There is no international consensus on the definition of the type of oncological resection that corresponds to each of the colectomies existing in the current literature.
The objective is to define for each colectomy described in the literature: embryological dissection plane, vascular pedicles in which to perform central ligation, the extent of the colectomy, and the need for resection of the greater momentum.
A consensus of experts is carried out through the Delphi methodology through two rounds from the Coloproctology Section of the Spanish Association of Surgeons. Study period: November 2021-January 2023. 120 experts were surveyed.
Degrees of consensus: Very strong: >90%, Strong: 80%–90%, Moderate: 50%–80%, No consensus: <50%.
The definition for each oncological colectomy was established by very strong, and strong recommendations.
Each oncological colectomy was established as Right hemicolectomy (RHC), RHC with D3 lymphadenectomy, Extended-RHC, transverse colon segmental colectomy, splenic flexure segmental colectomy, subtotal colectomy, total colectomy, left hemicolectomy (LHC), extended-LHC, sigmoidectomy.
No existe consenso internacional en la definición del tipo de resección oncológica que corresponde a cada una de las colectomías existentes en la bibliografía actual.
El objetivo es definir para cada colectomía descrita en la literatura: plano de disección embriológico, pedículos vasculares en los que realizar ligadura central, la extensión de la colectomía y la necesidad de resección de epiplón mayor.
Se realiza un Consenso de expertos a través de la metodología Delphi mediante dos rondas desde la Sección Coloproctología de Asociación Española de Cirujanos. Período de estudio: noviembre 2021-enero 2023. Fueron encuestados 120 expertos.
Grados de consenso: Muy fuerte: >90%, Fuerte: 80%–90%, Moderado: 50%–80%, No consenso: <50%.
La definición para cada colectomía oncológica quedó establecida mediante recomendaciones muy fuertes y fuertes.
Colectomías definidas: hemicolectomía derecha (HCD), HCD con linfadenectomía D3, HCD-ampliada, colectomía segmentaria de colon transverso, resección segmentaria ángulo esplénico, colectomía subtotal, total, hemicolectomía izquierda (HCI), HCI-ampliada y sigmoidectomía.
Widely recognised standards for oncological resections in colon cancer were defined in 2008 by Hohenberger et al. who described the complete mesocolic excision technique (CME) in detail.1 These standards are based on dissection through the embryological plane to keep the mesocolon intact, high or central ligation of tumour feeding vessels, a 10-cm bowel safety margin, and incorporation of the greater omentum into the resection.
The D3-lymphadenectomy (LD3) is based on the same principles as the CME, in addition to the need to include the venous drainage territories of the mesocolon that are not accompanied by an artery in the surgical specimen.2
Nevertheless, as yet there is no international consensus on the precise definition of the type of oncological resection that corresponds to each of the existing colectomies in the current literature.3 Consequently, the methodology of the studies carried out to date has not been consistent with that of the CME technique.
Indeed, differences between European, and American guidelines can even be noted, which are the result of the surgical legacy of the various schools of surgery.
Material and methodsStudy designed and conducted by the Board of Coloproctology of the Spanish Association of Surgeons (AEC-CP, for its acronym in Spanish).
Expert consensus was used by means of the Delphi methodology, which involves the application of a sequence of questionnaires, complemented by summaries, and reviews based on previous responses.4 Study period November 2021 to January 2023.
Phase ISelection of experts to participate in the studyExpert selection criteria: Surgeons who are members of the AEC-CP section holding the European Board of Surgical Qualification (EBSQ) in Coloproctology (EBSQ) and/or authors of T-1 publications related to cancer of the colon in the last five years.
Selection of colectomies to be definedRight hemicolectomy (RHC), RHC with D3 lymphadenectomy (RHC-LD3), extended RHC, segmental transverse colectomy (STC), splenic flexure segmental colectomy (SFSC), subtotal (SUBTC), and total (TOTC) colectomy, left hemicolectomy (LHC), extended LHC, and sigmoidectomy (SIG).
Depiction of arterial, and venous surgical vascular anatomy is presented in Fig. 1A/B.
Phase IIElaboration of the first-round questionnaireMultiple choice questions on each of the technical points of oncological colectomies.
For colectomies involving the right, and transverse colon including both flexures, questions were also posed as to whether to include the greater omentum or not with or without the inclusion of the gastro-omental artery.
In addition, there is a question regarding the venous vasculature to be included in the surgical specimen in the RHC-LD3, and SFSC. The reason for this exception is that these are the only two areas of the mesocolon where there is venous return without satellite arterial vascularisation.
In order to create the multiple questions, a member of the AEC-CP was assigned to each colectomy on a voluntary basis; this person reviewed the literature to present the various options reported in the literature.
The recommended guidelines for this bibliographic review were as follows:
Databases: PubMed, Medline, Google Scholar, and Cochrane.
Inclusion criteria for the bibliographic search were:
- -
Articles published since 2000,
- -
Full text readings,
- -
Meta-analyses, reviews, books, and journals,
- -
In the Google Scholar database, language filters were put in place, including those articles that were only in Spanish, and in English.
Degrees of consensus:
Very strong: more than 90% agreement, Strong: 80%–90%, Moderate: 50%–80%, No consensus: less than 50%.
Responses with very strong, strong, or moderate consensus were set as final responses.
Those for which there was moderate or no consensus were posed again in round 2, but with a different format, in an attempt to achieve a higher degree of consensus.
Phase IV: Elaboration of the second-round questionnaireTo elaborate the second-round questionnaire, two new question formats were put forth for consideration: Using line drawings and/or statistically significant data based on the bibliography.
The members of the AEC-CP opted for the new format by means of voting during a telematic meeting.
Phase VDistribution of the second-round questionnaire: Questionnaires were sent out by official e-mail from the AEC-CP secretary’s accountSecond-round response storage. By means of the Typeform applicationFirst-round response analysisEach colectomy was defined using the recommendations for which there was a degree of recommendations exceeding 50% (very strong, strong, or moderate).
ResultsInvitations to complete the questionnaires were extended to 120 experts. Of them, 86 (71.6%) participated fully (they responded to the entire two rounds).
The type of embryological plane; arterial and venous vascular flaps to be ligated at their origin; the extension of the colectomy; the need and amount of the greater omentum to be resected, in addition to the need to include the gastro-omental artery were defined for each of the colectomies.
Tables 1–4 present the questions that were asked for each of the colectomies; the results of the first round (FR) are displayed, as well as the results of second round with respect to the question that received the most votes and the degree of consensus obtained.
Questions posed with respect to the right hemicolectomy, right hemicolectomy with D3 lymphadenectomy, and extended right hemicolectomy. The answers that received the most votes in the second round are presented, in addition to the right hemicolectomy. In addition, the percentage of votes in both the first and the second round are illustrated, as well as the degree of consensus.
| Resection | Question | Result | Round 1 (%) | Round 2 (%) | Consensus |
|---|---|---|---|---|---|
| Right hemicolectomy | Embryological plane dissection | Toldt + Fredet access | 81.6 | Strong | |
| Vascular and arterial flaps (and satellite veins) | Ileocolic + right colic* + right branch of the middle colic | 77 | 80.2 | Strong | |
| Greater omentum and gastro-omental | Right inclusion of the greater omentum without the gastro-omental | 72.4 | 77.9 | Moderate | |
| Right hemicolectomy with D3 lymphadenectomy | Embryological dissection | Toldt + Fredet access | 92 | Very strong | |
| Vascular and arterial flaps (and satellite veins) | Ileocolic + right colic* + right branch of the middle colic | 57.5 | 73.3 | Moderate | |
| Greater omentum and gastro-omental | Right inclusion of the greater omentum without the gastro-omental | 52.9 | 62.8 | Moderate | |
| Venous territories | Lymphofatty tissue overlying the superior mesenteric vein and gastrocolic trunk of Henle | 88.5 | Strong | ||
| What vein of the gastrocolic trunk of Henle should be incorporated? | Right superior colic vein at its junction with the gastrocolic trunk of Henle | 80.05 | Strong | ||
| Extended right hemicolectomy | Embryological dissection | Toldt + Fredet access | 85.1 | Strong | |
| Vascular and arterial flaps (and satellite veins) | Ileocolic + right colic* + middle colic | 81.6 | Strong | ||
| Greater omentum and gastro-omental | Inclusion up to the splenic flexure of the greater omentum without the gastro-omental | 50.6 | 38.4 | No | |
| Extension | Extension of the transversal colectomy without including the splenic flexure | 64.4 | 65.1 | Moderate | |
Questions asked about the segmental colectomy of the transverse colon and segmental colectomy of the splenic flexure. Answers receiving the most votes in the second round are displayed. In addition, the percentage of votes received in both the first and second rounds and the degree of consensus are presented.
| Resection | Question | Result | Round 1 (%) | Round 2 (%) | Consensus |
|---|---|---|---|---|---|
| Segmental colectomy of the transverse colon | Embryological plane dissection | Access to the omental transcavity. No coalescence fascia | 73.6 | 72.1 | Moderate |
| Vascular and arterial flaps (and satellite veins) | Middle colic | 92 | Very strong | ||
| Greater omentum and gastro-omental | Entire greater omentum, including flexures without the gastro-omental | 35.6 | 54.7 | Moderate | |
| Extension | Transverse colon including flexures | 23 | 66.3 | Moderate | |
| Segmental colectomy of the splenic flexure | Embryological plane dissection | Toldt in descending colon + transcavity in transverse | 100 | Very strong | |
| Vascular and arterial flaps (and satellite veins) | Left branch middle colic + left colic | 89.7 | Strong | ||
| Greater omentum and gastro-omental | Greater omentum from splenic flexure without the gastro-omental | 70.1 | 72.1 | Moderate | |
| Venous territories | Portion of inferior mesenteric vein between the origin of the mesenteric artery and inferior edge of the pancreas | 72.4 | 77.9 | Moderate | |
| Extension | Distal portion of transverse colon + descending colon | 95.3 | Very strong | ||
Questions asked about the subtotal and total colectomy. Answers receiving the most votes in the second round are displayed. In addition, the percentage of votes received in both the first and second rounds and the degree of consensus are presented.
| Resection | Question | Result | Round 1 (%) | Round 2 (%) | Consensus |
|---|---|---|---|---|---|
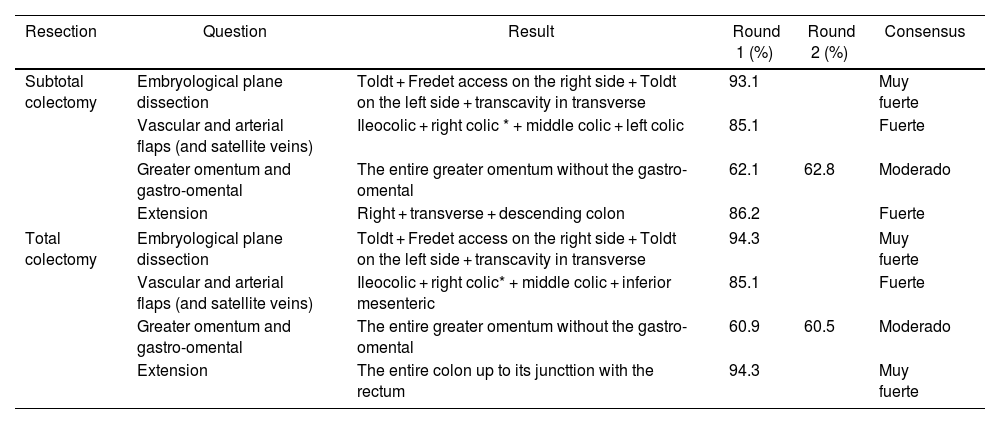
| Subtotal colectomy | Embryological plane dissection | Toldt + Fredet access on the right side + Toldt on the left side + transcavity in transverse | 93.1 | Muy fuerte | |
| Vascular and arterial flaps (and satellite veins) | Ileocolic + right colic * + middle colic + left colic | 85.1 | Fuerte | ||
| Greater omentum and gastro-omental | The entire greater omentum without the gastro-omental | 62.1 | 62.8 | Moderado | |
| Extension | Right + transverse + descending colon | 86.2 | Fuerte | ||
| Total colectomy | Embryological plane dissection | Toldt + Fredet access on the right side + Toldt on the left side + transcavity in transverse | 94.3 | Muy fuerte | |
| Vascular and arterial flaps (and satellite veins) | Ileocolic + right colic* + middle colic + inferior mesenteric | 85.1 | Fuerte | ||
| Greater omentum and gastro-omental | The entire greater omentum without the gastro-omental | 60.9 | 60.5 | Moderado | |
| Extension | The entire colon up to its juncttion with the rectum | 94.3 | Muy fuerte | ||
Questions asked about the left hemicolectomy, extended left hemicolectomy, and sigmoidectomy. Answers receiving the most votes in the second round are displayed. In addition, the percentage of votes received in both the first and second rounds and the degree of consensus are presented.
| Resection | Question | Result | Round 1 (%) | Round 2 (%) | Consensus |
|---|---|---|---|---|---|
| Left hemicolectomy | Embryological plane dissection | Toldt access | 100 | Very strong | |
| Vascular and arterial flaps (and satellite veins) | Inferior mesenteric | 28.7 | 95.3 | Very strong | |
| Extension | Descending colon + sigma | 21.8 | 95.3 | Very strong | |
| Extended left hemicolectomy | Embryological plane dissection | Toldt access | 100 | Very strong | |
| Vascular and arterial flaps (and satellite veins) | Inferior mesenteric + left branch of the middle colic | 51.7 | 94.2 | Very strong | |
| Extension | Distal third of the transverse colon + descending colon + sigma | 39.1 | 94.2 | Very strong | |
| Sigmoidectomy | Embryological plane dissection | Toldt access | 83.9 | Strong | |
| Vascular and arterial flaps (and satellite veins) | Inferior mesenteric at point of origin | 63.2 | 65.1 | Moderate | |
| Proximal extension | Descending colon and sigma junction | 41.4 | 68.6 | Moderate | |
| Distal extension | Superior rectus including rectosigmoid junction | 92 | Very strong | ||
Table 5 depict the final definition for each of the colectomies based on the responses that received more than 50% consensus (moderate, strong, or very strong).
Presents the definitive definition obtained after both rounds for each of the oncological colectomies. This definition is the results of the answers for which there was a consensus > 50%.
| Type of resection | Definitive definition | Figure |
|---|---|---|
| Right Hemicolectomy | Dissection plane: Toldt + Fredet access. | |
| Central arterial ligation: Ileocolic + right colic + right branch of the middle colic. | ||
| Inclusion of the greater omentum: Right inclusion of the greater omentum without the gastro-omental vessels. | ||
| Right Hemicolectomy with D3 lymphadenectomy | Dissection plane: Toldt + Fredet access. | |
| Central arterial ligation: Ileocolic + right colic + right branch of the middle colic | ||
| Venous central ligation: lymphoadipose tissue overlying the superior mesenteric vein + superior right colic vein where it empties into Henle’s gastrocolic trunk | ||
| Inclusion of the greater omentum: Right inclusion of the greater omentum without the gastro-omental vessels. | ||
| Extended Right Hemicolectomy | Dissection plane: Toldt + Fredet access. | |
| Central arterial ligation: Ileocolic + colic right + colic media. | ||
| Extension of the colectomy: Colon transversa without including the splenic flexure. | ||
| Inclusion of the greater omentum: No consensus. Most widely voted answer: Inclusion up to the splenic flexure of the greater omentum without the gastro-omental vessels.* | ||
| Segmental Transverse Colon Colectomy | Dissection plane: Access to the omental transcavity. There is no coalescence fascia. | |
| Central arterial ligation: Colic media | ||
| Extension of the colectomy: Transverse colon including flexures | ||
| Inclusion of the greater omentum: The entire greater omentum, including flexures without gastro-omental. | ||
| Segmental Splenic Flexure Colectomy | Dissection plane: Toldt access in the descending colon + transcavity in the transverse colon. | |
| Central arterial ligation: Left branch of the middle colic + left colic. | ||
| Venous central ligation: Portion of the inferior mesenteric vein between the origin of the inferior mesenteric and the inferior edge of the pancreas. | ||
| Extension of the colectomy: Distal portion of the transverse colon/descending colon. | ||
| Inclusion of the greater omentum: Greater omentum of the splenic flexure without gastro-omental. | ||
| Subtotal Colectomy | Dissection plane: Toldt + Fredet access on the right + Toldt on the left + transverse transcavity | |
| Central arterial ligation: Ileocolic + right colic* + middle media colic + left colic. | ||
| Extension of the colectomy: Right + transverse + descending colon | ||
| Inclusion of the greater omentum: The entire greater omentum, including flexures without gastro-omental. | ||
| Total Colectomy | Dissection plane: Toldt + Fredet on the right side + Toldt on the left + transcavity in transverse. | |
| Central arterial ligation: Ileocolic + right colic* + middle colic + inferior mesenteric. | ||
| Extension of the colectomy: The entire colon up to its junction with the rectum. | ||
| Inclusion of the greater omentum: the entire greater omentum without gastro-omental. | ||
| Left Hemicolectomy | Dissection plane: Toldt access | |
| Central arterial ligation: Inferior mesenteric. | ||
| Extension of the colectomy: Descending colon + sigma. | ||
| Extended Left Hemicolectomy | Dissection plane: Toldt access | |
| Central arterial ligation: Inferior mesenteric + left branch of the middle colic | ||
| Extension of the colectomy: Distal third of the transverse colon + descending colon + sigma | ||
| Sigmoidectomy | Dissection plane: Toldt access | |
| Central arterial ligation: Inferior mesenteric at its origin. | ||
| Extension of the colectomy: From the juncture of the descending colon and sigma until the superior rectum including the rectosigmoid junction. |
Supplementary information can be found consisting of two documents with all the FR and SR answers in detail. (Supplementary Material).
Below, the reader will find a summary of the FR and SR results for each colectomy.
The RHC, RHC-LD3, and extended RHC exhibit a strong or very strong degree of consensus concerning the dissection plane through Toldt’s and Fredet’s fascia, as well as the central ligation of the ileocolic and colic right (when present) arterial vessels, and the right branch of the middle colic in the RHC and RHC-LD3, or middle colic vessels where they arise in the extended RHC.
In addition, there is an 88.5% consensus as to the inclusion of the lymphoadipose tissue located on the superior mesenteric vein (SMV) and on the gastrocolic trunk of Henle (GCTH) in the RHC-LD3. Furthermore, 80% of the experts feel that the superior right colic vein (SRCV) is the GCTH vein that should be included in the surgical specimen (Table 1).
Consensus as to inclusion of the right portion of the greater omentum in the surgical specimen is moderate.
As for the STC, 92% of the respondents believe that central ligation should be performed on the middle colic, whereas the consensus concerning resecting both flexures and including the entire greater omentum in the surgical specimen is moderate.
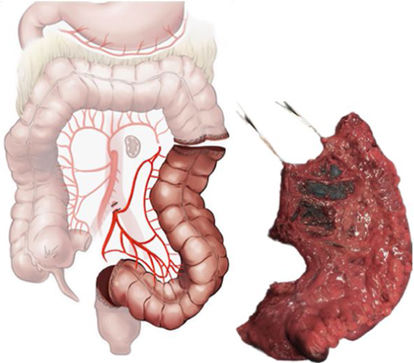
When it comes to the SFSC, a very strong or strong consensus was attained in the FR regarding the dissection plane through Toldt’s layer in the descending colon and the transcavity of the omenta in the transverse colon, the extension of the colectomy of the distal portion of the transverse colon and descending colon, and the central arterial ligation of the left branch of the middle colic and of the left colic artery. In contrast, after both rounds, consensus was moderate as regards including the greater omentum of the splenic flexure without including the gastro-omental arcade (72%), as well as including the portion of the inferior mesenteric vein between the origin of the inferior mesenteric artery and the inferior edge of the pancreas (78%) (Table 2).
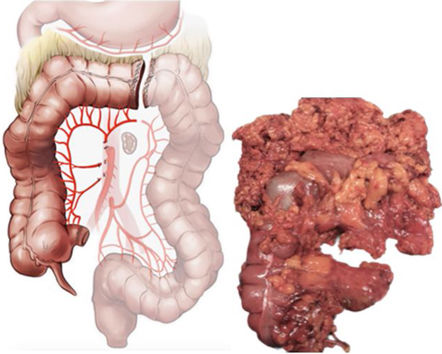
Already in the first round, there was a very strong or strong degree of consensus (85%–94%) for the dissection plane, the extent of the resection of the colon, and the central ligation of the vascular flaps in both the SUBTC and TOTC. Both techniques are performed through the same dissection plane –Toldt’s and Fredet’s fascia in the right colon, Toldt’s fascia in the left colon, and the transcavity of the omenta in the transverse colon. The extent of resection will be right, transverse, and descending colon for SUBTC and TOTC, but TOTC will add the sigmoid colon. Central ligation in both techniques coincide in the ileocolic vessels, right colic vessels if present, and middle colic vessels, but differ in that central ligation of the left colic artery is performed in CSBUT, and of the inferior mesenteric artery in TOTC. There is a moderate consensus (60%–62%) for both SUBTC and TOTC to include resection of the entire greater omentum without the gastro-omental arcade (Table 3).
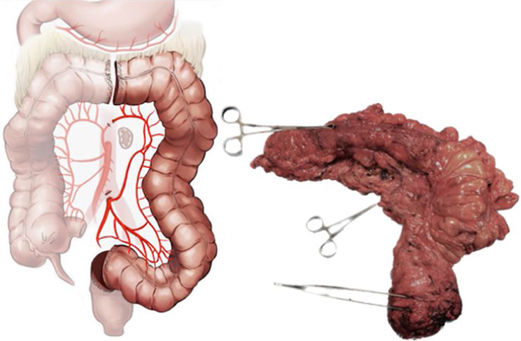
The first round of questioning, a high degree of consensus was found with regard to the dissection plane by Toldt's fascia in both the LHC, extended LHC, and the SIG (84%–100%). However, in this first round, consensus was not achieved for the extent of resection (21%–39%) or for the type of central vascular ligation (28%–51%) in the LHC and the extended LHC. Nevertheless, after drafting the new, second round questionnaire, very strong consensus (94%–95%) was attained for both items. In LHC, central vascular ligation of the inferior mesenteric artery (IMA) and extension of resection of the descending colon and sigma should be performed. In LHC, central vascular ligation is performed of the inferior mesenteric artery and left branch of the middle colic vessels with an extension of resection of the distal third of the transverse colon, descending colon, and sigma.
Finally, a moderate degree of consensus was observed for SIG concerning high ligation of the inferior mesenteric artery (65%) and extension of proximal resection at the junction of the descending colon and sigma (69%), and a very strong consensus (92%) for extension of distal resection up to the superior rectum including the rectosigmoid junction (Table 4).
Table 5 provides the final definition obtained after the two rounds for each of the oncological colectomies.
DiscussionDepending on the tumour location, different types of colectomies are described in the current literature.
However, the definition of each can vary according to the literature consulted.5 This may represent a methodological bias in those studies that assess both morbidity and mortality and oncological outcomes in the short and long term.
The aim of this study is not to indicate the type of colectomy to be performed on the basis of the tumour site, but to standardise the definition of each colectomy so as to unify criteria.
The first round of questions of the present study illustrated this lack of consensus, inasmuch as none of the colectomies could be fully defined.
Following the study published in 2009 by Dr Hohemberger, dissection through the embryological plane, high ligation of the feeding arteries, and a bowel safety margin of 10 cm proximal and distal to the lesion1 were accepted as quality standards for the surgical technique.
However, there are two points that are more controversial and not have not been fully settled in the literature: whether or not to include the greater omentum6 with or without the gastro-omental arcade7 and the extent of the colectomy as a function of the location of the tumour.8
Furthermore, the increasing adaptation of the D3 lymphadenectomy in the West promoted by the East adds new anatomo-surgical areas of confusion.9
In the present study, the embryological plane of dissection depending on the colectomy to be carried out appears to be unambiguous, as a strong or very strong consensus was reached on the first round in this respect.
In the ascending colon, this plane is Toldt's fascia and Fredet's fascia,10 and Toldt's fascia in the case of the descending colon.11 Fredet's fascia is not referred to as such in the article by Hohemberger, et al.; nevertheless, since the publication in 201910 in which its usefulness was shown to dissect the right mesocolon of the second duodenal portion and the pancreatic head, this fascia has gained importance to the point of being included in the definitive definition of RHC, RHC-LD3, and extended RHC.
The experts' selection of Toldt's fascia in the sigmoid is striking, given that due to embryological rotation, the sigmoid has no coalescence fascia.12 The reason for this choice may lie in the fact that the respondents considered that in the sigmoidectomy, the descending colon must also be freed from the retroperitoneum.
These coalescence fasciae are the result of embryological rotations of the primitive intestine, and not only have repercussions on the surgical technique used in colon cancer, but also on the anatomopathological study of the surgical specimens. In fact, if the dissection has been performed through the embryological surgical plane, the mesocolic surface will be smooth and the specimen will be classified as a proper mesocolic plane, whereas if the surface is rough, it will be classified as an improper mesocolic plane. There is, however, a point of contention with this system by which to classify surgical quality, as both the transverse colon13 and the sigmoid colon are devoid of any coalescence fascia with the retroperitoneum, and therefore all the specimens will have a smooth surface.
In terms of the high ligation of tumour feeding arteries, there is a clear consensus of definition in the RHC, extended RHC, and STC. Tumours of the proximal hepatic and transverse hepatic flexure entail difficulties in selecting which colectomy to perform, as there is no clear anatomical point to indicate when lymphatic drainage should be performed through the middle colic vessels and, consequently, when they should be ligated at their origin, as in extended RHC and STC. On the other hand, the literature shows that the STC is scantly carried out, although its definition should be included in the present work, as it is used in some retrospective studies of localised cancer in the transverse colon.
The high ligation of tumours located in the left colon obtained a clear lack of consensus in the first round. This fact is reflected in the various definitions that currently exist to define a high ligation of the left colic artery plus a high ligation of the left branch of the middle colic artery. Mike et al.14 opine that it would be called a left hemicolectomy, while the experts in the present study consider it to be a segmental colectomy of the splenic flexure.
The meta-analysis published by Wang et al.15 is a clear example of how this lack of a defining consensus means that the conclusions of the different studies can be contested. In this meta-analysis, the conclusion is that there are no differences in oncological survival in neoplasia located in the splenic flexure for different surgical techniques: subtotal colectomy, extended right hemicolectomy, left hemicolectomy, and segmental colectomy. However, according to the results of the present study, neither the extended right hemicolectomy nor the left hemicolectomy would include a tumour located in the splenic flexure in their resection and, as a result, should not be considered in this study.
An example of the need for the present work is the type of central ligation in the LHC. In the first round, 28% of the experts selected the AMI, whereas after including illustrations in the second questionnaire, this percentage rose to 95%, which is deemed a very strong consensus.
The Extended LHC achieved a 94% consensus of central ligation of the IMA and of the left branch of the middle colic vessels after both rounds. In this instance, it should be noted that in some cases the surgeon will decide to section the middle colic vessels, not for oncological reasons, but in order to be able to subsequently perform a tension-free colorectal anastomosis using the Deloyers procedure or the complete bowel derotation procedure.16
Oncological sigmoidectomy has three types of central ligation reported in the literature, none of which have demonstrated better oncological results to date. The first would be central ligation of the sigmoid vessels without including the IMA, which would be equivalent to a D2 lymphadenectomy in the East.17 The second would be central ligation of the IMA, which would be equivalent to D3 lymphadenectomy. However, the latter is further divided into two: high and low ligation.18,19 In the low ligation, IMA ligation is performed posterior to the origin of the ICA, whereas in the high ligation it is performed at the origin of the IMA from the aorta. In the present study, there is a moderate degree of consensus (65%) for high ligation and inclusion of the entire course of the IMA in the surgical specimen, while 32% voted for low ligation of the IMA and only 3% for central ligation of the sigmoid vessels.
The fact that the current literature has failed to demonstrate inferiority in terms of survival of central ligation of the sigmoid vessels could be the result of another problem with the anatomopathological classification system of the surgical specimens. It is not yet possible to know from the macroscopic view of the surgical specimen what type of ligation the surgeon has performed, and most publications classify the specimens according to the time out sheet.
The draining vein to be included in the surgical specimen is satellite to the arterial one, with the exception of two areas of the mesocolon: the SRCV in the right colon20 and the fragment of the inferior mesenteric vein between the origin of inferior mesenteric artery and inferior edge of the pancreas in the left colon.21 These areas take on even greater importance within the concept of the LD3, given the embryological venous origin of the lymphatic ducts.
In RHC-LD3, the inclusion of lymphoadipose tissue located above the SMV, and above the GCTH is strongly recommended. Regardless of the GCTH makeup, it is also strongly recommended that the vein to be apically ligated is the SRCV. This consensus is consistent with the latest publications highlighting the importance of this vein which drains from the hepatic flexure to the HCGCT,22 and is present in 95% of all patients.23
There are differing postures with respect to the SFSC toward whether the inferior mesenteric vein must be included in the surgical specimen in light of the possibility of adenopathies.24 In this work, 78% of the experts consulted recommend including the portion of the inferior mesenteric vein between the origin of the inferior mesenteric artery and the inferior edge of the pancreas.
Recently, Planellas et al.25 conducted a randomised, multicentre study in which they demonstrated that in the oncological sigmoidectomy, this fragment of the inferior mesenteric vein need not be included; nevertheless, the issue is not settled as it concerns localized tumours in the splenic flexure, and descending colon.
Another item of surgical doubt is the extension of the different colectomies. As regards the LHC, the left transverse, descending and sigmoid colon are included as per the Colorectal Surgery. Clinical Guidelines of the Spanish Association of Surgeons26; that being said, there was a very strong consensus in the present study about including only the descending and sigmoid colon. The distal transverse colon would comprise part of the Extended LHC.
The difference in how the TOTC and SUBTC are defined as pertains to the extension of the colectomy has always been cause for confusion. Some authors27 define the SUBTC as consisting of the right, transverse, descending, and sigmoid colon up to the rectosigmoid junction. Nevertheless, the results of the present study reveal a very strong consensus in the sense that this degree of extension would be synonymous with the total colectomy and not the subtotal colectomy. Therefore, if the high ligation of the AMI and resection of the sigmoid colon are not performed, the colectomy will be defined as SUBTC.
Concerning whether to include the greater omentum or not in the case of localised tumours in the hepatic flexure, transverse colon, and splenic flexure, several studies illustrate the need to include the greater omentum in light of the risk of tumour seeding in this structure,28 while others insist that including the gastro-omental arcade is mandatory due to the possibility of adenopathies,29 particularly in both colon flexures. Despite this, recent studies endorse not including the greater omentum, inasmuch as its embryological origin is not the same as the mesocolon and, hence, the risk of micro-metastasis is very low.30 In the present study, this discussion is evidenced by the fact that, despite the two rounds, there was still no consensus or moderate consensus for the RHC, RHC-LD3, extended RHC, STC, SFSC, SUBTC, and TOTC.
To conclude, thanks to the prospective study following the Delphi methodology by the Coloproctology Section of the Spanish Association of Surgeons, the surgical definition of every one of the oncological colectomies cited in the bibliography have been defined.
Therefore, criteria could be unified and prospective studies having better methodological quality could be conducted and, in that way, reach more accurate survival conclusions following surgical resection for colon cancer. Moreover, standardising the surgical technique for cancer of the colon will be highly useful.
Collaborative working group in the preparation of the paper and collaborative group of respondents: Supplementary material classified as collaborative groups.
Conflict of interestThe authors declare that they have no conflict of interest.
Illustrator: María Martín-Martí (mmartmararte@gmail.com).
J. Die Trill, Unidad de Cirugía Colorrectal, Hospital Universitario Ramón y Cajal, Madrid; M. Pascual, Unidad de Cirugía Colorrectal, Hospital del Mar, Barcelona; E. Peña Ros, Unidad de Cirugía Colorrectal, Hospital General Universitario Reina Sofía de Murcia; R.M. Jiménez Rodríguez, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocío, Sevilla, España; M. Hidalgo-Pujol, Unidad de Cirugía Colorrectal Hospital Universitario de Bellvitge, Barcelona; L.M. Jiménez, Unidad de Cirugía Colorrectal, Hospital Universitario Gregorio Marañón, Madrid; B. Arencibia Pérez, Unidad de Cirugía Colorrectal, Hospital Universitario de Gran Canaria Doctor Negrín; V. Vigorita, Unidad de Cirugía Colorrectal, Hospital Universitario Álvaro Cunqueiro Vigo; R. Colombari, Unidad de Cirugía Colorrectal, Hospital General Universitario Gregorio Marañón, Madrid; T. Pérez-Pérez, Unidad de Cirugía Colorrectal, Servicio de Cirugía General, Hospital Lluis Alcanyis de Xativa, Valencia, Spain.
AFFILIATION OF AUTHORS
M.T. García Martínez, Unidad de Cirugía Colorrectal, Hospital Universitario de A Coruña (CHUAC); J. Bauxali, Unidad de Cirugía Colorrectal, Clínica Universitaria de Navarra, Madrid; J. Cerdán, Unidad de Cirugía Colorrectal, Hospital Clinico Universitario de San Carlos, Madrid; J.C. García-Pérez, Unidad de Cirugía Colorrectal, Hospital Universitario Ramón y Cajal, Madrid; B. Martin-Perez, Unidad de Cirugía Colorrectal, Servicio Extremeño de Salud; N. Uribe Quintana, Unidad de Cirugía Colorrectal, Hospital Arnau de Vilanova, Valencia, España; R. Farrés Coll, Unidad de Cirugía Colorrectal, Departamento de Cirugía General y Digestiva, Hospital Universitario de Girona; F.J. González-Argenté, Unidad de Cirugía Colorrectal, Hospital Universitario Son Espases Islas Baleares, Palma de Mallorca, España; J.C. Bernal Sprekelsen, Unidad de Cirugía Colorrectal, Hospital Dr. Peset de Valencia, Valencia, España; D. Fraccalvieri, Unidad de Cirugía Colorrectal, Hospital Universitario de Bellvitge, Departamento de Cirugía General y Digestiva, Barcelona, España; E. Garcia Granero, Unidad de Cirugía Colorrectal, Hospital Universitario y Politécnico de La Fe Valencia, España; M. Gómez Ruiz, Unidad de Cirugía Colorrectal, Hospital Universitario Marqués de Vadecilla, Santander; A.M. García Cabrera, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocio, Sevilla; P. Palma, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen de las Nieves, Granada; V. Pla-Martí, Unidad de Cirugía Colorrectal, Hospital Clínico Universitario de Valencia; S. Mera Velasco, Unidad de Coloproctología, Hospital Regional Universitario Carlos Haya, Málaga; F. Blanco-Antona, Unidad de Cirugía Colorrectal, Hospital Clínico Universitario de Salamanca; A. Parajó, Unidad de Cirugía Colorrectal, Hospital Univeristario de Pontevedra; G. Salgado, Unidad de Cirugía Colorrectal, Hospital Santa Helena, Los Álamos, Málaga; J.M. Vázquez Monchul, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocío, Sevilla; J. Ocaña Jiménez, Unidad de Cirugía Colorrectal, Hospital Universitario Ramón y Cajal, Madrid; F. Jiménez-Escobar, Unidad de Cirugía Colorrectal, Hospital Universitario Galdakao- Usansolo, Vizcaya; M. Martí-Gallostra, Unidad de Cirugía Colorrectal, Hospital Universitario Vall d´Hebrón, Barcelona; J.M. Díaz Pavón, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocío, Sevilla, España; C. Salvador-Morales, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen Macarena, Sevilla; S. Biondo, Unidad de Cirugía Colorrectal, Hospital Universitario de Bellvitge, Barcelona; A. Espí, Unidad de Cirugía Colorrectal, Hospital Clínico Universitario de Valencia; A. Solana-Bueno, Unidad de Cirugía Colorrectal, Hospital de Sagunto; G. Marín, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocío, Sevilla; C. Pastor Idoate, Unidad de Cirugía Colorrectal, Clínica Universitaria de Navarra, Madrid; E.D. Valle-Hernández, Unidad de Cirugía Colorrectal, Hospital Universitario Gregorio Marañón, Madrid; P. Tejedor, Unidad de Cirugía Colorrectal, Hospital Universitario Gregorio Marañón, Madrid; R. Alós Company, Unidad de Cirugía Colorrectal, Hospital Universitario y Politécnico La Fe, Valencia; T. Elosua, Unidad de Cirugía Colorrectal, Hospital Universitario de León; J.A. Rueda Orgaz, Unidad de Cirugía Colorrectal, Hospital Universitario Fundación de Alcorcón; J. García Septiem, Unidad de Cirugía Colorrectal, Hospital Universitario de La Princesa, Madrid; C. Ballester Ibánez, Unidad de Cirugía Colorrectal, Hospital Universitario y Politécnico La Fe, Valencia; M. Frasson, Unidad de Cirugía Colorrectal, Hospital Universitario y Politécnico La Fe, Valencia; J.V. Hernandis Villalba, Unidad de Cirugía Colorrectal, Hospital General Universitario de Elda, Alicante; I. Pascual Miguelañez, Unidad de Cirugía Colorrectal, Hospital Universitario de La Paz, Madrid; J.M. García-González, Unidad de Cirugía Colorrectal, Hospital Universitario de Cruces, Vizcaya; M. Jimenez-Toscano, Unidad de Cirugía Colorrectal, Hospital del Mar, Barcelona; J.A. Benavides Buleje, Unidad de Cirugía Colorrectal, Hospital Universitario Reina Sofía, Murcia; J.M. Enríquez-Navascués, Unidad de Cirugía Colorrectal, Hospital Universitario de Donostia; M.L. Reyes Díaz, Unidad de Cirugía Colorrectal, Hospital Universitario Virgen del Rocío, Sevilla; M. Millan, Unidad de Cirugía Colorrectal, Hospital Universitario y Politécnico La Fe, Valencia; L. Sánchez-Guillén, Unidad de Cirugía Colorrectal, Hospital Universitario de Elche; J.V. Roig Vila, Unidad de Cirugía Colorrectal, Hospital Nisa 9 de Octubre, Valencia; P.A. Parra-Baños, Unidad de Cirugía Colorrectal, Hospital Universitario Reina Sofía de Murcia; C. Fernánde, Unidad de Cirugía Colorrectal, Hospital Universitario Marqués de Valdecilla, Santander; R. Cantero-Cid, Unidad de Cirugía Colorrectal, Hospital Universitario de La Paz, Madrid; N. Truán Alonso, Unidad de Cirugía Colorrectal, Hospital Universitario Central de Asturias, Oviedo; E.M. Nogués-Ramia, Unidad de Cirugía Colorrectal, Hospital Universitario de Gran Canaria Dr. Negrín, Universidad de Las Palmas de Gran Canaria, Las Palmas de Gran Canaria; S. Serra Pla, Unidad de Cirugía Colorrectal, Hospital Universitario Parc Taulí, Sabadell, Barcelona; M. Climent-Agustín, Unidad de Cirugía Colorrectal, Hospital del Mar, Barcelona; F. Marinello, Unidad de Cirugía Colorrectal Hospital Universitario Vall d´Hebron, Barcelona; D. Moro-Valdezate, Unidad de Cirugía Colorrectal, Hospital Clínico Universitario de Valencia; R. Frago, Unidad de Cirugía Colorrectal, Hospital Universotario de Bellvitge, Barcelona; E. Espin, Unidad de Cirugía Colorrectal Hospital Universitario Vall d´Hebron, Barcelona; M. Pera-Román, Unidad de Cirugía Colorrectal, Hospital del Mar, Barcelona; C.J. Álvarez Laso, Unidad de Cirugía Colorrectal, Hospital Universitario de Cabueñes, Gijón; C. Placer-Galan, Unidad de Cirugía Colorrectal, Hospital Universitario de Donosti; M. Labalde Martínez, Unidad de Cirugía Colorrectal, Hospital Universitario 12 de Octubre. Madrid; J.J. García-Armengol, Unidad de Cirugía Colorrectal, Hospital Vithas- Nisa 9 de Octubre, Valencia; A. Codina, Unidad de Cirugía Colorrectal, Hospital Universitario de Girona; L.C. Capitan-Morales, Unidad de Cirugía Colorrectgal, Hospital Universitario Virgen Macarena, Sevilla; J. Garcia-Aguilar, Unidad de Cirugía Colorrectal, Memorial Sloan Kettering Cancer Center, New York; J.M. Fernández-Cebrián, Unida de Cirugía Colorrectal, Hospital Universitario Ramón y Cajal. Madrid; M. Fernández-Hevia, Unidad de Cirugía Colorrectal, Hospital Universitario Central de Asturias; L.J. García-Flórez, Unidad de Cirugía Colorrectal, Hospital Universitario Central de Asturias; G. Pellino, Unidad de Cirugía Colorrectal, Hospital Universitario Vall d´Hebrón, Barcelona; C. Martínez-Pérez, Unidad de Cirugía Colorrectal, Hospital General Universitario de Valencia; F. Fernández-López, Unidad de Cirugía Colorrectal; Complejo Hospital Universitario de Santiago de Compostela.
The names of the members of the Board of the Coloproctology Section of the AEC and collaborators in the preparation of this study are listed in Appendix 1.