Dopamine agonists (DA) are the first-line therapy in prolactinomas, but they fail to decrease prolactin (PRL) levels and/or tumor size in some of these tumors, which are labeled as resistant prolactinomas (RP). To date, risk factors for DA resistance are not fully understood and management of DA-RP is not well established.
MethodsWe retrospectively recorded clinical, biochemical and radiological features, as well as management and outcome, of all cabergoline (CAB)-RP attended at the Endocrinology department of a tertiary hospital between 1995 and 2016. CAB resistance was defined as the failure to normalize PRL (biochemical resistance, BR) or reduce tumor size by at least 50% (morphological resistance, MR) with a CAB dose up to 2mg/week (or 3mg/week in cases where lower doses were not tested) for at least 3 months.
ResultsTen CAB-RP were found. The mean age of the cohort was 30.6 years and 50% of subjects were male. The average tumor size was 1.78cm (80% macroadenomas). The mean maximal dose of CAB was 3.8mg/week. Five patients showed isolated MR, four combined MR+BR and only one isolated BR. MR patients were more often males and older than MR+BR patients. Transsphenoidal surgery achieved normalization of PRL and/or disappearance of tumor in three of seven patients. At the end of follow up all patients had controlled PRL levels (with or without CAB) and most of them bore a visible although stable tumor.
ConclusionsIsolated MR and combined MR+BR are the most frequent patterns of DA resistance whereas isolated BR seems to be uncommon. Our data support a high tumor size but not male gender as a risk factor for DA resistance.
Los agonistas dopaminérgicos (AD) son el tratamiento de elección de los prolactinomas, pero en algunos casos no logran normalizar los niveles de prolactina (PRL) o disminuir el tamaño del tumor, y estos casos se etiquetan como prolactinomas resistentes (PR). Los factores de riesgo de resistencia a los AD y el manejo de los PR no están bien establecidos.
MétodosAnalizamos retrospectivamente las características clínicas, bioquímicas y radiológicas, así como el manejo y evolución de los PR a cabergolina (CAB) atendidos en el departamento de Endocrinología de un hospital terciario entre 1995 y 2016. La resistencia a CAB se definió como persistencia de PRL elevada (resistencia bioquímica, RB) o reducción tumoral inferior al 50% (resistencia morfológica, RM) tras al menos 3 meses de tratamiento con una dosis de CAB de hasta 2mg/semana (o 3mg/semana en los casos que no recibieron dosis inferiores)
ResultadosSe incluyeron 10 pacientes, edad media 30.6 años, 50% varones. El tamaño medio del tumor fue 1.78cm (80% macroadenomas) y la dosis máxima media de CAB 3.8mg/semana. Cinco pacientes presentaron RM aislada, cuatro RM+RB y uno RB aislada. La prevalencia de sexo masculino y la edad fueron superiores en el grupo RM comparado con el grupo RM+RB. La cirugía transesfenoidal logró normalización de PRL y/o desaparición del tumor en tres de siete pacientes. Al final del seguimiento la PRL era normal (con o sin CAB) en todos los casos y la mayoría presentaba un tumor visible de tamaño estable.
Conclusionesla RM aislada y la RM+RB combinadas son los patrones más frecuentes de resistencia a los AD. Nuestros datos apoyan la asociación del tamaño tumoral pero no del sexo masculino con la resistencia a los AD.
Among the functioning-pituitary tumors, prolactinomas are the most common, making up approximately 40% of all pituitary adenomas.1,2 Dopamine agonists (DA) are the first-line therapy for these tumors. However, about 10–15% of patients do not achieve normalization of prolactin (PRL) levels with DA and a higher percentage does not experience a reduction in tumor size.3,4
The concept of resistance to DA was established for the first time by Pellegrini et al. in 1989.5 However, there is not clear consensus on the definition of DA-resistant prolactinomas (DA-RP) yet. Furthermore, the molecular mechanisms underlying the escape from dopaminergic regulation in DA-RP are not fully elucidated. The dopamine receptor D2 (DRD2) itself is thought to be the main factor involved in resistance to DA. To date, no point mutation in the DRD2 gene has been identified in DA-RP,2,3,6,7 but a reduced number of binding sites,5 reduced gene expression,8 impaired balance between short and long receptor isoforms,9 and genetic polymorphisms in the DRD210 have been found. Other genes probably also influence the sensibility of lactotroph cells to DA. For example, MEN1-related prolactinomas are more frequently resistant than sporadic prolactinomas,11 and AIP mutations are associated with aggressive prolactinomas both in familiar isolated pituitary adenomas and in young patients with sporadic tumors.12
When the DA resistance is evident from the beginning of the DA treatment it is named primary resistance and it is the most common form. Secondary resistance is defined as the one that develops after a period of adequate response to DA treatment. This is fairly uncommon, and other causes of apparent lack of response, such as non-compliance with treatment or concomitant therapy with testosterone or estrogens, must be ruled out before diagnosing a true secondary DA resistance. Although somewhat anecdotic, malignant transformation of the pituitary adenoma must also be ruled out in this scenario.13
Another important point to consider in DA-RP is whether the lack of response involves clinical features, PRL levels or tumor size. Clinical resistance is the most difficult to define because some symptoms, such as the decreased libido or menstrual cycle disorders, are difficult to measure and/or are influenced by other variables. The resistance to PRL-lowering effects of DA is well-known and widely described in literature, and it is usually defined as the failure to achieve normoprolactinemia.2,14 When related to tumor size, the most accepted definition of DA resistance is a failure to achieve a reduction of at least 50% in tumor size.2,14 In contrast to the resistance to PRL-lowering effects, the resistance to mass-reducing effects has been poorly addressed.
A DA dose threshold is needed to be attained before labeling a prolactinoma as DA resistant. Different authors apply different thresholds but the most universally accepted are 15mg/day for bromocriptine and 2mg/week for CAB3,15–18 as they are approximately twofold higher than the average dose able to control hyperprolactinemia in patients with macroprolactinoma (macroP).
Risk factors for DA resistance in prolactinomas are not fully defined and management of DA-RP is not well established. With the aim of achieving further knowledge about these tumors, we describe the clinical, biochemical and morphological characteristics of a cohort of ten CAB-RP. We also describe the treatment applied and the long-term outcome.
Material and methodsPatientsWe retrospectively reviewed all patients with a prolactinoma diagnosis who were treated at our Endocrinology department in a tertiary care center between 1995 and 2016 and met the criteria of BR or/and MR described below. Ten patients with DA-RP were identified. The mean follow-up since the diagnosis of RP was 102 (26–237) months.
Hormonal and image studiesThe baseline hormonal study included the measurement of serum PRL after 30minutes of rest, plasma ACTH and serum cortisol, IGF-1, FSH, LH, estradiol or testosterone (according to gender), TSH and FT4. The normal ranges for serum PRL in our laboratory were<20ng/ml in men and<30ng/ml in women.
In all patients, pituitary magnetic resonance imaging (MRI) was used to characterize the pituitary lesion, specifying the maximum diameter of the lesion, presence or absence of suprasellar extension, invasion of cavernous sinuses, signs of tumor bleeding, chiasmatic compression and cystic changes. Changes in tumor size were calculated based on variations in the major diameter.
DefinitionsWe defined biochemical resistance (BR) as the failure to normalize PRL levels with a CAB dose up to 2mg/week (or 3mg/week in cases where this was the minimum dose tested) for at least 3 months. Morphological resistance (MR) was defined as the failure to achieve a reduction of at least 50% in the major tumor diameter at the same CAB dose thresholds and for the same period of time.
Surgical cure was considered when normal PRL levels and complete disappearing of tumor were achieved by transsphenoidal surgery (TSS).
Remission was defined as a situation in which normal PRL levels and complete disappearing of tumor were achieved by one or more treatment modalities (DA, TSS, radiotherapy (RT)), whether DA maintenance treatment was needed or not.
Normal PRL levels (achieved by one or more treatment modalities) along with persistence of a visible tumor was termed biochemical control (BC), whether DA maintenance treatment was needed or not.
Recurrence was considered when one or more of the following were identified after 6 months of remission: (1) a visible tumor, (2) an unequivocally high PRL level (>200ng/ml), (3) a PRL level of 20/30–200ng/ml, on at least two occasions, and not justified by any other reason.
Statistical analysisThe statistical analysis was performed with STATA 15.0. U-Mann–Whitney and Fisher test were used for comparison of quantitative and qualitative variables respectively, and Spearman correlation to analyze relationship between variables. Statistical significance was defined as a p value<0.05.
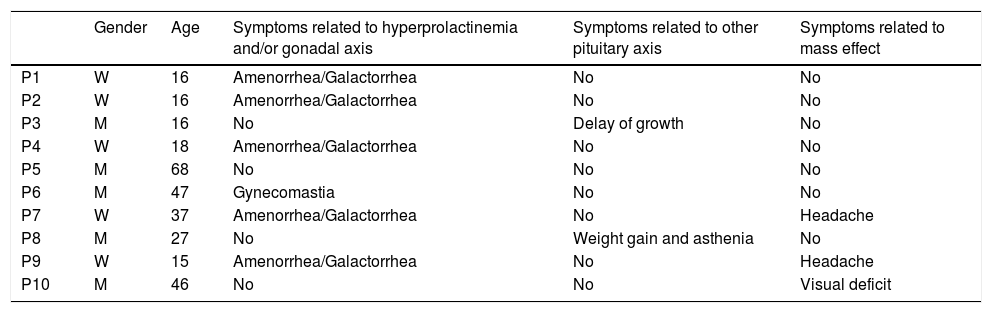
ResultsBaseline clinical characteristicsThe median age of the cohort at diagnosis was 22.5 years (range: 15–68). Five patients were males and they were older than women (median age 36 vs 19 years) but the difference did not reach statistical significance (p=0.072). At baseline, all women presented menstrual cycle dysfunction and galactorrhea; among males none had erectile dysfunction or decreased libido and only one had gynecomastia (P6). Three patients had symptoms related to mass effect: headache in two females (P7, P9) and bitemporal hemianopsia in one male (P10). One patient (P3), diagnosed at the age of 16, suffered growth delay related to GH deficiency, and another one (P8) presented with signs and symptoms of hypothyroidism due to TSH deficiency. None of the patients were under testosterone or estrogen treatment at the time of diagnosis (Table 1).
Baseline clinical characteristics.
| Gender | Age | Symptoms related to hyperprolactinemia and/or gonadal axis | Symptoms related to other pituitary axis | Symptoms related to mass effect | |
|---|---|---|---|---|---|
| P1 | W | 16 | Amenorrhea/Galactorrhea | No | No |
| P2 | W | 16 | Amenorrhea/Galactorrhea | No | No |
| P3 | M | 16 | No | Delay of growth | No |
| P4 | W | 18 | Amenorrhea/Galactorrhea | No | No |
| P5 | M | 68 | No | No | No |
| P6 | M | 47 | Gynecomastia | No | No |
| P7 | W | 37 | Amenorrhea/Galactorrhea | No | Headache |
| P8 | M | 27 | No | Weight gain and asthenia | No |
| P9 | W | 15 | Amenorrhea/Galactorrhea | No | Headache |
| P10 | M | 46 | No | No | Visual deficit |
W=woman, M=man.
In no case a familial genetic background was suspected due to the familiar history or association with other endocrine neoplasms. Genectic testing for AIP and MEN1 germline mutations was performed in one patient (P9) with negative result.
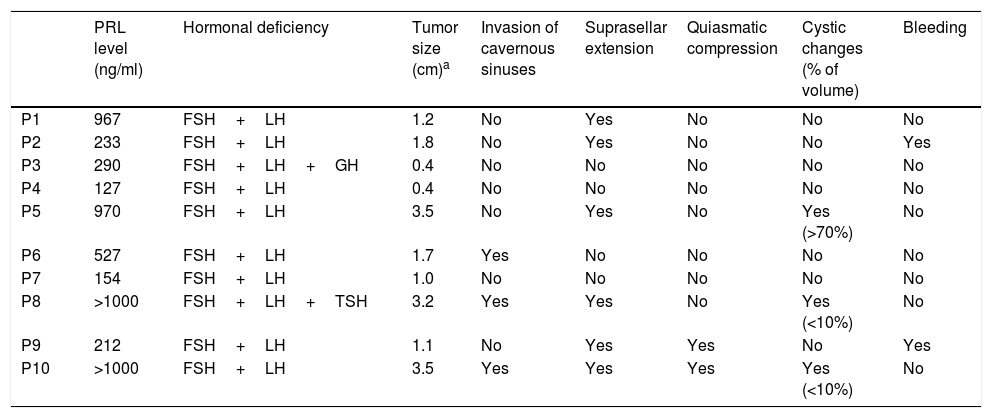
Baseline hormonal and radiological characteristicsThe baseline PRL levels ranged from 127 to >1000ng/ml, but the exact upper limit of the range is unknown as the serum PRL sample was not diluted in two cases (Table 2).
Hormonal and radiological characteristics.
| PRL level (ng/ml) | Hormonal deficiency | Tumor size (cm)a | Invasion of cavernous sinuses | Suprasellar extension | Quiasmatic compression | Cystic changes (% of volume) | Bleeding | |
|---|---|---|---|---|---|---|---|---|
| P1 | 967 | FSH+LH | 1.2 | No | Yes | No | No | No |
| P2 | 233 | FSH+LH | 1.8 | No | Yes | No | No | Yes |
| P3 | 290 | FSH+LH+GH | 0.4 | No | No | No | No | No |
| P4 | 127 | FSH+LH | 0.4 | No | No | No | No | No |
| P5 | 970 | FSH+LH | 3.5 | No | Yes | No | Yes (>70%) | No |
| P6 | 527 | FSH+LH | 1.7 | Yes | No | No | No | No |
| P7 | 154 | FSH+LH | 1.0 | No | No | No | No | No |
| P8 | >1000 | FSH+LH+TSH | 3.2 | Yes | Yes | No | Yes (<10%) | No |
| P9 | 212 | FSH+LH | 1.1 | No | Yes | Yes | No | Yes |
| P10 | >1000 | FSH+LH | 3.5 | Yes | Yes | Yes | Yes (<10%) | No |
There were 8 macroP and 2 microprolactinomas (microP). The average tumor size of the cohort was 1.78cm (SD=1.21) and there was a strong correlation between PRL levels and tumor size (r=0.80, p=0.005). Among the macroP, seven had suprasellar extension, three invasion of cavernous sinuses, three cystic changes, two signs of bleeding and two chiasmatic compression. All patients had secondary hypogonadism; in addition, one male had secondary hypothyroidism and another one GH deficiency.
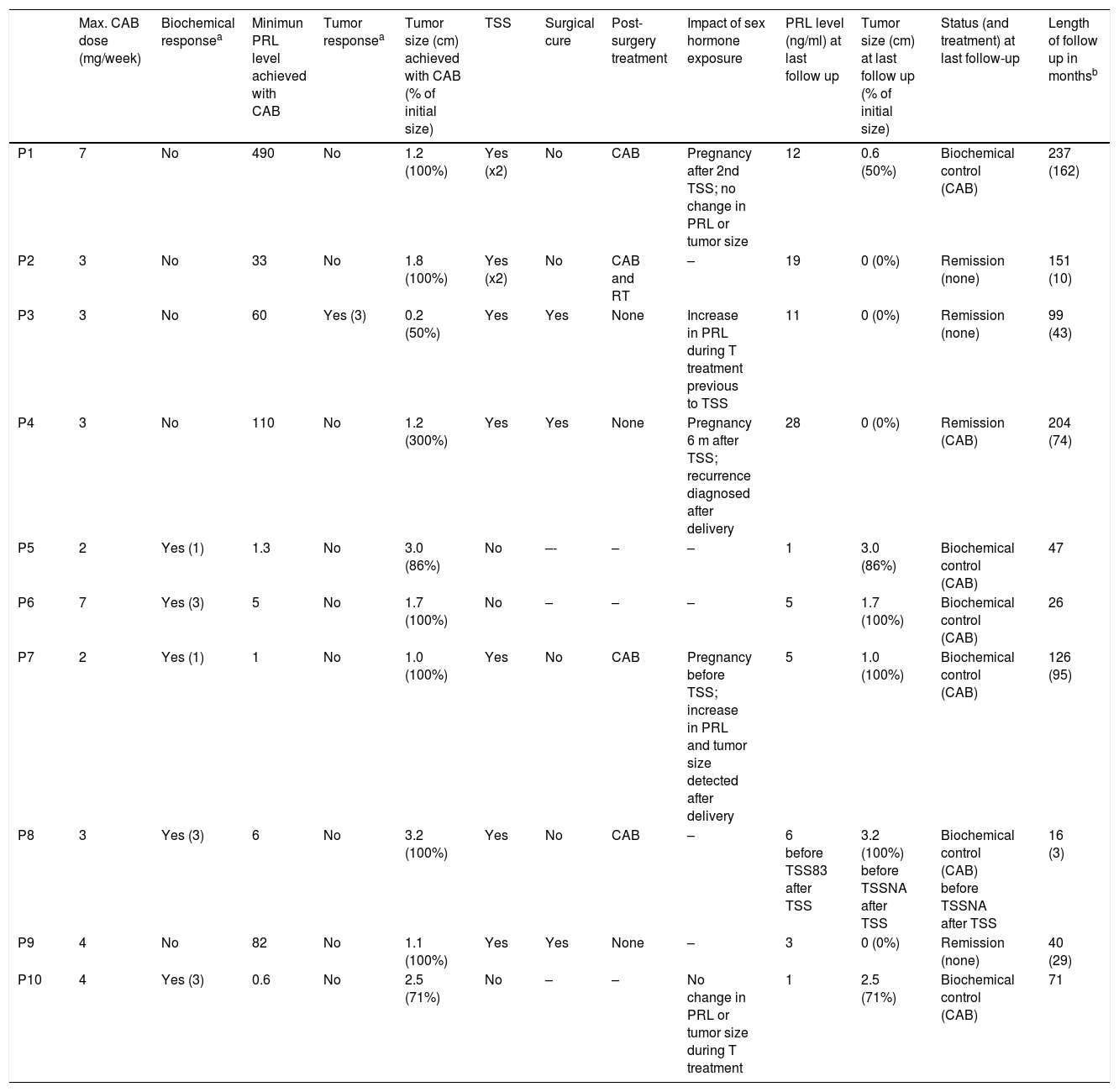
Treatment and responseFive patients were treated with CAB from the beginning. Three patients were initially treated with bromocriptine and two with quinagolide; afterwards all five were switched to CAB due to the lack of tumor and/or PRL response. The mean maximal dose of CAB used was 3.8mg/week and there were no differences between men and women (3.8mg/week in both). In all patients an adequate adherence to treatment was confirmed through anamnesis in each visit. Cabergoline was well tolerated and there was no need to decrease the dose or discontinue the treatment.
All patients showed primary resistance. Five patients (P5–P8, P10) showed isolated MR, one (P3) isolated BR and four (P1, P2, P4, P9) combined MR+BR (Tables 3 and 4). Among the five patients with isolated MR there were four males, whereas the four patients with combined MR+BR were all females, a difference that was statistically significant (p=0.048). Median age was significantly higher in MR than in MR+BR patients (46 vs 16 years, p=0.014). However median tumor size in MR patients (3.2cm) did not significantly differ from that in MR+BR patients (1.15cm) (p=0.14).
Treatment and response attained.
| Max. CAB dose (mg/week) | Biochemical responsea | Minimun PRL level achieved with CAB | Tumor responsea | Tumor size (cm) achieved with CAB (% of initial size) | TSS | Surgical cure | Post-surgery treatment | Impact of sex hormone exposure | PRL level (ng/ml) at last follow up | Tumor size (cm) at last follow up (% of initial size) | Status (and treatment) at last follow-up | Length of follow up in monthsb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 7 | No | 490 | No | 1.2 (100%) | Yes (x2) | No | CAB | Pregnancy after 2nd TSS; no change in PRL or tumor size | 12 | 0.6 (50%) | Biochemical control (CAB) | 237 (162) |
| P2 | 3 | No | 33 | No | 1.8 (100%) | Yes (x2) | No | CAB and RT | – | 19 | 0 (0%) | Remission (none) | 151 (10) |
| P3 | 3 | No | 60 | Yes (3) | 0.2 (50%) | Yes | Yes | None | Increase in PRL during T treatment previous to TSS | 11 | 0 (0%) | Remission (none) | 99 (43) |
| P4 | 3 | No | 110 | No | 1.2 (300%) | Yes | Yes | None | Pregnancy 6 m after TSS; recurrence diagnosed after delivery | 28 | 0 (0%) | Remission (CAB) | 204 (74) |
| P5 | 2 | Yes (1) | 1.3 | No | 3.0 (86%) | No | –- | – | – | 1 | 3.0 (86%) | Biochemical control (CAB) | 47 |
| P6 | 7 | Yes (3) | 5 | No | 1.7 (100%) | No | – | – | – | 5 | 1.7 (100%) | Biochemical control (CAB) | 26 |
| P7 | 2 | Yes (1) | 1 | No | 1.0 (100%) | Yes | No | CAB | Pregnancy before TSS; increase in PRL and tumor size detected after delivery | 5 | 1.0 (100%) | Biochemical control (CAB) | 126 (95) |
| P8 | 3 | Yes (3) | 6 | No | 3.2 (100%) | Yes | No | CAB | – | 6 before TSS83 after TSS | 3.2 (100%) before TSSNA after TSS | Biochemical control (CAB) before TSSNA after TSS | 16 (3) |
| P9 | 4 | No | 82 | No | 1.1 (100%) | Yes | Yes | None | – | 3 | 0 (0%) | Remission (none) | 40 (29) |
| P10 | 4 | Yes (3) | 0.6 | No | 2.5 (71%) | No | – | – | No change in PRL or tumor size during T treatment | 1 | 2.5 (71%) | Biochemical control (CAB) | 71 |
CAB=cabergoline, TSS=transsphenoidal surgery, RT=radiotherapy, T=testosterone, NA=not available.
Clinical and tumor size differences according to the resistance pattern.
| BR (n=1) | MR (n=5) | MR+BR (n=4) | p value (MR vs MR+BR) | |
|---|---|---|---|---|
| Sex (ratio M/F) | 4/1 | 0/4 | 0.048 | |
| Age at diagnosis “M(R)” | 46 (41) | 16 (3) | 0.014 | |
| Size tumor (cm) “M(R)” | 3.2 (2.5) | 1.15 (1.4) | 0.14 |
M=median value; R=range; BR=biochemical resistance; MR=morphological resistance.
The five patients with isolated MR achieved normal PRL with a mean dose of CAB of 2.2mg/week (range 1–3mg/week). There was not any tumor shrinkage in three of them (P6–P8) with a mean CAB dose of 4mg/week (range 2–7mg/week); one patient (P10) experienced a tumor reduction of 30% with a CAB dose of 3mg/week; and the patient with a partially cystic prolactinoma (P5) showed a minor reduction of the solid component with a CAB dose of 2mg/week. The patient with isolated BR achieved a shrinkage>50% of the tumor with 3mg/week of CAB and the lowest PRL level he achieved was 60ng/ml, also with 3mg/week of CAB. The maximum CAB dose reached in the patients with combined MR+BR ranged from 3 to 7mg/week (mean 4.25mg/week). The lowest PRL achieved with these doses ranged from 33 to 490ng/ml (mean 179ng/ml) and tumor size showed minor or no reduction.
In two patients who did not respond to 3mg/week of CAB (P1 and P6), the dose was increased to 7mg/week. In P1 (combined BR+MR) there was not any change in tumor size, and PRL levels even increased from 382 to 499ng/ml. In P6 (isolated MR) there were also no changes in tumor size.
During the follow-up, testosterone replacement was started in two men due to persistence of low levels of testosterone despite CAB treatment. In one of them (P10), a patient with a macroP who had shown isolated MR (thus, a mass effect on the gonadotroph cells accounted for the hypogonadism), no significant change in the sensitivity to CAB was evident during testosterone exposure. The second case (P3) was a patient with a microP who had shown isolated BR before testosterone treatment (therefore the hypogonadism was attributed to persistent hyperprolactinemia). During testosterone replacement, PRL levels increased from 68 to 460ng/ml and the patient was submitted to TSS.
Three women became pregnant after the prolactinoma diagnosis. One of them (P1) had a macroP and became pregnant while she was in situation of BC after two TSS. During the pregnancy she remained in the same dose of CAB as before (3mg/week) and no relevant change in PRL level or tumor size were noted during or after pregnancy. Another woman (P4), who had a microP and got pregnant while she was in remission after TSS, showed a PRL level around 40ng/ml after delivery (without breastfeeding). Although a visible tumor could not be detected, PRL level remained high for more than two years and the patient developed amenorrhea, so the patient was diagnosed with a recurrence. CAB was then started and normal PRL level and restoration of the menstrual cycle were achieved. The third woman (P7) bore a macroP that had shown MR and became pregnant while she was in situation of BC (with 2mg/week of CAB). During pregnancy, CAB treatment was discontinued and pregnancy was uneventful but after delivery, a PRL level of 152ng/ml and a significant increase in tumor size was found as compared to before pregnancy. CAB treatment was restarted at 2mg/week, without control in PRL levels nor tumor size, so the patient was submitted to TSS. After surgery BC was achieved with a CAB dose lower than the pre-surgical one.
Overall, seven patients underwent TSS: two with isolated MR, the one with isolated BR and the four with combined BR+MR. Reasons for TSS were persistence of hypogonadism (P1–P3, P9), tumor growth (P4, P7) and invasive tumor and growth (P8). After TSS mean PRL levels decreased significantly from 425ng/ml before surgery to 66ng/ml after surgery, which meant an 84% reduction. However, surgical cure was achieved in only three patients. One of these three cases was diagnosed with a recurrence after surgery (and after pregnancy) and the two others remain in remission after 43 and 29 months of follow up.
Four cases did not achieve surgical cure and two of them underwent a second TSS, again without attaining remission. All four cases were treated post-operatively with DA and one of them also received fractionated external beam RT. A debulking effect of surgery was seen in two of them as both achieved BC with a lower postoperative CAB dose (1 and 0.25mg/week) than the preoperative one (3 and 2mg/week). The patient who received RT could stop CAB nine years after the treatment. The fourth patient was lost for follow-up so response to CAB after surgery could not be evaluated.
In the 3 non-operated patients, all of them with isolated MR, an adequate clinical and hormonal control was achieved with CAB treatment, but tumor was still evident, so they met criteria of BC.
DiscussionAlthough the majority of prolactinomas respond correctly to CAB treatment, some of them fail to achieve normalization of PRL and/or reduction in size despite high doses of the drug. These tumors are considered CAB-resistant and are fairly uncommon as they represent 3–14% of all CAB-treated prolactinomas.3,7,16
CAB is a long-acting DA with better efficacy than bromocriptine to control PRL levels and tumor size in prolactinoma patients.19,20 There is somewhat a consensus that at least 15mg/day of bromocriptine must be given to a prolactinoma patient before labeling the tumor as resistant to this drug.5,17 By contrast, a cut-off dose for resistance to CAB is not clearly established, although the most accepted is between 1.5 and 2mg/week.14,16 In order to select patients with a more clear phenotype of DA resistance, treatment with CAB at the more stringent criteria of >2mg/week (or 3mg/week if a lower dose was not tested) was required in our series to define DA resistance.
It is noteworthy that the age of our patients, with a mean of 30.6 years, is similar to that found in other DA-RP series,7,15 and also similar to the age reported for unselected prolactinomas.3,21 Thus, in agreement to other reports,21 our findings do not point at age as a risk factor for DA resistance. Interestingly, however, mean age at diagnosis in our cohort was 20 years higher in males than in females, at the expense of a remarkable low age in women. A younger age in woman as compared to males is also common in unselected prolactinomas but the difference is not so marked21 and relies mainly on the high prevalence of microP during the fertile period. Since most women in our study had macroP instead of microP, our data suggests that a young age could negatively influence DA sensitivity in women but not in men. However, this is in contrast to previous data, as a younger age in men22,23 and an older age in women24 were reported to be associated to DA resistance. A different definition of resistance or, maybe, the reduced size of our cohort could account for the difference between these data and ours.
In contrast to unselected prolactinomas, where the prevalence of macro and microadenomas is quite similar,3,4,21 macroP were more prevalent than microP in our cohort (80% vs 20%). These figures are almost identical to that reported in DA-RP by other authors4,7 and support the size of the adenoma as a risk factor for DA resistance, as previously found.3,4,21
Regarding to gender, we found a male prevalence of 50% in our study, a proportion between the 45% reported by Vroonen et al. in a cohort of resistant microP and macroP7 and the 69% found by Delgrange et al. in resistant macroP.16 Bearing in mind that the prevalence of male gender in unselected prolactinomas is around 30%,3,4,21 our data seem to point to a higher frequency of DA resistance in male patients. However, the preponderance of female gender in the global population of prolactinoma patients is at the expense of microP as they are clearly more common in females than in males.21 If macroP are analyzed separately the rate male:female is close to 1:1.21 Thus, the male prevalence we found in our global cohort, that included mainly macroP patients, is according to that reported for unselected macroP. Therefore, our data do not support the higher risk of resistance to DA in males found by Delgrange et al.16 On the contrary, they are in agreement with the data reported by Colao et al.,21 who did not find a different rate response to DA according to gender, and by Verhelst et al.,4 who also did not find differences related to gender after adjusting to the size of the adenoma.
Among macroP, 37% were invasive, a proportion higher than in non-RP patients,22 but surprisingly quite lower than the 71% reported by Delgrange et al.16 In contrast to ours, the latter study did not include isolated MR but this fact did not account for the differences between them since the prevalence of invasiveness among macroP after excluding patients with isolated MR in our study was 33%. Maybe the higher proportion of females in our series compared with others (50 vs 30%) is the reason for the difference, as DA resistant macroP in females seem to be less invasive than in males.16
Several germline mutations predisposing to pituitary tumors are currently known, and some of them such as AIP and MEN1 mutations are associated with more aggressive phenotypes and in certain cases with a poor treatment response.11,12 However a recent Spanish study has found that DA resistance in prolactinoma is not a useful criterion to identify patients harboring these mutations.25 Accordingly, no patient in our study was suspected to harbor predisponing germline mutations and the only patient tested for AIP or MEN1 mutations was negative.
Most prolactinoma patients with a morphological DA response also normalize their PRL levels. However, a considerable percentage of patients with a biochemical response do not present a decrease in tumor size ≥50%.2 In our cohort the most frequent pattern of DA resistance was isolated MR, followed by combined MR+BR, and isolated BR. An absence of a linear relationship between PRL levels decrease and tumor size changes and a very low frequency of isolated BR have also been described by other authors.26
In our series, MR was more frequent in men and MR+BR in women. As male gender was associated to an older age and female gender to a younger age, we could not conclude from our data if the different resistance patterns are linked to gender, age or both. Beyond these features, we did not find other important differences between the different patterns of DA resistance, possibly related to the limited number of patients in our cohort. At present it is not known if different molecular mechanisms are responsible for different forms of DA resistance.
Occasionally very high doses of CAB (≥7mg/week) have been prescribed for RP. Some studies in patients with Parkinson's disease have suggested that the use of high doses of CAB for a long time could lead to restrictive valvular disease.3,14,16,27 Although no risk of valve failure associated with the use of CAB in prolactinoma patients was observed in most studies,28 the safety of the doses employed for the treatment of RP is still controversial. Therefore, to minimize the risk of valvular disease it is important to define the CAB dose threshold above which no further response is expected. Some studies have suggested that 3.5mg/week could represent such a threshold since increasing CAB dose from 3.5 to ≥7mg/week normalized PRL only in a limited number of previously resistant patients.16 In two of our patients the CAB dose was increased from 3 up to 7mg/week, without further response. In addition, late responses to CAB are unusual and three months is a period of time considered sufficient to observe the effect of the drug. Therefore, if a PRL drop is not seen three months after increasing the dose, it should be reduced to the minimum that achieves the lowest PRL levels.
No patient in our series was under testosterone or estrogen treatment at the time that DA resistance was diagnosed. Estrogens increase PRL secretion by directly stimulating PRL gene transcription and mitotic activity of lactotroph cells18,29 and also by increasing expression of the less active long isoform of the DRD2.30 It is also known that macroP can increase in size during pregnancy6,18,31 most probably as a consequence of lactotroph cell hyperplasia due to the estrogen stimulation. Thus, treatment with estrogens in prolactinoma patients could lead to DA resistance. Testosterone may induce a similar effect, probably mediated by estrogens derived from peripheral aromatization. In fact, two cases of prolactinoma have been described in which testosterone appeared to decrease DA sensitivity.6,31 In line with these observations, we found that PRL level and/or tumor size were higher after pregnancy than before in two women. Although in one of them the increase might be related to the DA discontinuation during pregnancy, the treatment resumption at the same dose that had previously worked failed to control PRL level and tumor growth. In addition, one male experienced an increase in PRL levels after initiating testosterone treatment. Although the three patients had developed DA resistance before exposure to the sex hormone increase, pregnancy and treatment with testosterone were factors possibly associated with an increase in DA resistance in our series. This observation supports a relationship between sex hormones and DA resistance in prolactinoma patients. Therefore, it may be advisable to avoid pregnancy until the tumor is controlled, and carefully consider the risk-benefit balance of replacement therapy with testosterone.
As for the management of RP, the first step is to confirm resistance to DA. Although some cases of CAB-RP response to bromocriptine have been reported,32 this is very uncommon.14 By contrast up to 85% of bromocriptine-RP and 90% of quinagolide-RP respond to high doses of CAB.27 Thus a trial of CAB treatment is mandatory before labeling the tumor as DA resistant.
Once the CAB resistance is confirmed, another treatment modality must be selected if PRL levels or tumor size are accompanied by negative clinical consequences. TSS is usually the treatment of choice, reserving RT/radiosurgery, temozolomide and other experimental treatments for specific cases. The reported curation rate obtained by TSS in RP is around 35%,33 and a similar rate of 43% was observed in our series. This rate is lower than that reported for non-RP, that stands around 75%.33 The difference might be explained by the higher prevalence of macroadenomas between RP as surgical success in RP is equivalent to that of non-RP when micro and macroadenomas are analyzed separately.34
Although TSS is not always curative, the reduction of tumor mass can help to achieve biochemical control with DA. Two recent studies have shown that partial resistance to CAB can be overcomed after surgical debulking with a lower dose of CAB than the one used before surgery.7,35 Accordingly, at least two out of four patients from our series not cured after TSS, achieved adequate hormonal control with a CAB dose lower than the preoperative one.
After a mean follow-up of 102 months all patients with BR (isolated or associated to MR) showed an adequate biochemical control after surgery alone or surgery plus CAB o RT. Furthermore, although disappearance of tumor was infrequent, no progression in size was evident during follow up. These data are in contrast to other series that reported uncontrolled tumor growth in some patients and a high prevalence of uncontrolled hyperprolactinemia in spite of multimodal therapy.7 Given the limited number of patients in our series, chance itself could be the reason for these differences, but a lower proportion of invasive tumors is another possible explanatory factor.
As a limitation of our study, it should be noted that the real number of prolactinomas controlled in the same time period is not known, and therefore, it was not possible to estimate what percentage the resistant prolactinomas represented.
ConclusionIn conclusion, combined MR+BR and isolated MR were the most frequent patterns of DA resistance whereas isolated BR was uncommon. Combined MR+BR seems to be more prevalent in females and isolated MR in males, although the pattern of DA resistance can also be influenced by age. The prevalence of macroadenomas was higher in our RP patients than that reported in non-RP, supporting the value of this feature to predict DA resistance, as suggested previously. In contrast, male sex does not seem to be a risk factor for DA resistance.
FundingThere is no funding source.
Conflict of interestThe authors declare that they have no conflict of interest.