Type 1 diabetes (T1DM) is a common childhood disease for which parents bear considerable responsibility for its management. Twenty-four hour care and monitoring, with nocturnal blood glucose controls and the correction of hypo- and hyperglycaemia, can contribute to sleep disturbances for carers and to chronic sleep deprivation.1
A cross-sectional descriptive study was conducted. Its aim was to analyse the quality of sleep in carers of children with T1DM under 17 years of age assessed in consultation between November 2021 and January 2022 undergoing treatment with multiple doses of insulin (MDI) and flash glucose monitoring (FGM), hybrid closed-loop (HCL) systems or insulin pump (continuous subcutaneous insulin infusion [CSII]) with FGM, with a minimum of three months between the start of treatment and stable control. The control group was randomly obtained from those carers of healthy children without relevant chronic diseases who consulted during the study period. Clinical and glucometric data were collected. Sleep quality was assessed in all carers using the validated Pittsburgh questionnaire (0–21 scale), with poor sleep quality defined as a score greater than five.2
A total of 81 patients (48.7% women, with a participation rate of 93.8%) were analysed. The main carer was the mother in 80.3% (mean age 45.9±5.9 years). In total, 42.1% of the main carers did not work outside the home, while 57.9% had a job outside the home (of these, 36.8% worked part-time, 11.1% worked shifts and 52.1% worked full time without shifts).
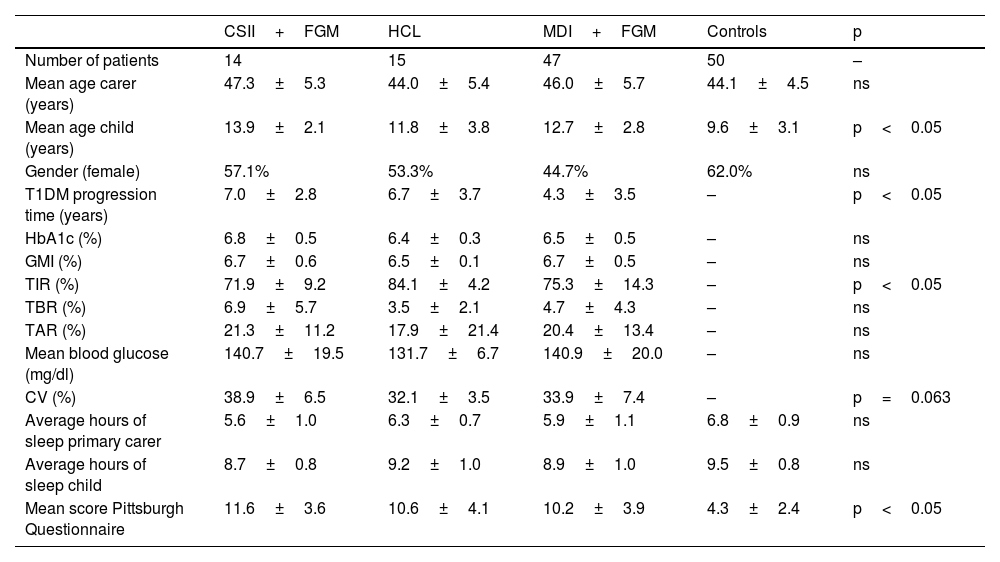
Clinical characteristics and metabolic control are shown in Table 1, with a longer time in range (TIR) and a trend towards a better coefficient of variation (CV) being noteworthy in those patients treated with HCL, and a shorter T1DM progression time in those treated with MDI+FGM. Mean sleep duration was 8.9±1.0h for children with T1DM and 5.9±1.0h for the main carer. Overall, 90.7% of the carers of children with T1DM and 20% of the carers of the controls had poor sleep quality (score greater than five), p<0.001. Similarly, the score on the questionnaire was higher in carers of children with T1DM than in controls (10.6±3.9 vs 4.3±2.4), reflecting the poorer sleep quality of carers of children with T1DM (p<0.05). No differences were found between sleep quality and the different types of treatment. In patients with T1DM, a multivariate analysis evaluating the sleep scale as the dependent variable found that only the main carer working outside the home maintained statistical significance (β=9.628, p<0.05), with no differences found in the remainder of the evaluated parameters (gender, age, TIR, time below range [TBR], number of children, shift work and type of treatment).
Clinical and metabolic control characteristics of patients with type 1 diabetes (T1DM).
| CSII+FGM | HCL | MDI+FGM | Controls | p | |
|---|---|---|---|---|---|
| Number of patients | 14 | 15 | 47 | 50 | – |
| Mean age carer (years) | 47.3±5.3 | 44.0±5.4 | 46.0±5.7 | 44.1±4.5 | ns |
| Mean age child (years) | 13.9±2.1 | 11.8±3.8 | 12.7±2.8 | 9.6±3.1 | p<0.05 |
| Gender (female) | 57.1% | 53.3% | 44.7% | 62.0% | ns |
| T1DM progression time (years) | 7.0±2.8 | 6.7±3.7 | 4.3±3.5 | – | p<0.05 |
| HbA1c (%) | 6.8±0.5 | 6.4±0.3 | 6.5±0.5 | – | ns |
| GMI (%) | 6.7±0.6 | 6.5±0.1 | 6.7±0.5 | – | ns |
| TIR (%) | 71.9±9.2 | 84.1±4.2 | 75.3±14.3 | – | p<0.05 |
| TBR (%) | 6.9±5.7 | 3.5±2.1 | 4.7±4.3 | – | ns |
| TAR (%) | 21.3±11.2 | 17.9±21.4 | 20.4±13.4 | – | ns |
| Mean blood glucose (mg/dl) | 140.7±19.5 | 131.7±6.7 | 140.9±20.0 | – | ns |
| CV (%) | 38.9±6.5 | 32.1±3.5 | 33.9±7.4 | – | p=0.063 |
| Average hours of sleep primary carer | 5.6±1.0 | 6.3±0.7 | 5.9±1.1 | 6.8±0.9 | ns |
| Average hours of sleep child | 8.7±0.8 | 9.2±1.0 | 8.9±1.0 | 9.5±0.8 | ns |
| Mean score Pittsburgh Questionnaire | 11.6±3.6 | 10.6±4.1 | 10.2±3.9 | 4.3±2.4 | p<0.05 |
CSII, continuous subcutaneous insulin infusion - insulin pump; CV, coefficient of variation; FGM, flash glucose monitoring; GMI, glucose management indicator; HCL, hybrid closed-loop; MDI, multiple doses of insulin; ns, not significant; TAR, time above range; TBR, time below range; TIR, time in range.
In our study, the average number of hours of daily sleep of the carers of both children with T1DM and healthy children is lower than recommended. This finding was also reported by Jaser et al.,3 who found that 51% of parents of children with T1DM sleep less than the recommended seven to nine hours. In our series, the quality of sleep in the majority of carers of children with T1DM is disturbed, which is evident when compared with a control population, even with the children in their care being significantly younger. Our results are significantly worse than those of Macaulay et al.4 and Jaser et al.,3 who analysed sleep quality in parents of children with T1DM using the Pittsburgh scale and found 65% and 53% had poor sleep quality, respectively.
Whether or not the implementation of new technologies such as continuous glucose monitoring (CGM) or automated insulin delivery systems can improve the quality of sleep of carers of patients with T1DM has been the subject of debate. In the case of CGM, increased access to information on glucose levels can overwhelm some carers, especially those who have only been using the device for a short time, leading to a greater number of controls and sleep disturbances.4,5 In addition, device alarms and technical problems can contribute to being woken up and poorer sleep quality.4 Recent studies on sleep quality and automated insulin delivery systems6 demonstrate how after 12–16 weeks of use, HCL systems improve the sleep quality of carers of children with T1DM who had poor sleep quality at the beginning, which they relate to improved metabolic control with a significant increase in TIR, especially at night.
In our study, no differences were found in the quality of carer sleep according to the type of treatment received, probably due to the smaller sample size of patients in the subgroups of treatment with CSII+FGM and HCL, and the shorter period of time since the introduction of HCL systems, despite their better outcomes obtained in TIR and CV. Moreover, we did not find a correlation between the sleep quality of carers and the metabolic control of patients, unlike Jaser et al.,3 who describe a correlation between poor sleep quality and poor metabolic control, as well as between the poor sleep quality of parents and that of their children.3 In addition, the real-life nature of this study cannot obviate the fact that the indication for HCL systems was probably restricted to those patients more prone to hypoglycaemia, and probably worse baseline sleep quality, which could have affected/influenced the results obtained by not knowing the quality of sleep prior to the start of treatment.
In conclusion, T1DM may contribute to sleep disturbance in people with T1DM and their carers. The incorporation of HCL systems into the treatment of these patients can improve quality of sleep, although further studies are needed that incorporate objective measures such as actigraphy and polysomnography, as well as longitudinal studies that include a control population.