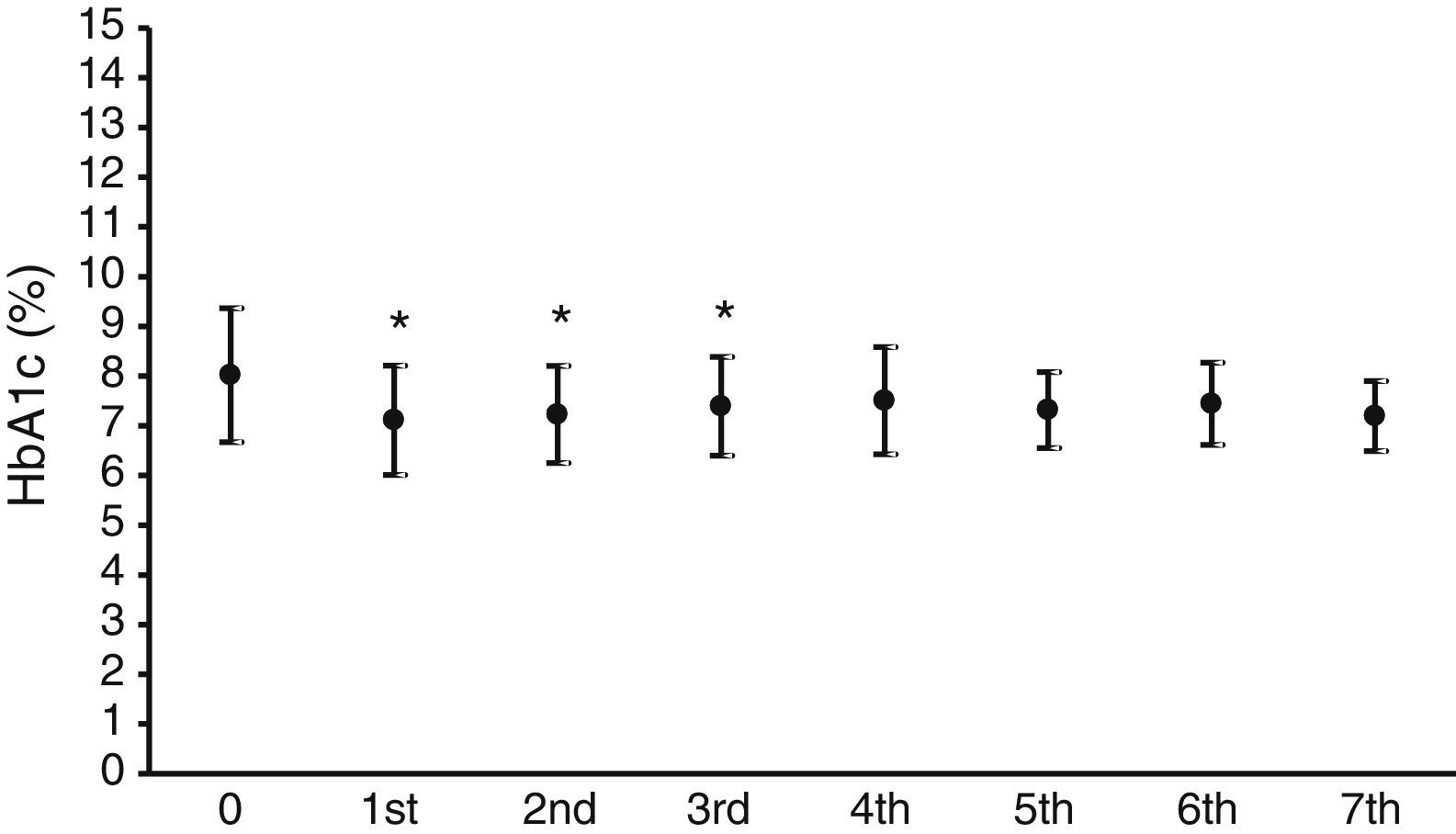
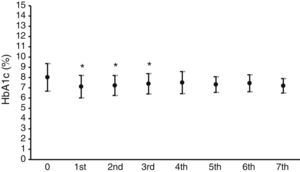
Fig. 1 shows the mean HbA1c value in the year prior to the start of CSII therapy, which was 8±1.3%, and the mean HbA1c values over the seven years of follow-up. A statistically significant (p<0.05) decrease in HbA1c levels ranging from 0.7% to 0.9% was seen in the first three years.
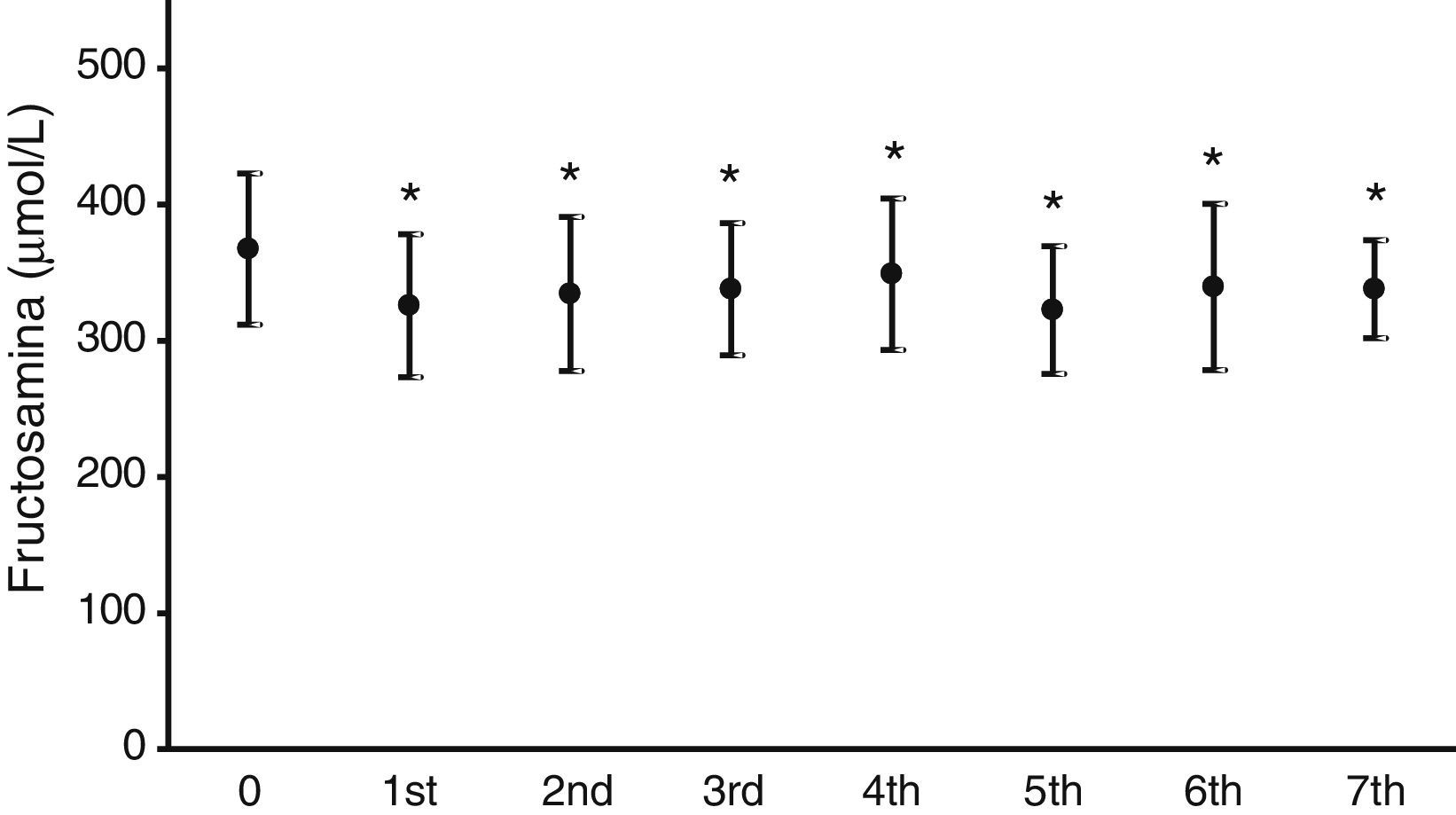
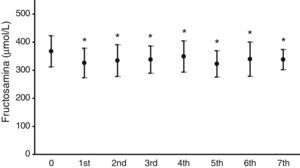
Fig. 2 illustrates the mean serum fructosamine levels in the year prior to treatment start (367.2±55.5μmol/L) and in the following seven years. A statistically significant decrease (p<0.05) was found in all subsequent years. Fructosamine levels decreased by 5.1–12.2% as compared to the mean value in the year prior to pump treatment.
Nine percent of patients had hypoglycemic episodes weekly, 24% every 15 days, and 48% had hypoglycemic events monthly. Nineteen percent of patients experienced no hypoglycemic events. The clinical signs of hypoglycemia were assessed based on the presence of adrenergic symptoms alone (in 69% of patients who experienced hypoglycemia) and neuroglycopenic manifestations (in 10%). Asymptomatic and unaware hypoglycemia occurred in 21%. Hypoglycemia was more frequent soon after CSII treatment was started, but its frequency rapidly decreased after the subsequent adjustment of the basal algorithms and bolus doses of insulin based on the daily, dietary, and occupational habits of the patients.
Of the 50 patients selected based on the indication of frequent or unaware, severe hypoglycemia, 18% had asymptomatic hypoglycemic events, 6% hypoglycemia with neuroglycopenic symptoms, and 76% hypoglycemia with adrenergic symptoms. Forty-six percent had hypoglycemic episodes monthly, 24% every 15 days, and 10% weekly, while 20% had no hypoglycemic episodes.
Four patients experienced a ketoacidotic complication during treatment, but had no other complications: two of them due to severe gastrointestinal and renal infection, and the other two for involuntary discontinuation of insulin infusion due to problems with the catheter. Transient local complications at the subcutaneous site of needle insertion occurred in three patients, but disappeared when the insertion site was changed. All these data were retrospectively collected from the clinical records of the patients.
DiscussionThe treatment of diabetic patients with the subcutaneous insulin infusion system has represented a great therapeutic advance because it allows for a more physiological insulin administration. This has led to improved blood glucose control as compared to other insulin therapy models, which may result in a lower prevalence of metadiabetic complications and greater quality of life.
For treatment with CSII to be approved in the Castile and León autonomous community, patients must have at least one of the indications required and must meet all of the above listed selection criteria. The most common indication in this series was unstable diabetes with wide glycemic fluctuations (74.1% of patients). Frequent or unaware hypoglycemia was another common indication (44.6%). In fact, CSII was indicated in some patients based on these types of hypoglycemia despite the presence of mean HbA1c values not higher than 7% in the year prior to the start of CSII (12.5% of the total). Both indications can easily be understood because they result from the failure to achieve adequate blood glucose control with multiple insulin doses, and insulin pump is an alternative therapeutic procedure which is, at least theoretically, better at achieving the desired metabolic control or at decreasing hypoglycemic events, which are always poorly tolerated by patients and decrease their quality of life.
It should be noted that, in this series, the hypoglycemia rate varied little in patients previously treated with the basal/bolus regimen and in whom the indication for CSII was precisely the presence of severe, frequent or unaware hypoglycemia. Our results showed that only 19% of the patients experienced no hypoglycemia (or had very isolated, brief, and extremely sporadic hypoglycemic episodes). It should be noted, however, that hypoglycemic events were uncommon in most patients (48% experienced hypoglycemia once monthly), although a small but not negligible proportion of patients (9%) experienced hypoglycemia every week. It should be stressed that hypoglycemia was more frequent soon after pump treatment had been started and rapidly decreased in frequency thereafter after the adjustment of basal algorithm and bolus doses of insulin based on the daily, dietary, and occupational habits of the patients. This occurred despite the fact that insulin infusion was started at a total daily dose at least 20–30% lower than the dose given to the patient before pump placement. Most patients noticed hypoglycemia because of adrenergic symptoms, which allowed for the adoption of training measures to prevent future hypoglycemic events. However, these continue to represent a complication that should not be disregarded, not only due to their potential occurrence, particularly at the beginning of treatment as discussed, but also because there was a non-negligible proportion of unaware hypoglycemic episodes. Various studies have found no significant differences in the hypoglycemia rate and its severity. Mild hypoglycemia rates were very similar (CSII 92%, MDI 94%), and severe hypoglyemia was uncommon.15 There are series reporting a decrease by 0.9 episodes/patient/year of hypoglycemia requiring external help with CSII therapy.16 In contrast to the above studies, other series reported a 9% increase in hypoglycemic events as compared to MDI therapy.17
As regards blood glucose control measured by the regular assessment of HbA1c and fructosamine, the results shown in Figs. 1 and 2 reveal a significant improvement in both parameters. HbA1c decreased by 0.7–0.9%, which agrees with the results in other series, reporting decreases ranging from 0.3 to 0.8%.16,18,19 Fructosamine levels decreased by 5.1–12.3% as compared to the mean value in the year prior to pump treatment. Hirsch et al. also reported a significant, but lower, decrease in serum fructosamine levels,15 but the duration of pump treatment in their patient series was only five weeks. HbA1c decreases were statistically significant in the first three years of treatment. Subsequently, although levels continued to be lower as compared to the mean value in the year prior to pump treatment, their statistical significance disappeared. The interpretation of these results is debatable, but they may be explained by a relaxation in strict blood glucose self-monitoring by the patient over time after these three initial years. By contrast, the significant decrease in serum fructosamine levels was maintained throughout follow-up. Although this is also arguable, regular controls of diabetic patients routinely show a stricter self-monitoring the closer the date of the hospital visit. Both test parameters are routinely monitored, thus supplying more complete information on blood glucose control over time, because HbA1c does not provide information close to the date of the patient visit. Such information is achieved by measuring fructosamine, which mainly reflects the degree of glycosylated albumin, having a much shorter half-life than that of the erythrocyte.
To sum up, treatment with a continuous subcutaneous insulin infusion system represents a therapeutic advance in type 1 DM and provides better blood glucose control. However, specific patient training and a fine adjustment of insulin dose are required to prevent blood glucose fluctuations and, particularly, hypoglycemic events, which are the most common complications. Transient local complications at the subcutaneous site of needle insertion occurred in only three patients in our series, and disappeared when the insertion site was changed.
AuthorshipPapargyri P. and Ojeda Rodríguez S. have contributed equally to this study.
Conflicts of interestThe authors state that they have no conflicts of interest.
Authors thank Estefanía Cortés García and Ruth Ortiz Sánchez, educational nurses in the Department of Endocrinology and Nutrition.
Please cite this article as: Papargyri P, Ojeda Rodríguez S, Corrales Hernández JJ, Mories Álvarez MT, Recio Córdova JM, Delgado Gómez M, et al. Estudio observacional de infusión subcutánea continua de insulina a lo largo de 7 años en el tratamiento de la diabetes mellitus tipo 1. Endocrinol Nutr. 2014;61:141–146.