Special considerations are warranted in management of thyroid nodule and thyroid cancer during pregnancy. The diagnostic and therapeutic approach of thyroid nodules follows the standard practice in non-pregnant women. On the other hand, differentiated thyroid cancer management during pregnancy poses a number of challenges for the mother and fetus. The available data show that pregnancy is not a risk factor for thyroid cancer development or recurrence, although flare-ups cannot be completely ruled out in women with active disease. If surgery is needed, it should be performed during the second term or, preferably, after delivery. A majority of pregnant patients with low-risk disease only need adjustment in levothyroxine therapy. However, women with increased serum thyroglobulin levels before pregnancy or structural disease require regular thyroglobulin measurements and neck ultrasound throughout pregnancy. Pregnancy is an absolute contraindication for radioactive iodine administration.
La conducta a seguir con el nódulo tiroideo y el cáncer de tiroides en la paciente embarazada requiere especiales consideraciones. El abordaje diagnóstico y terapéutico del nódulo tiroideo se rige por los criterios habituales en las pacientes no embarazadas. Por su parte, el manejo del cáncer diferenciado de tiroides durante la gestación implica una serie de retos para la madre y el feto. Los datos disponibles muestran que el embarazo no supone un aumento de riesgo de aparición o recurrencia de cáncer de tiroides, pero no está completamente descartado que la gestación pueda representar un estímulo en pacientes con enfermedad activa. Es importante tener en cuenta que en caso de ser necesario el tratamiento quirúrgico se recomienda llevarlo a cabo durante el segundo trimestre o, preferentemente, tras el parto. La mayoría de las gestantes con enfermedad de bajo riesgo solo requieren el ajuste del tratamiento con levotiroxina. Sin embargo, en mujeres que tienen valores elevados de tiroglobulina antes de la gestación o con datos morfológicos de persistencia de enfermedad deberá realizarse un seguimiento periódico mediante la determinación de tiroglobulina y realización de ecografías cervicales. La gestación supone una contraindicación absoluta para la administración de 131I.
Both the American Thyroid Association (ATA) and the Endocrine Society (ENDO) have recently published clinical guidelines reporting the main advances in recent years in the management of thyroid disease in pregnant women.1,2 These documents, especially the ATA guidelines, are comprehensive, and their recommendations largely overlap.
We think that such a profusion of guidelines is unnecessary, because they may confound healthcare professionals.3 Thus, we do not intend to provide an additional guideline, but to adapt and summarize for Spanish-speaking professionals the information included in the two American guidelines and, where appropriate, discuss any peculiarities specific to our situation. If a more in depth discussion of any concept is required, or the strength of or evidence for any particular recommendation needs to be known, the abovementioned guidelines may be consulted.
The Clinical guidelines for the management of subclinical thyroid dysfunction during pregnancy were published a few years ago in Endocrinología y Nutrición.4 As a continuation of this document, the Clinical guidelines for the management of thyroid nodular disease in pregnancy have now been published. In order to allow for rapid consultation, the recommendations are presented in a clear, practical, and manageable question and answer format.
Interpretation of thyroid function and goiter during pregnancyChanges occurring in the thyroid gland during pregnancy have been thoroughly studied in recent years and may be both morphological and functional.5 There are specific reference levels for both thyroid-releasing hormone (TSH) and other thyroid hormones during pregnancy, which should be taken into account and should be flagged by each laboratory to avoid erroneous interpretations.1,6,7 This is of paramount importance when monitoring patients with a history of differentiated thyroid cancer, as will be seen later.
Thyroid cancer should be suspected in any patient consulting for goiter. It should therefore be taken into account that, depending on iodine intake, thyroid volume may increase during pregnancy by 10% (in areas with normal-high iodine intake) to 40% (in low intake areas). A study was conducted on 35 pregnant women in Valle de Arán, an area where iodine deficiency is traditionally endemic. The authors measured thyroid volume during the first and third trimesters of pregnancy. Median thyroid volume increased in these women from 7.5 to 9.5mL during pregnancy (p<0.001). The difference was also seen to be greater in women with multiple pregnancies. In agreement with studies in other geographical areas, this Spanish study concluded that both iodine deficiency and multiparity are goitrogen factors during pregnancy.8
Pregnancy and thyroid nodulesThyroid nodule management during pregnancy is usually based on the standard diagnostic and treatment criteria for the condition. The special characteristics of pregnancy are associated with changes in nodule prevalence, size, and growth, and to indications for treatment, particularly surgery.
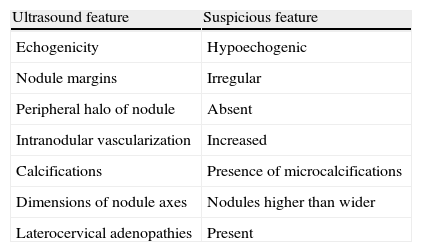
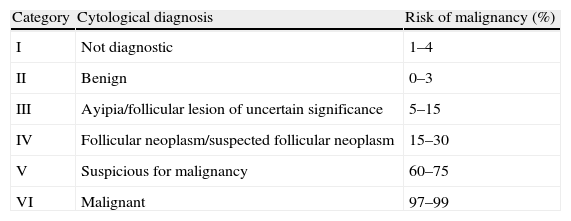
As in non-pregnant women, ultrasound examination is essential in any thyroid nodule. Ultrasonography provides valuable information regarding the benign or malignant nature of nodules9 (Table 1). In accordance with general criteria, cytological evaluation using fine needle aspiration (FNA) is indispensable in assessing thyroid nodules. Obviously, pregnancy has no effect on the cytological diagnosis of thyroid nodules. Table 2 summarizes the most commonly used classification, the Bethesda system.10
Ultrasound features of thyroid nodules suggesting malignancy.
| Ultrasound feature | Suspicious feature |
| Echogenicity | Hypoechogenic |
| Nodule margins | Irregular |
| Peripheral halo of nodule | Absent |
| Intranodular vascularization | Increased |
| Calcifications | Presence of microcalcifications |
| Dimensions of nodule axes | Nodules higher than wider |
| Laterocervical adenopathies | Present |
Cytological diagnostic criteria “Bethesda Classification”.
| Category | Cytological diagnosis | Risk of malignancy (%) |
| I | Not diagnostic | 1–4 |
| II | Benign | 0–3 |
| III | Ayipia/follicular lesion of uncertain significance | 5–15 |
| IV | Follicular neoplasm/suspected follicular neoplasm | 15–30 |
| V | Suspicious for malignancy | 60–75 |
| VI | Malignant | 97–99 |
Both the appearance of new nodules and the volume of those already existing have been reported to increase during pregnancy.1 However, nodules usually return to their baseline size after delivery11 In a Spanish study, however, Jaén Díaz et al.12 found a 33.2% prevalence of thyroid nodules during the first trimester of pregnancy, which was slightly lower than the prevalence seen in controls (38.5%), although the difference was not statistically significant. This suggests that pregnancy does not promote the occurrence of thyroid nodules, in contrast to findings in studies in other countries.11,13,14 However, the high prevalence of thyroid nodules in pregnant women found in the Jaén Díaz et al. study12 is also noteworthy because it was markedly higher than that reported in the majority of similar studies, where it ranged from 3% to 12%.
- 1.
Does pregnancy cause any morphological disorder in the thyroid gland?
Most research studies suggest that pregnancy increases the risk of developing new thyroid nodules and also promotes an increase in the size of previously existing nodules.1 The size of pre-gestational nodules may increase by up to 50%, and a de novo nodule occurs during pregnancy in up to 20% of patients. This risk is also enhanced both by age and a higher number of pregnancies (multiparity is associated with a 10–15% increase).
- 2.
What precautions may be taken to prevent the occurrence of thyroid nodules during pregnancy?
Epidemiological data suggest that low iodine intake predisposes to the development of thyroid nodules. It is therefore highly recommended that potassium iodide supplements be taken throughout pregnancy. This supplementation also protects against the occurrence of hypothyroidism in the mother and fetus, in addition to providing the fetus with the iodine needed for thyroid hormone synthesis.
The clinical management of thyroid nodules during pregnancy- 3.
How should thyroid nodules be managed in pregnant women?
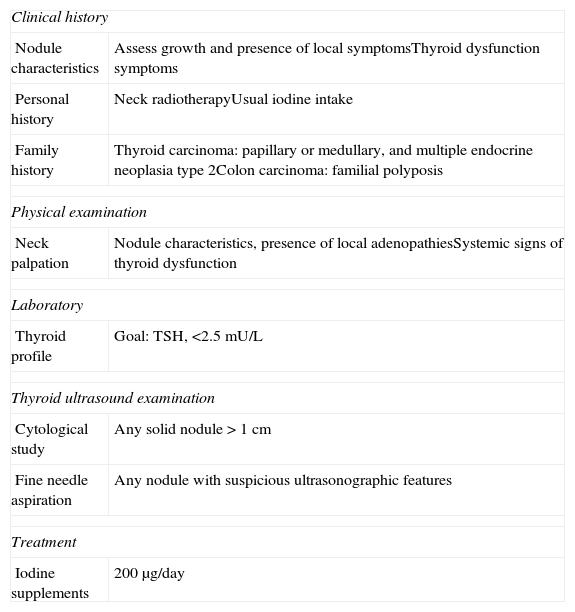
If a thyroid nodule is found during pregnancy, a thorough clinical history and physical examination should be performed. The history should include: (a) any family history of thyroid diseases (autoimmune and nodular) and carcinomas (medullary thyroid cancer, multiple endocrine neoplasia 2A, and colon cancer); (b) personal history, especially iodine intake, prior neck irradiation, and obstetric history; (c) signs and symptoms of thyroid dysfunction, especially changes over time in the thyroid nodule, must also be investigated; and (d) careful neck examination (Table 3).
Action protocol in the first visit of a pregnant patient with thyroid nodules.
| Clinical history | |
| Nodule characteristics | Assess growth and presence of local symptomsThyroid dysfunction symptoms |
| Personal history | Neck radiotherapyUsual iodine intake |
| Family history | Thyroid carcinoma: papillary or medullary, and multiple endocrine neoplasia type 2Colon carcinoma: familial polyposis |
| Physical examination | |
| Neck palpation | Nodule characteristics, presence of local adenopathiesSystemic signs of thyroid dysfunction |
| Laboratory | |
| Thyroid profile | Goal: TSH, <2.5mU/L |
| Thyroid ultrasound examination | |
| Cytological study | Any solid nodule>1cm |
| Fine needle aspiration | Any nodule with suspicious ultrasonographic features |
| Treatment | |
| Iodine supplements | 200μg/day |
The general recommendation to perform thyroid function tests in any patient with thyroid nodules also applies to pregnant women.15 Thus, a pregnant woman with a thyroid nodule should undergo a functional study including tests to measure TSH and free T4 levels.
If morphological changes found at examination are confirmed, thyroid ultrasound examination should be performed and, if required, work-up should be completed with FNA. Pregnancy is not a contraindication for FNA, which may be performed at any time during pregnancy. The ultrasonographic findings suggesting the need for cytological analysis are summarized as follows: (a) any nodule greater than 1cm in size; (b) nodules with ultrasonographic characteristics suggesting malignancy; (c) the rapid growth or clinical suspicion of malignancy; (d) the discovery by ultrasonography of potentially metastatic neck adenopathies; and (e) the presence of risk factors such as a family history of thyroid cancer or a history of cervical radiotherapy. However, if ultrasonographic findings suggest a benign condition, FNA of nodules may be performed after delivery.
Scintigraphy is contraindicated during pregnancy. However, according to the available information, an involuntary scintigraphy performed before week 12 of pregnancy does not appear to damage the fetal thyroid gland.1
The presence of thyroid cancer does not usually cause impaired thyroid gland function. The measurement of circulating thyroglobulin levels is of no diagnostic or prognostic interest in this setting. There is no adequate information regarding the convenience of measuring calcitonin levels during pregnancy, unless there is a family history of medullary thyroid carcinoma. Pentagastrin stimulation is contraindicated in pregnancy.
- 4.
Should pregnant women be screened for thyroid changes?
There is increasing evidence to support the advisability of thyroid function screening and physical examination of the neck as soon as the patient knows that she is pregnant.16 The benefit of correcting thyroid dysfunctions detected during the first trimester of pregnancy clearly outweighs the cost of screening.17
Few studies are available on the relationship between thyroid nodules and autoimmunity during pregnancy, and there are thus no recommendations regarding the advisability of measuring thyroid antibodies during pregnancy. Moreover, because of immune privilege during pregnancy, circulating levels of thyroid antibodies decrease during pregnancy, which may complicate the interpretation of the results.18
- 5.
What reference levels of thyroid hormones should be considered sufficient during pregnancy?
For the abovementioned reasons, circulating TSH levels should range from 0.1 to 2.5mU/L during the first trimester of pregnancy. During the second and third trimesters, levels should be 0.2–3.0 and 0.3–3.0mU/L respectively.1
- 6.
How should benign nodules be monitored during pregnancy?
No special monitoring is required during pregnancy for thyroid nodules suspected of being benign based on ultrasound characteristics or found to be benign at cytological study and causing no compressive symptoms.
Repeat ultrasound examination should be performed if a nodule grows rapidly. Both in these cases and when ultrasonographic changes suggesting malignancy occur, FNA should be repeated.
The treatment of thyroid nodules during pregnancyOnce clinical, ultrasonographic, or cytological diagnosis has been made, benign thyroid nodules require no treatment.
- 7.
Is levothyroxine suppression treatment useful during pregnancy?
The treatment of nodular goiter with supraphysiological doses of levothyroxine is not recommended, because the suppression of TSH secretion may induce untoward adverse effects in both the mother and the fetus.19
- 8.
When is surgery indicated for a thyroid nodule?
If ultrasonographic or cytological changes occur in previously benign nodules or the course of the nodule casts doubt on its nature, surgery is recommended. Surgery is also indicated when compressive symptoms occur.
Nodules with indeterminate (Bethesda categories 3, 4, and 5) or malignant (category 6) FNA results should undergo surgery (Table 2). Specific indications for surgery are addressed in question 12.
- 9.
How should hyperfunctioning thyroid nodules be treated?
The treatment of hyperthyroidism in pregnant women is complex. Recommendations for the management of this condition are beyond the scope of these guidelines and have been reviewed in another publication.4 It should, however, be noted that treatment with radioactive iodine is contraindicated throughout pregnancy.
The management of differentiated thyroid cancer diagnosed during pregnancyDifferentiated thyroid cancer is the second leading malignant tumor during pregnancy after breast cancer, with a prevalence of 14 per 100,000births.20 In addition, 10% of all thyroid cancers occurring during childbearing age are diagnosed during pregnancy or within one year of delivery, and the most common histological type is papillar.21 The management of differentiated thyroid cancer during pregnancy poses a number of diagnostic and therapeutic challenges for both the mother and fetus.
- 10.
Is there an increased frequency of thyroid cancer in pregnant women with thyroid nodules?
Three retrospective studies have reported an increased risk of malignancy in pregnant women with thyroid nodules. However, these studies had a patient selection bias, because they were conducted in tertiary reference hospitals where women with cancer may be overrepresented.22–24
Pregnancy may however be expected to promote the occurrence and growth of malignant nodules because of the relative iodine deficiency experienced by the mother, increased growth factors, the appearance of hormones with a stimulating activity similar to that of TSH, and high estrogen levels. The management of these patients therefore requires special consideration.
- 11.
Does pregnancy affect the prognosis of thyroid cancer?
Most studies suggest that pregnancy does not worsen the prognosis of women diagnosed with differentiated thyroid cancer.25–31 Only one of the seven studies published addressing this issue found an increased risk of disease persistence/recurrence in women diagnosed during pregnancy or within one year of delivery.30 This finding was related to the presence of high estrogen receptor expression levels in thyroid tumor cells. However, the patient sample in this study was small and poorly representative.
In conclusion, no adequate evidence which could lead us to conclude that pregnancy worsens the prognosis of thyroid cancer is currently available. Consequently, thyroidectomy may be delayed in most pregnant patients until the postpartum period without this affecting the risk of recurrence or mortality.1
The impact of pregnancy in women with anaplastic or medullary carcinoma has not been analyzed.
- 12.
How should differentiated thyroid cancer diagnosed during pregnancy be managed?
It should be taken into account that a conflict between the optimum treatment of the mother and fetal well-being very often exists. There is therefore an ethical dilemma. The decision as to which is the best therapeutic option should preferably be taken by a multidisciplinary team consisting of an endocrinologist, a surgeon, an obstetrician, a nuclear medicine specialist, and a neonatologist.
There are two special considerations that should be taken into account in the management of women with differentiated thyroid cancer diagnosed during pregnancy: the absolute contraindication of the administration of radioactive iodine and the choice of the time of thyroidectomy. As noted above, it is usually advisable to delay surgery until the postpartum period, as there is no adequate evidence showing that pregnancy worsens cancer prognosis.1
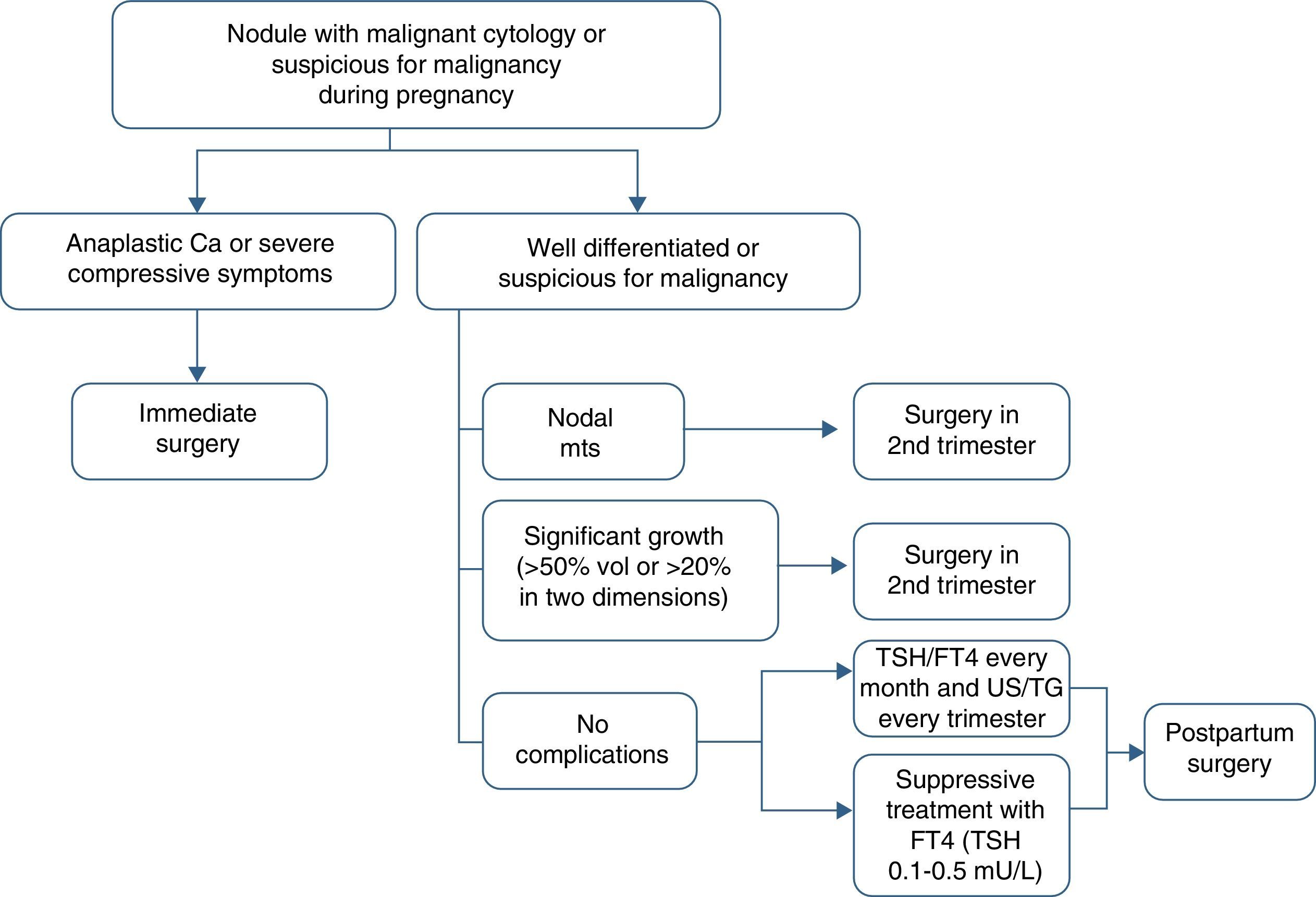
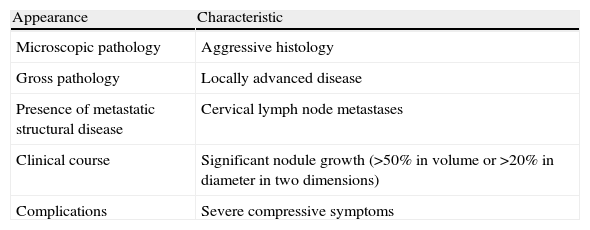
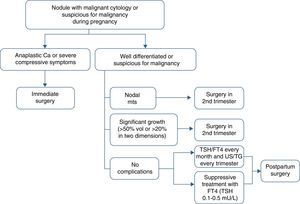
Thyroidectomy is recommended in the second trimester in the following cases: (a) aggressive or locally advanced histology (e.g. anaplastic or poorly differentiated carcinoma), (b) metastatic cervical lymph nodes (diagnosed by cytology), (c) severe compressive symptoms (e.g. tracheal obstruction), and (d) the significant growth of a malignant nodule (>50% in volume or >20% in diameter in two dimensions) before week 24 of pregnancy (Table 4).
Indications for surgery of a thyroid nodule in the second trimester of pregnancy.
| Appearance | Characteristic |
| Microscopic pathology | Aggressive histology |
| Gross pathology | Locally advanced disease |
| Presence of metastatic structural disease | Cervical lymph node metastases |
| Clinical course | Significant nodule growth (>50% in volume or >20% in diameter in two dimensions) |
| Complications | Severe compressive symptoms |
If the malignant nodule does not meet the above conditions or is diagnosed toward the end of the second half of pregnancy, surgery may be delayed until the postpartum period. In such cases, it is recommended that levothyroxine suppression treatment aimed at maintaining TSH levels in the lower normal limit (0.1–1.5mU/L) be started.
Circulating TSH and free T4 levels should be monitored every month, and ultrasound examination and thyroglobulin tests should be performed every three months (Fig. 1).
- 13.
What are the perioperative risks for the mother and fetus of thyroidectomy during pregnancy?
Surgery in the first trimester involves an unacceptable risk of miscarriage and impaired organogenesis. On the other hand, surgery during the third trimester is associated with an increased risk of preterm delivery. Thyroidectomy during the second trimester does therefore involve the lowest risk for the mother and fetus.32 However, if surgery is performed during pregnancy, the risk of postoperative maternal hypothyroidism (and potential hypoparathyroidism) should be taken into account.
The management of pregnant women with a history of differentiated thyroid cancerSpecific considerations are required in the management of women with a history of differentiated thyroid cancer who want to become pregnant again. Table 5 summarizes these considerations.
Considerations regarding pregnancy and lactation in women with a history of differentiated thyroid cancer.
| Before pregnancy |
| - Pregnancy should be avoided in the 6–12 months following the administration of therapeutic radioiodine doses- A history of radioiodine treatment involves no increased risk of infertility, miscarriage, neonatal mortality, congenital malfomations, premature delivery, low birthweight, death during the first year of life, or cancer development- Pregnancy involves no risk of recurrence in women with no evidence of biochemical or structural disease before pregnancy. However, it may represent a stimulus in patients with active disease |
| Monitoring during pregnancy |
| - In any woman with hypothyroidism, it is essential to maintain thyroid hormone levels adequate for the time of pregnancy- Low-risk patients: these only require TSH monitoring and treatment adjustment when needed, and examination every 3 months- High-risk patients: if TSH suppression was recommended before pregnancy due to the risk of disease, it should be maintained during pregnancy, with the levothyroxine dose being adjusted. In women with high thyroglobulin levels before pregnancy or with morphological evidence of persistent disease, neck ultrasound should be performed every trimester |
| Monitoring during breast-feeding |
| - Breast-feeding is safe in women with a history of differentiated thyroid cancer- Radioiodine should not be administered during lactation and for at least four weeks after breast-feeding has stopped |
- 14.
What information should be given to women with a history of differentiated thyroid cancer who desire pregnancy?
Women should be informed that pregnancy should be avoided in the 6–12months following the administration of the therapeutic dose of radioactive iodine in order to achieve the stabilization of the levothyroxine dose and to verify disease remission.2,33–35
- 15.
What are the potential risks in women previously receiving radioiodine therapy?
Studies conducted on women previously given radioiodine for the treatment of differentiated thyroid cancer have not reported an increased risk of complications such as infertility, miscarriage, newborn mortality, congenital malformation, premature delivery, low birthweight, death in the first year of life, or cancer development.36,37
- 16.
Can a new pregnancy increase the risk of recurrence in women with a history of thyroid cancer?
Often, the patient is mainly interested in knowing whether cancer recurrence may or may not occur as a consequence of a new pregnancy.
From the pathophysiological viewpoint, it should be noted that both HCG and estrogens induce changes in serum TSH, FT4, and thyroglobulin levels during pregnancy.7,38 Some studies have reported that estrogens may stimulate thyroglobulin gene expression, particularly during the third trimester, increasing potential thyroglobulin production in differentiated thyroid cancer. This occurs without stimulating proto-oncogene c-Myc and without promoting rapid cell proliferation.39,40 Moreover, some clinical studies suggest that circulating thyroglobulin levels may increase in the absence of a concomitant tumor, which may be a confounding factor.40.41
Several studies, three of them published in the past five years, have assessed the impact of pregnancy in women with a history of differentiated thyroid cancer.40,42–44 Leboeuf et al.40 studied 36 women who had become pregnant a mean of 4.3 years after initial treatment for differentiated thyroid cancer. After delivery, eight of these women had thyroglobulin levels 20% greater than those found before pregnancy. Three of these women had active disease, and five had no evidence of disease. However, no recurrence was found in the early postpartum period in women with negative neck ultrasound whose serum thyroglobulin levels were less than 3.2ng/mL.
Data from these studies suggest that pregnancy involves no risk of recurrence in women with no evidence of biochemical or structural disease before pregnancy. However, as previously noted (question 11), it has not been completely ruled out that pregnancy may represent a stimulus in patients with active disease.1
Monitoring during pregnancy of women with a history of differentiated thyroid cancerIn any woman with hypothyroidism, it is crucial to maintain thyroid hormone levels within the specific reference intervals throughout pregnancy. Various studies have shown that even mild hypothyroidism is associated with adverse effects for the mother and fetus.45 Thyroid hormone replacement requirements usually increase by 20–40%, which makes adjustment indispensable in the first eight weeks.46,47 In addition, other routinely used supplements, such as iron and calcium, may alter levothyroxine absorption.
- 17.
How should the levothyroxine dose be adjusted?
Thyroid hormone levels should always be measured when pregnancy is documented. Thyroid function tests should initially be repeated every four weeks until week 16–20, and then once between weeks 26 and 32. If an adjustment of the levothyroxine dose is required, thyroid hormones should be assessed again four weeks later.1
To provide guidance, it may be noted that mean increases in levothyroxine dose as compared to the pregestational dose over the three trimesters of pregnancy are 9%, 21%, and 26% respectively.
- 18.
Should TSH suppression be maintained during pregnancy?
Goal TSH levels during pregnancy do not change as compared to before pregnancy. In low risk patients (T1-T2, N0, M0, with no aggressive histology), the goal is to achieve TSH levels ranging from 0.3 to 1.5mU/L. In patients with no evident biochemical or structural disease but with risk factors at diagnosis (T3-T4, N1, M1, or with an aggressive histology), the goal is to maintain circulating TSH levels ranging from 0.1 to 0.5mU/L. It should be noted, however, that if the risk of disease before pregnancy makes the maintenance of suppressed circulating TSH levels advisable, the same caution should be exercised during pregnancy, the levothyroxine dose being adjusted as appropriate.1 A study conducted on 25,765 women, 433 of whom had subclinical hyperthyroidism, was very illustrative of the adverse effects of this measure. Low TSH levels did not correlate to adverse effects in any of the women.48
- 19.
Should iodine supplements be used?
Despite prior thyroidectomy, patients with a history of thyroid cancer, like all other pregnant women, should take at least 250μg/day of iodine during pregnancy and breast-feeding.49 In these cases, iodine supplements are administered to the mother to cover the requirements of the fetal thyroid.1
- 20.
How should monitoring be performed?
Most pregnant women with low-risk differentiated thyroid cancer only require monitoring of circulating TSH levels and adjustments of treatment, when needed, as well as an examination every three months.45 Neither thyroglobulin measurement nor ultrasonography is required.1
However, women with high thyroglobulin levels before pregnancy or with morphological evidence of disease should be monitored with thyroglobulin tests and neck ultrasound in every trimester of pregnancy.1
The management of women with differentiated thyroid cancer during lactation- 21.
What considerations should be taken into account during breast-feeding?
Breast-feeding may be safe in women with a history of differentiated thyroid cancer.15 It should, however, be noted that radioiodine should not be administered during lactation and until at least four weeks after its discontinuation,2 because the lactating breast concentrates a significant amount of iodine.15 However, it is advisable to delay radioiodine administration until three months after breast-feeding has been stopped to ensure the normalization of the increased activity of the sodium-iodine symporter caused by lactation.50 If urgent treatment is needed, 123I scintigraphy may be performed to assess breast residual uptake. Treatment should be delayed if a higher than normal radioactivity level is found.
Involution of the lactating breast is variable, and there is evidence that bromocriptine is able to accelerate it.51 This drug may be prescribed in special cases “outside of its specified indications for use”.15 Breast-feeding cannot, of course, be resumed after radioiodine administration.
Conflicts of interestThe authors state that they have no conflicts of interest.
The other members of the Working Group on Thyroid Cancer of the Spanish Society of Endocrinology and Nutrition are: Elías Álvarez García, Emma Anda Apiñaniz, Amparo Calleja, Sergio Donnay, José Manuel Gómez-Sáez, Edelmiro Menéndez Torre, Pablo Moreno Llorente, María Angustias Muros, Elena Navarro González, Vicente Pereg, Begoña Pérez Corral, Javier Santamaría Sandi, Pilar Santisteban, and Carles Zafón Llopis.
Please cite this article as: Galofré JC, Riesco-Eizaguirre G, Álvarez-Escolá C, en representación del Grupo de Trabajo de Cáncer de Tiroides de la Sociedad Española de Endocrinología y Nutrición. Guía clínica para el manejo del nódulo tiroideo y cáncer de tiroides durante el embarazo. Endocrinol Nutr. 2014;61:130–138.