The only curative treatment of pelvic recurrence of rectal cancer is radical resection. The aim of this paper is to analyze our experience in surgery for local recurrence of rectal cancer.
MethodsWe performed a descriptive retrospective analysis of patients treated with curative intent for local recurrence of rectal cancer from May 2000 to January 2014. The presence of resectable liver or lung metastases was not an exclusion criterion. The descriptive results, overall survival and disease free survival are presented.
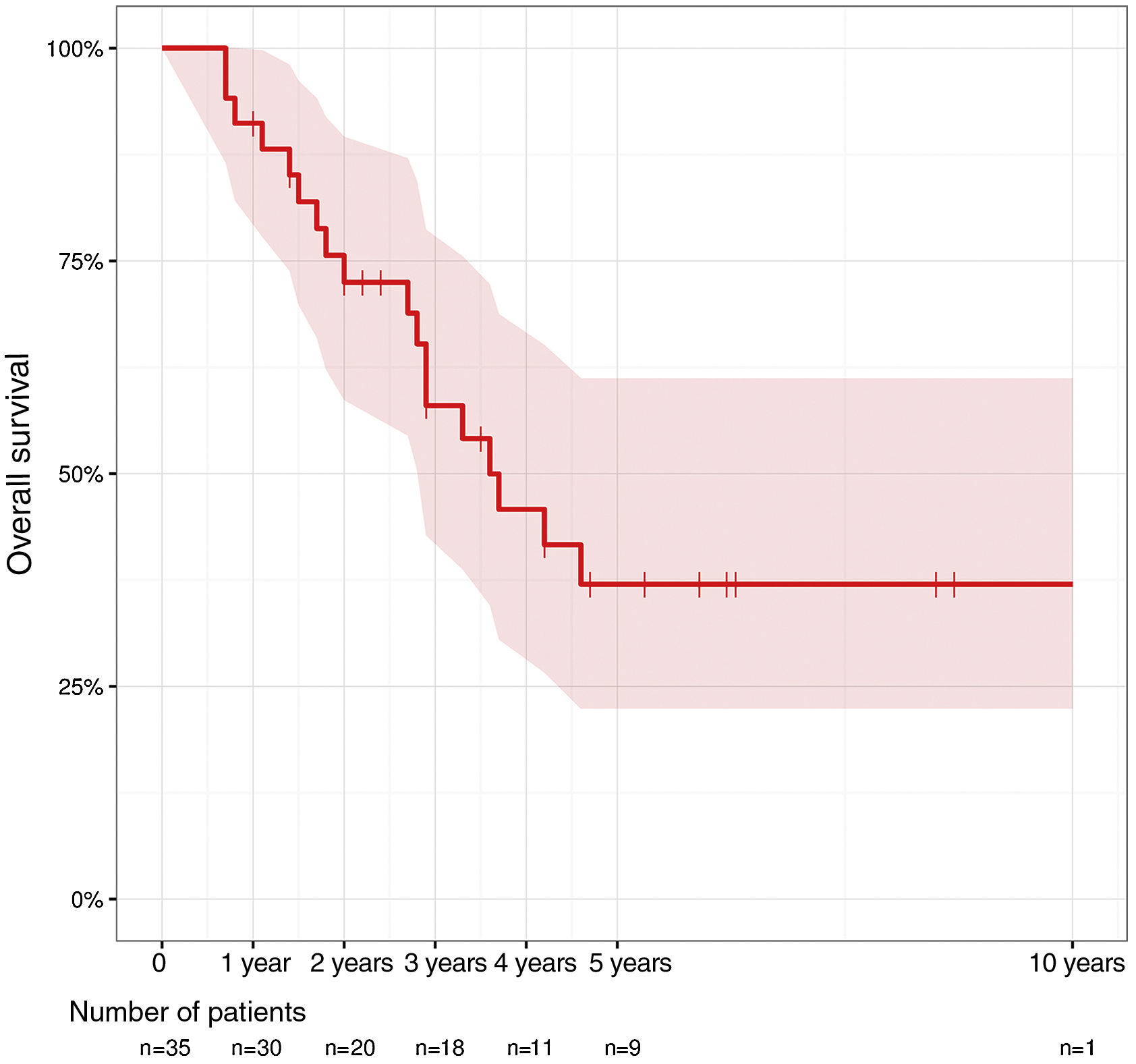
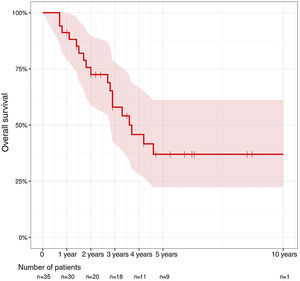
ResultsA total of 35 patients were included. In 18 patients an abdominoperineal resection of the remaining rectum was performed. Two of them included excision of lower sacral vertebrae, while in 17 patients, sphincter sparing surgery was performed. The most frequent postoperative complications were pelvic collection and postoperative ileus. Seven patients required reoperation and one patient died. Overall survival at 1 year was 91.2%, at 2 years 75.6% and at 5 years 37%.
ConclusionsLocal recurrence of rectal cancer is a disease with high curability rate. The only curative option is radical surgery, with acceptable mortality.
Actualmente, el único tratamiento curativo de la recurrencia pélvica del cáncer de recto es la resección radical. El objetivo de este trabajo es realizar un análisis de nuestra experiencia en la cirugía de la recidiva local del cáncer de recto.
MétodosRealizamos un análisis descriptivo retrospectivo de los pacientes intervenidos con intención curativa por recidiva local de cáncer recto desde mayo de 2000 hasta enero de 2014. La presencia de metástasis hepáticas o pulmonares resecables no fue criterio de exclusión. Se presentan los resultados descriptivos y los tiempos de supervivencia y libres de enfermedad.
ResultadosSe incluyó a 35 pacientes. En 18 pacientes se realizó una amputación del remanente del recto, en 2 de ellos con exéresis de vértebras sacras inferiores, y en 17 pacientes se realizó cirugía preservadora de esfínteres. Las complicaciones postoperatorias más frecuentes fueron la colección pélvica y el íleo paralítico postoperatorio. Siete pacientes requirieron reintervención y uno falleció. La supervivencia global al año fue del 91,2%, a los 2 años del 75,6% y a los 5 años del 37%.
ConclusionesLa recidiva local del cáncer de recto es una enfermedad con alta tasa de curabilidad. La única opción curativa es la cirugía radical, con una mortalidad aceptable.
The locoregional recurrence of rectal cancer after curative treatment for the primary tumor is an important therapeutic challenge for colorectal surgeons, and radical resection is the only option for a cure.1
Over the last 2 decades, the incidence of local recurrence of rectal cancer has been decreasing thanks to the advances made in neoadjuvant treatment and in surgical technique.2 Prior to total mesorectal excision (TME), recurrences ranged between 20% and 40%.3,4 However, its association with neoadjuvant oncologic treatment has had an influence on the reduction in the recurrence rate to between 2.6% and 10%.5–7 In the majority of cases, recurrence occurs within the first years after primary tumor surgery,8 although it may present later in some 36% of cases.9
Furthermore, there has been a change in the location pattern of pelvic recurrences, as total mesorectal excision entails a lower risk for central relapse and a predominance of lateral or posterior recurrences.10
Mean survival after diagnosis of local recurrence is 7 months without treatment, and 5-year survival is less than 5%.11 Nonetheless, overall survival under multidisciplinary treatment can reach 40%.12 Between 15% and 20% of patients present metastasis at the time of diagnosis, and 30% will develop distant metastasis after radical surgery.13
Currently, the only curative treatment is radical resection in order to achieve complete excision (R0), which may or may not be associated with specific oncologic treatment.2
The objective of this study is to analyze our experience in surgery for local recurrence of rectal cancer. Special interest has focused on survival related to the residual tumor classification.
MethodsA retrospective descriptive analysis was conducted of all the local recurrences of rectal cancer treated surgically at our institution from May 2000 until January 2014.
The colorectal cancer unit at our hospital is comprised of a multidisciplinary team of surgeons specialized in colorectal surgery, oncologists, radiotherapists, a clinical nurse specialist, an administrative assistant, gastroenterologists and pathologists. Once a week, a session is held to decide on the treatment of all the patients with colorectal cancer in the reference population.
The inclusion criteria of the study were: patients with local recurrence of rectal cancer who could benefit from surgical treatment; patients with a second local recurrence of rectal cancer susceptible to radical resection. The presence of distant recurrence (hepatic or pulmonary) susceptible to radical resection was not considered an exclusion criterion.
Local recurrence was defined as the appearance of tumor cells originating from the primary cancer in the pelvis minor after surgery with curative intent.
In cases with suspected local recurrence of rectal cancer, diagnostic studies included computed tomography (CT) scans of the thorax, abdomen and pelvis as well as pelvic magnetic resonance imaging. Recently, we have also used positron-emission tomography (PET)/CT in cases with uncertain diagnosis as well as endoanal ultrasound in patients with previous sphincter-preserving surgery.
Biopsy was reserved for patients with uncertain diagnosis after diagnostic imaging studies whose lesions were accessible by CT-guided biopsy.
We classified recurrences in accordance with the Leeds group14: central (relapse located in the pelvic organs with no invasion of the lateral wall); lateral (contact with lymphovascular structures or with the lateral pelvic wall); sacral (posterior, invading the sacrum); mixed (sacrum and sidewalls).
As the pelvic lesions did not infiltrate vascular structures or sacral nerve roots at S2 or higher, we decided to treat the pelvic recurrences by surgery. In the first stage of sacral resection, we performed the necessary anterior dissection, closed the abdominal wall and completed the operation with the patient in prone position. A trauma surgeon specialized in spinal surgery collaborated in the approach of the sacral resection.
Once the operation was finalized, the surgeon classified the surgery macroscopically as R0 or R2. In the pathology study, R0 was considered a curative resection, R1 the presence of microscopic residual tumor and R2 macroscopic residual tumor.
The patient variables analyzed in the study were age, sex and American Society of Anesthesiologists (ASA) classification. In the recurrence surgery, we analyzed tumor location, postoperative complication according to the Clavien–Dindo classification, readmission, pathology results and long-term follow-up.
We defined overall survival as the period of time between the initial treatment of the recurrence and death. Disease-free survival was considered the time transpired between the surgery for pelvic recurrence and the appearance of another local or distant recurrence.
Statistical AnalysisWe present the descriptive results of the patients included; survival times and disease-free (local or distant) survival times have been calculated. In the descriptive analysis, the case count and percentage have been used for the qualitative variables. For the quantitative variables, the means and standard deviation (SD) or median and range are presented. Survival analysis curves have been calculated with the Kaplan–Meier method and compared with the log-rank test. A P-value of less than .05 was considered statistically significant. The statistical analysis was carried out with the R 3.1.1 program (R Project for Statistical Computing, Vienna, Austria).
ResultsA total of 50 patients were included in the study who had been treated surgically for local recurrence of rectal cancer. Excluded from the analysis were 8 patients due to unresectable lesions at the time of surgery and 7 patients in whom no evidence of adenocarcinoma was observed in the pathology study.
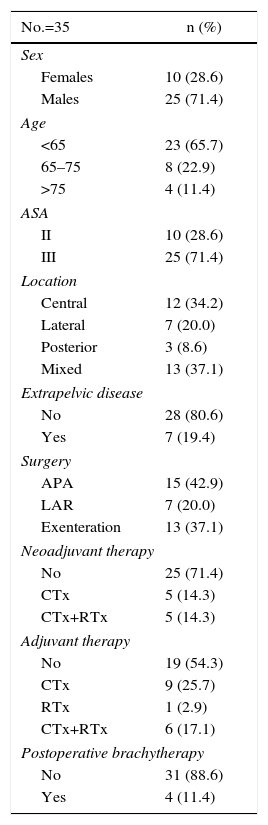
Table 1 reports the patient characteristics, recurrence locations and types of treatment received.
Patient Characteristics and Treatment of the Rectal Cancer Recurrence.
| No.=35 | n (%) |
|---|---|
| Sex | |
| Females | 10 (28.6) |
| Males | 25 (71.4) |
| Age | |
| <65 | 23 (65.7) |
| 65–75 | 8 (22.9) |
| >75 | 4 (11.4) |
| ASA | |
| II | 10 (28.6) |
| III | 25 (71.4) |
| Location | |
| Central | 12 (34.2) |
| Lateral | 7 (20.0) |
| Posterior | 3 (8.6) |
| Mixed | 13 (37.1) |
| Extrapelvic disease | |
| No | 28 (80.6) |
| Yes | 7 (19.4) |
| Surgery | |
| APA | 15 (42.9) |
| LAR | 7 (20.0) |
| Exenteration | 13 (37.1) |
| Neoadjuvant therapy | |
| No | 25 (71.4) |
| CTx | 5 (14.3) |
| CTx+RTx | 5 (14.3) |
| Adjuvant therapy | |
| No | 19 (54.3) |
| CTx | 9 (25.7) |
| RTx | 1 (2.9) |
| CTx+RTx | 6 (17.1) |
| Postoperative brachytherapy | |
| No | 31 (88.6) |
| Yes | 4 (11.4) |
A total of 35 patients were analyzed (10 women and 25 men), with a mean age of 58.6. All had positive histology confirmation for adenocarcinoma. Preoperative comorbidity was evaluated by means of the ASA classification. A total of 19 patients (54.3%) had been treated surgically for the primary tumor at another hospital and were referred to our department for recurrence surgery.
In most patients, the primary tumor was located in the mid-rectum (15 patients) and lower rectum (12 patients). In 21 patients, neoadjuvant treatment had not been administered to treat the primary rectal tumor. After radical surgical treatment, 10 patients did not require cytostatic treatment, while 17 patients completed treatment with chemotherapy and 7 with chemoradiotherapy. This information was not available for one patient who had been treated for the primary tumor at another hospital.
The most frequently performed surgery was anterior resection of the rectum (24 patients). Five patients had been treated with a Hartmann procedure, 4 patients with local transanal resection, one patient with abdominoperineal amputation and another with combined Turnbull–Cutait procedure in association with resection of a single liver metastasis.
The majority of the patients presented pT3–4 pN0–1 tumors, with no distant metastasis (27 patients). In 23 patients, resection of the primary tumor was considered R0, in 5 patients R1, and no data were obtained for this parameter in 5 patients who had been referred from other hospitals.
The pelvic recurrences were diagnosed during follow-up after a mean of 3.2 years (SD 3.03) after the initial rectal cancer surgery. The diagnosis was made in 8 patients by detecting elevated CEA levels, in 10 patients with imaging tests and in 9 patients by means of colonoscopy. These 27 patients presented no symptoms at the time of diagnosis. In 8 patients, the diagnosis of recurrence was symptom-based: 2 bowel obstructions, and 6 rectal bleeding.
The location of the local recurrence of rectal cancer was of the mixed type in 13 patients, central relapse in 11 patients, lateral recurrence in 7, and posterior in 3. We do not have this information available for one patient who had been treated surgically for re-recurrence, whose first relapse had been treated at another hospital.
At the time of diagnosis of the recurrence, 7 patients presented treatable extrapelvic distant disease.
As for treatment, in the presence of local recurrence in which resection might have inadequate margins (3 patients), the surgical intervention was completed with the intraoperative placement of specific plastic guides for postoperative brachytherapy needles.
In 18 patients, the rectal remnant was resected; in 2, this was associated with excision of the inferior sacral vertebrae, and sphincter-preserving surgery was performed in 17. The urinary tract was reconstructed in 7 patients: 2 partial cystectomies, one Bricker and 4 double-barreled wet colostomies.15
The most frequent postoperative complications were pelvic collection (11 patients) and postoperative paralytic ileus (10 patients). Seven patients required reoperation: 2 for intraperitoneal hemorrhage, 4 for anastomotic dehiscence and one patient for pelvic collection. Five patients were readmitted with the diagnosis of pelvic collection, one of whom required surgery. The readmission rate was 31.4% (11 patients).
After radical surgery, mean hospital stay was 23.9 days (range 5–70 days, SD 15.8). Postoperative complications were observed in 26 patients (75%). According to the Clavien–Dindo classification, 4 patients presented grade I complications, 14 grade II, 4 grade IIIa, 4 grade IIIb and one patient grade V (death).
In terms of residual tumor, 3 patients (8.5%) were R2, 14 (40%) R1, and 14 patients R0 (40%). In 4 patients, the margins were not evaluable due to fragmentation of the surgical specimen.
Mean follow-up was 4.2 years (range 1–8.7 years). Eleven patients did not present any disease recurrence. Out of the 23 patients who were diagnosed with another tumor relapse, 7 patients already had distant disease at the time of surgery of the first pelvic recurrence. As for the type of relapse, 8 patients presented pelvic local re-recurrence alone (5 were operable), 8 patients were diagnosed with distant metastasis and 7 presented new local and distant recurrences.
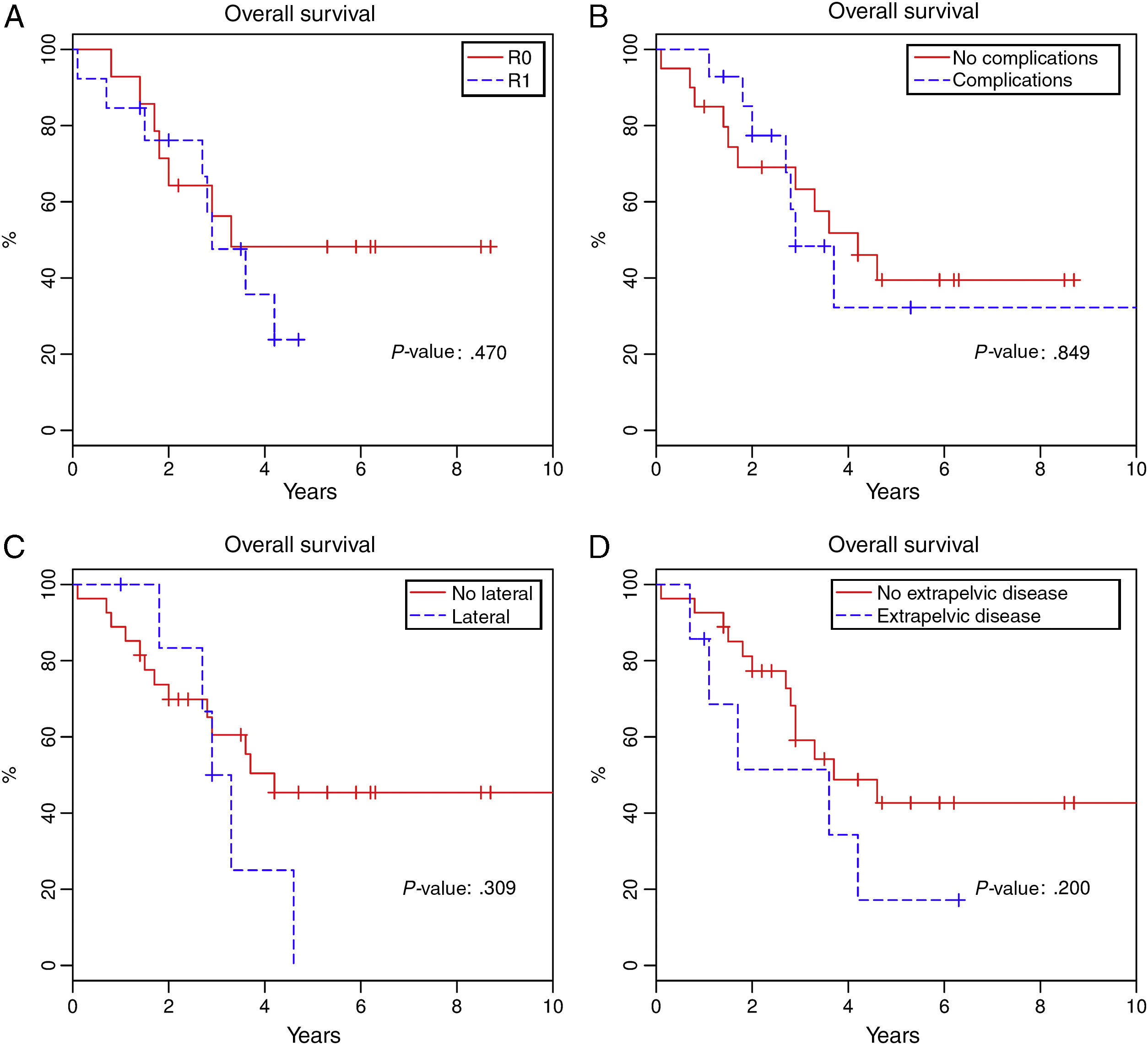
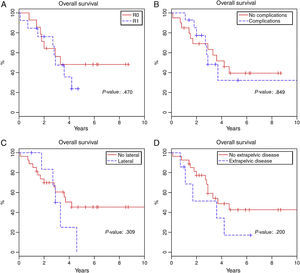
Total 1-year survival was 91.2%, 2-year survival was 75.6%, and 5-year survival was 37% (Fig. 1). Mean survival was 3.6 years (95% CI 2.9–4.6). Fig. 2 shows the survival curves related with several parameters. In the period studied, no differences were found in survival when R0 and R1 resections were compared. Survival rates were similar among the patients who presented some type of postoperative complication. There were no differences with regards to the location of the recurrence in the pelvis. We found no differences in the prognosis between patients with resectable distant or local recurrence versus those who only presented local recurrence of rectal cancer.
Out of the patients of the present series, 15 are alive, and 9 continue to be disease-free. Twenty patients died during follow-up: one patient during the early postoperative period, 2 from non-tumor causes and 17 due to progression of the disease.
We studied patients with rectal cancer re-recurrence treated surgically at our institution. The second pelvic recurrence was diagnosed at a mean of 2.2 years (SD 0.96) during the postoperative follow-up of the first intervention due to relapse.
Five patients underwent surgery for re-recurrence. The locations were: one central, 2 mixed and 2 posterior. R0 was only achieved in the central re-relapse, and no patient presented extrapelvic disease. One patient required resection of the sacral vertebrae. Mean hospital stay was 30.4 days (SD 17.9); 2 patients presented postoperative paralytic ileus and pelvic collection.
During follow-up, the patients with macroscopic residual tumor presented local progression and died from this cause. Out of the 2 patients with R1, one had no follow-up beyond 6 months at our hospital; the other presented progression of the distant disease and died 6 years after surgery. The patient with R0 continues to be disease-free 4 years after the third radical surgery.
DiscussionIn the present series of surgical patients treated for rectal cancer recurrence, no significant differences have been observed in survival related with resection type or different locations of the recurrence.
Several studies have demonstrated that radical surgery can reach or surpass survival rates between 35% and 50%, with an acceptable morbidity rate in the preoperative period.16–19 Nonetheless, it should be mentioned that the published series include heterogeneous patients, as not all have been treated with TME in the primary tumor surgery. In these patients, the recurrence usually originates from the mesorectal remnant, unlike recurrences after TME, which usually infiltrate the pelvic wall or adjacent organs. This latter aspect implies the need for highly complex surgical techniques.1,20
Reaching R0 in local recurrence of rectal cancer surgery in the post-TME era is a constant challenge for surgeons, especially in lateral recurrences with infiltration of the pelvic wall. In our series, we achieved 40% R0 and 40% R1, results that are similar to those published to date in the literature.14,16,17,20,21 Furthermore, no differences are observed for survival when patients with R0 and R1 are compared, although the tendency of the survival curve indicates that patients with R0 present better prognoses. These results differ from those reported in the international literature.6,8,20,21 We believe that the reason for finding no differences is due to the limited number of patients studied.
Recently, Rahbari et al.2 have conducted a study with 92 patients who had undergone surgery for local recurrence after TME. The authors maintain that, due to its highly complex nature, radical surgery should be performed in specialized hospitals and by multidisciplinary teams. In their experience, they observed a perioperative morbidity rate of 42.4% and a mortality rate of 3.3%. Similar results were obtained by Melton et al.22 in their series of 29 patients in whom they performed pelvic exenteration with partial sacrectomy. In our series, morbidity was 77%, with 51.4% of grades I–II Clavien–Dindo complications and 25.7% grades above III. The mortality rate was 2.8%.
The most frequent postoperative complications observed in the published series are the presence of intra-abdominal or pelvic postoperative abscesses and surgical wound infections.8,14,16 We have observed similar data in this present series.
One aspect that could have an impact both on the difficulty of the surgical resection as well as the risk for re-recurrence is the lateral location of the pelvic relapse. In these cases, the indication for postoperative brachytherapy could be considered. There are retrospective studies that have observed that, in patients with R1 risk, adding brachytherapy improves prognosis, with a 5-year survival that reaches 70%–94%.23,24
The existence of extra pelvic disease is another point of controversy. Published studies report lower survival in these patients.5,18,25 The difficulty to reach a therapeutic decision is linked with correct patient selection, as surgery for local recurrence of rectal cancer can also improve survival in patients with treatable distant disease. In our experience, we found no difference in patients selected with curable extrapelvic disease who were therefore treated surgically for the pelvic relapse.
In the present series, absolute contraindications were defined as lesions that infiltrated vascular structures or sacral nerve roots in the S2 region or higher. These contraindications have traditionally been considered absolute26,27; however, there continues to be much debate on this subject, and some authors currently classify them as relative contraindications.28
The number of patients diagnosed with local recurrence with no final evidence of malignancy after the pathology analysis of the specimen was actually quite important. Not much emphasis is given to these patients in the medical literature. In the present study, the 7 patients in whom no evidence for adenocarcinoma was found were excluded from the analysis.
The diagnosis of local recurrence of rectal cancer is difficult, and thorough preoperative studies are important. With the evolution of diagnostic methods and the experience acquired by surgeons and all the specialists involved, we believe that the percentage of false positives can be significantly reduced. In order to achieve this improvement, it is important to concentrate patients with suspected local recurrence at hospitals equipped with multidisciplinary teams that are experts in this disease.29–31
In conclusion, local recurrence of rectal cancer is a disease with a high cure rate. The only curative option is radical surgery, which involves an acceptable mortality rate and an important morbidity rate. All patients with suspected local recurrence of rectal cancer should be studied, treated and followed up at specialized hospitals by multidisciplinary teams.
Authorship/CollaboratorsBiondo: study design, analysis and interpretation of the results, article composition.
González-Castillo: data collection, analysis and interpretation of the results, article composition.
Kreisler: interpretation of the results, article composition, critical review.
García-Granero: data collection, critical review.
Cambray: critical review, approval of the final version.
Martínez-Villacampa: critical review, approval of the final version.
Conflicts of InterestThe authors have no conflicts of interest to declare.
The authors would like to thank Bernat Miguel, data manager of the Colorectal Surgery Unit of the General and Gastrointestinal Surgery Department at the Hospital Universitario de Bellvitge, for his data management and statistical study.
Please cite this article as: González-Castillo A, Biondo S, García-Granero Á, Cambray M, Martínez-Villacampa M, Kreisler E. Resultados de la cirugía de la recidiva pélvica de cáncer de recto. Experiencia en un centro de referencia. Cir Esp. 2016;94:518–524.