Surgical treatment of gallbladder cancer is still controversial. The extent of the radical surgery and its therapeutic efficacy continue to be debated.
ObjectiveAnalyse the efficacy of radical resection in patients with incidental gallbladder cancer evaluating the presence of residual disease in the resection specimen and analysing the associated factors of survival.
MethodsA retrospective analysis of patients with incidental GC between June 1999 and June 2010 was performed. Incidental (I) tumours were included. Data covering demographic features, clinical characteristics, local pathological stage, histological features and factors for long term survival were analysed. P<.05 were considered significant.
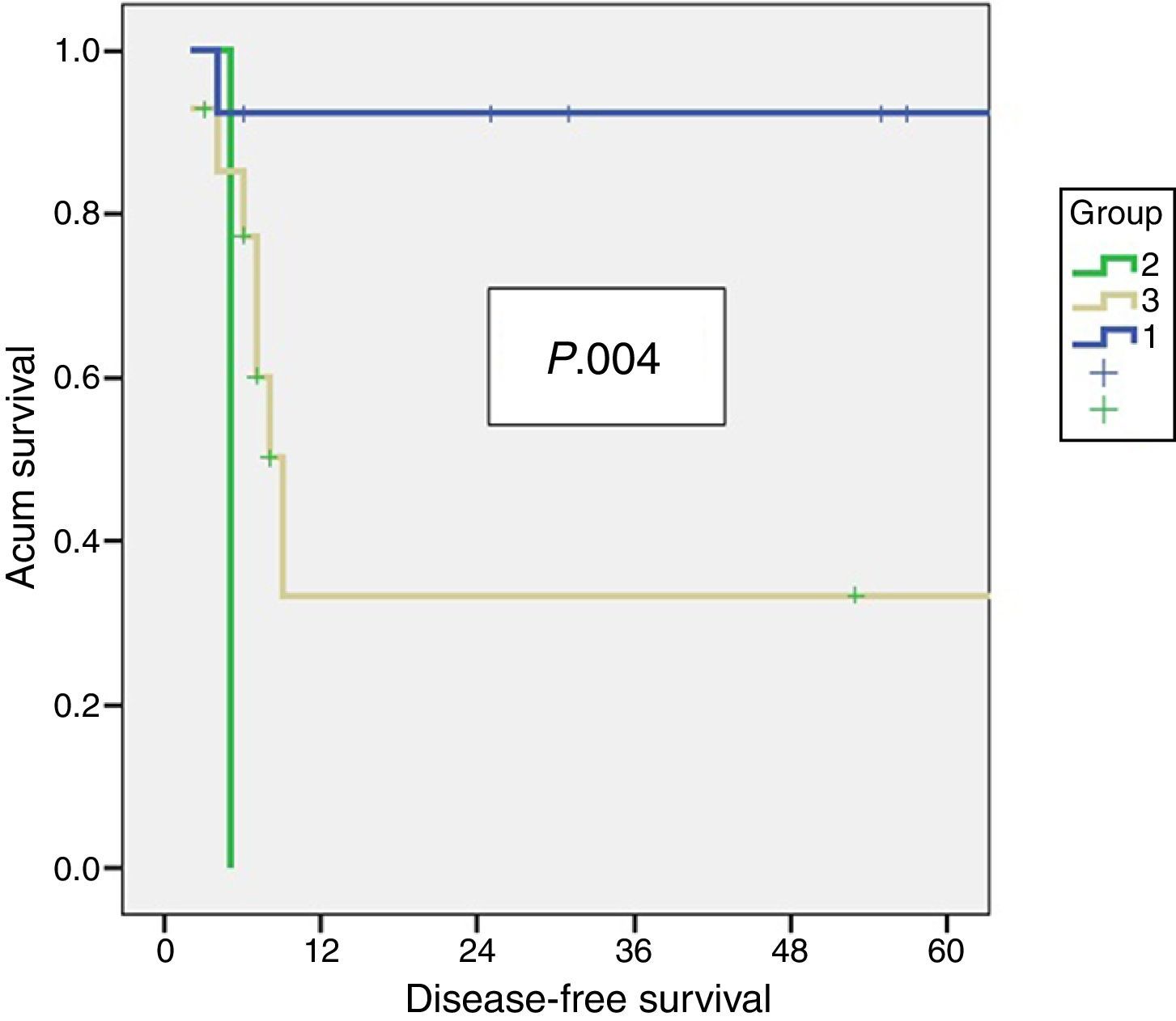
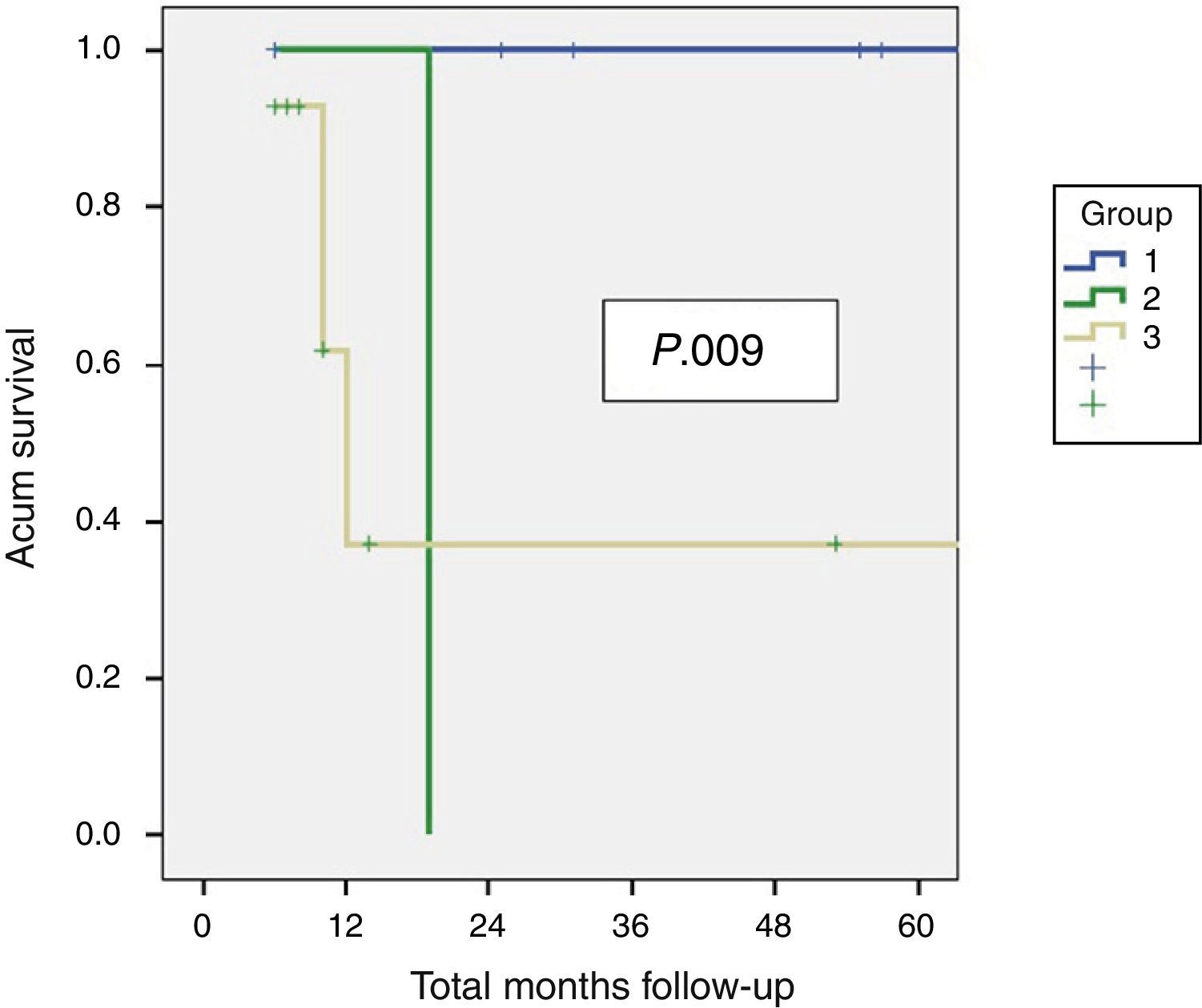
ResultsA total of 28 patients: 78.6% females. Median age 56 years. All treated by resection of segments 4b/5 and lymphadenectomy. Histological examination revealed residual disease in 42% (37% liver), residual disease was related to tumoural (T) stage (P .001). Patients with residual disease presented a DSS and DFS of 10 and 6.5 months respectively vs 56 months in those without residual disease (P .001). Variables associated with survival were T stage (P .006), TNM stage (P<.001), and residual disease in the resected specimen (P<.001).
ConclusionsAggressive re-resection of incidental GC offers the only chance for cure but its efficacy depends on the extent of the disease found at the time of repeated surgery and in the deferred pathological study.
El tratamiento quirúrgico del cáncer de vesícula continúa siendo controvertido. La extensión de la cirugía radical y su eficacia terapéutica todavía están en debate.
ObjetivoValorar la efectividad de la resección radical en el cáncer de vesícula incidental, evaluando la presencia de enfermedad residual en la pieza de resección, los factores asociados y su impacto en la supervivencia alejada.
MétodoSe analizó en forma retrospectiva a 43 pacientes adultos con diagnóstico de cáncer incidental de vesícula entre junio de 1999 y junio de 2011. Se incluyeron tumores incidentales con resección R0. Las variables analizadas fueron datos demográficos, características clínicas, estudio histopatológico de la colecistectomía previa, indicación de resección, tipo de resección, morbilidad, mortalidad, estudio histopatológico, presencia de enfermedad residual y supervivencia. Se consideró significativa una p< 0,05.
ResultadosSe identificó a 43 pacientes con cáncer incidental de vesícula: 28 pacientes fueron tratados mediante resección, el 78,6% era de sexo femenino, la edad mediana fue de 56 años (rango: 38–78). En todos los pacientes se realizaron resecciones 4b/5, más linfadenectomía, en 6 de 8 casos se realizó la resección de los puertos de laparoscopia. No hubo mortalidad perioperatoria, 25% de morbilidad (71% tipo I). Se identificó enfermedad residual en el estudio histopatológico en el 42% (37% en el hígado), la cual se relacionó con el estadio tumoral (T) (p 0,001) Los pacientes con enfermedad residual presentaron una SG y SLE de 6,5 y 10 meses respectivamente vs 56 meses en aquellos sin enfermedad residual (p 0,001). Los factores independientes relacionados con la supervivencia fueron la presencia de enfermedad residual, el estadio T y el TNM.
ConclusiónLa cirugía radical del cáncer incidental de vesícula es el único procedimiento con intención curativa pero su eficacia depende de la extensión de la enfermedad tumoral hallada durante la resección y en el estudio patológico diferido.
Cancer of the gallbladder (GBC) is the most frequent cancer of the biliary tract worldwide and the sixth most frequent cancer of the gastrointestinal tract, presenting a variable incidence in different regions, from 2.5 for every 100000 inhabitants in USA, 6.7 in Salta, 17.8 in Chile, and up to 21.5 in some regions in India.1 However, radical surgery is performed in less than 20%2 of cases. Due to low frequency and the extent of the disease on diagnosis, experience in its surgical treatment has determined that prognostic variable analysis on long-term survival is limited.3–5 The best survival rates are for patients with incidental tumours who undergo radical surgery.6 Despite the recommendations regarding hepatic resection and lymphadenectomy in patients with localised disease, this practice is not routinely used. A recent study showed that in patients with resectable cancer of the gallbladder, only 9% were treated with a hepatic resection and 5% with appropriate lymphadenectomy.7 Controversy still remains regarding the extent of hepatic resection, lymphadenectomy, use of laparoscopy and resection associated with the biliary tract.4,8,9 From the prognostic viewpoint, the T and N stages have been predictive variables of long-term survival after resection in different studies.10–12 Although the rationale behind performing further surgery for incidental gallbladder cancer is based on eradicating possible residual disease, controversy still persists regarding the selection of patients who would genuinely benefit from another surgery.6 The discovery of residual liver disease has been described from early stages (T1b-T2).13,14 Lymphadenectomy (N1) is also considered a relevant factor as treatment for residual lymph node cancer and the number of lymph nodes removed has shown correlation with survival in patients who have undergone radical surgery.4
The aim of this study was to evaluate the effectiveness of radical resection in incidental gallbladder cancer, assessing the presence of residual disease in the resection specimen, associated factors and their impact on long-term survival.
Material and MethodsBetween June 1999 and June 2011, 62 gallbladder cancer patients were treated in the Liver Transplant Unit of the Dr. Cosme Argerich Hospital. Forty-three of them (67.7%) had incidental tumours, which were defined as those diagnosed in histopathological analysis of the cholecystectomy piece. All patients with resected incidental gallbladder cancer with R0 margin were included. Diagnostic methodology has already been previously described by the authors in a study which previously presented.15 In all patients, surgical treatment involved: anatomic liver resection of segments 4b/5, lymphadenectomy (N1) (cystic lymph nodes, pericholedochal, liver artery and periportal artery) and resection of the laparoscopic ports in 6 of 8 patients who had undergone prior laparoscopic cholecystectomy. Reoperations were performed for definitive oncological resection in all patients with incidental cancer of the gallbladder which had spread beyond the submucosa (T1b, T2 and T3). Complications were grouped according to the classification described by Dindo et al. The 7th edition of the AJCC classification16 was used to stage the excised specimen.
Curative (R0) resection was defined as a resection without clinical evidence of tumour lesion and negative pathological margins. Histological spread of the resection margin was defined as R1 resection. The presence of microscopic spread in the deferred histopathological study of the material obtained from radical resection (hepatic parenchyma, laparoscopic lymph node incisions) was denominated residual disease. Selective adjuvant chemotherapy was used on those patients at greater risk of recurrence (residual disease, perineural spread, vascular spread). If recurrence presented, oncological treatment was indicated. Follow-up was based on clinical examination, a hepatogram, CA 19-9, scan and CT every 6 months the first 2 years and annually thereafter.
The variables analysed were: demographic data, diagnosis prior to surgery, histopathological study of previous cholecystectomy, indication for resection, type of resection, operating data, morbidity, mortality, hospital stay, histopathological study and evolution. An analysis on survival was performed, comparing different variables with prognostic value.
Overall survival (OS) and disease-free survival (DFS) were defined as death and the loss of follow-up and time between resection and first presentation of recurrence, respectively. Categorical variables expressed in percentages were compared using the Chi-square test and numerical variables of mean and standard deviation using the Student t-test. Survival analysis was performed using the Kaplan–Meier method. The log-rank test was used for univariate analysis of survival curves. Multivariate analysis was performed using the Cox proportional risks regression to identify separate risk factors associated with survival which had been statistically significant in the univariate analysis. Differences were considered significant with P<.05. The SPSS v15 Programme was used for statistical analysis.
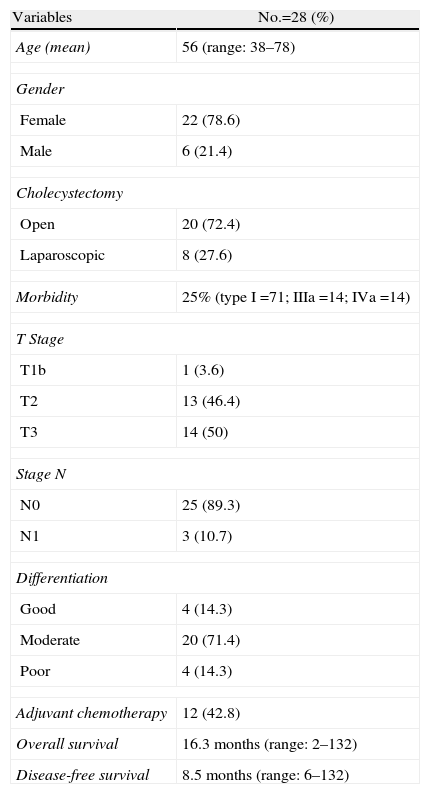
ResultsDemographic and Histopathological CharacteristicsDemographic and histopathological characteristics are summarised in Table 1. Mean age was 56: 22 patients (78.6%) were female. Of the 43 patients who presented with incidental cancer, 28 of them (65.1%) underwent surgery with curative intention. Twenty-six patients (92.8%) were from another institution, after histopathological diagnosis from the cholecystectomy specimen, with open surgery cholecystectomy being performed on 20 patients (72.4%) and 8 via laparoscopy (28.6%). Only 2 patients underwent cholecystectomy at our hospital and were subsequently referred to our unit. Gallbladder cancer diagnosis was carried out during cholecystectomy (2) or later in deferred histopathological analysis (26). An anatomopathological analysis was performed on the cholecystectomy specimen to confirm the level of tumour invasion (T). On admittance to our hospital (mean 57 days: range: 0–128), 15 patients were not candidates for curative resection, 13 of them due to disease progression (6 hepatic metastasis, 4 biliary infiltrations, 3 peritoneal carcinomatosis) and 2 refused surgery. Only one patient was rejected during surgery when the discovery of peritoneal invasion was made. All patients who were not treated with radical surgery died between 3 and 6 months from disease diagnosis. Anatomic hepatic resection of segments 4b/5 was carried out according to the described technique, subsequent to lymphadenectomy of lymph nodes N1 of the hepatic pedicule.3,23 Of the 8 patients with a history of previous laparoscopic cholecystectomy, resection of the laparoscopy ports was performed in only 6. There was no surgical mortality and morbidity was 25% (4 surgical wound infections, 2 biliary fistulas, one spontaneous pneumothorax), the most frequent being Dindo type I. Mean hospital stay was 5.5 days (range: 4–14).
Demographic and Histopathological Characteristics.
| Variables | No.=28 (%) |
| Age (mean) | 56 (range: 38–78) |
| Gender | |
| Female | 22 (78.6) |
| Male | 6 (21.4) |
| Cholecystectomy | |
| Open | 20 (72.4) |
| Laparoscopic | 8 (27.6) |
| Morbidity | 25% (type I=71; IIIa=14; IVa=14) |
| T Stage | |
| T1b | 1 (3.6) |
| T2 | 13 (46.4) |
| T3 | 14 (50) |
| Stage N | |
| N0 | 25 (89.3) |
| N1 | 3 (10.7) |
| Differentiation | |
| Good | 4 (14.3) |
| Moderate | 20 (71.4) |
| Poor | 4 (14.3) |
| Adjuvant chemotherapy | 12 (42.8) |
| Overall survival | 16.3 months (range: 2–132) |
| Disease-free survival | 8.5 months (range: 6–132) |
Mean OS and DFS values are expressed.
The histopathological analysis showed adenocarcinoma in 12 patients, 71.4% moderately differentiated. All resections were R0. The median of lymph node resections was 3 (range: 1–10). The histopathological analysis of the specimen showed parenchyma infiltration in 10 (35.7%) patients with liver resection (all T3), lymph invasion in 3 (10.7%), vascular invasion in 2 (7.1%) and perineural in 2 (7.1%). Those patients who presented residual disease received chemotherapy treatment with Gemcitabine (9) or Gemcitabine and Capecitabine (3); none of them had improved survival.
The median follow-up was 16.5 months (6–132 months) for the entire series and 53 months for the patients alive at the study date. During follow-up, 9 (32.1%) patients presented recurrence. The median OS and DFS were 16.5 and 8.5 months respectively.
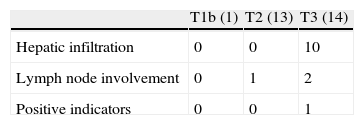
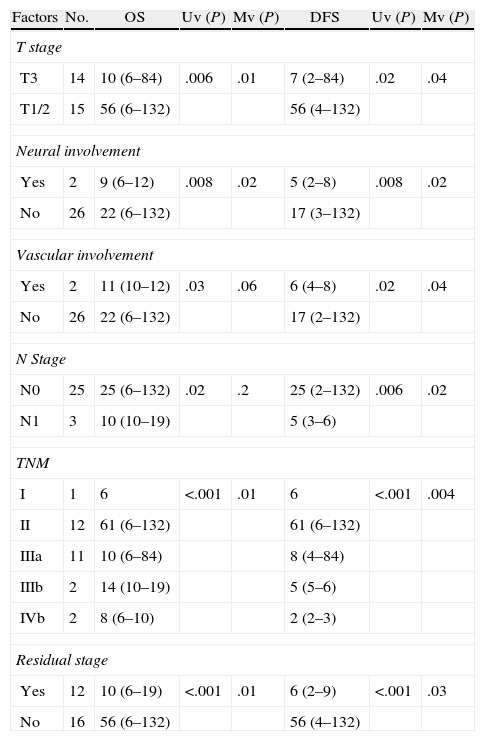
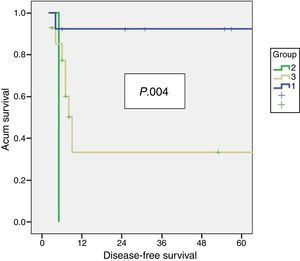
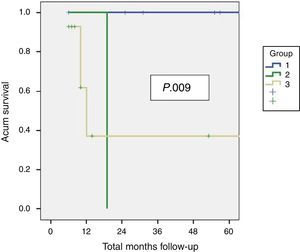
Residual Disease and SurvivalResidual disease was found in 12 resected patients (42.9%): hepatic parenchyma infiltration in 10, lymph node infiltration in 3 and infiltration of laparoscopic ports in one. The presence of residual disease was correlated with the tumoural stage (T): T1b=0%; T2=8.3%; T3=91.6% (P .001, Table 2). Significant differences were found with a lower OS and DFS in line with the presence of residual disease in patients treated using resective surgery. Those who presented with residual disease in the histopathological study showed an OS and DFS of 10 and 6.5 months respectively, whilst in patients with residual disease, both were 56 months (P .001). Differences in survival were also defined when the T2 patients with and without residual disease were analysed. It is of note that patients with stage T3 cancer presented better OS and DFS than patients with stage T2 and residual disease (Figs. 1 and 2). Univariate and multivariate analysis of factors relating to DFS and OS are shown in Table 3.
Uni and Multi Varied Analysis of Factors Relating to SG and SLE.
| Factors | No. | OS | Uv (P) | Mv (P) | DFS | Uv (P) | Mv (P) |
| T stage | |||||||
| T3 | 14 | 10 (6–84) | .006 | .01 | 7 (2–84) | .02 | .04 |
| T1/2 | 15 | 56 (6–132) | 56 (4–132) | ||||
| Neural involvement | |||||||
| Yes | 2 | 9 (6–12) | .008 | .02 | 5 (2–8) | .008 | .02 |
| No | 26 | 22 (6–132) | 17 (3–132) | ||||
| Vascular involvement | |||||||
| Yes | 2 | 11 (10–12) | .03 | .06 | 6 (4–8) | .02 | .04 |
| No | 26 | 22 (6–132) | 17 (2–132) | ||||
| N Stage | |||||||
| N0 | 25 | 25 (6–132) | .02 | .2 | 25 (2–132) | .006 | .02 |
| N1 | 3 | 10 (10–19) | 5 (3–6) | ||||
| TNM | |||||||
| I | 1 | 6 | <.001 | .01 | 6 | <.001 | .004 |
| II | 12 | 61 (6–132) | 61 (6–132) | ||||
| IIIa | 11 | 10 (6–84) | 8 (4–84) | ||||
| IIIb | 2 | 14 (10–19) | 5 (5–6) | ||||
| IVb | 2 | 8 (6–10) | 2 (2–3) | ||||
| Residual stage | |||||||
| Yes | 12 | 10 (6–19) | <.001 | .01 | 6 (2–9) | <.001 | .03 |
| No | 16 | 56 (6–132) | 56 (4–132) | ||||
Mv.: multivariate; N: lymph node involvement; OS overall survival expressed in median months (rage); DFS: disease-free survival, expressed in median months (range); TNM: TNM stage; Uv: univariate.
Although cholecystectomy is an appropriate treatment for T1a patients, radical resection has shown benefits in the survival of patients in more advanced tumoural stages.17 However, treatment of gall bladder cancer is still controversial. When it is feasible, further surgery with radical resection offers the only chance for cure, but the appropriate selection of patients according to the extent of tumour spread and the most effective type of resection is still the subject of debate. In a recent study in the United States with more than 4631 patients, only 2% were treated with the recommended surgery.18 We should highlight that a significant percentage of patients with incidental cancer were not candidates for resective surgery and this has been demonstrated in this study, with 35% presenting widespread disease on referral. Although the most standard treatment is radical resection consisting of a hepatic resection with lymphadenectomy, the debate continues as to the appropriate extent of parenchyma resection.19 In our hospital we systematically perform resection of segments 4b/5 in incidental gallbladder cancer unless tumour spread determines a larger resection. A recent German multicentre study showed that in T2 tumours, resection of segments 4b/5 would be recommended due to its association with improved long-term survival.17 This procedure has low morbidity and would have the added advantage of treating micro metastasis located in the cystic vein area.20–23 Lymphadenectomy itself provides major information for staging gallbladder cancer and may reduce the incidence of local recurrence in incidental tumours where hidden lymph node metastases are found in 10% and 45%25 of cases. In general, lymphadenectomy is confined to N1 lymph nodes of the hepatoduodenal ligament (cystic, pericholedocian, periarterial and periportal). More extensive lymphadenectomies are not routinely performed24,25 and are only supported by some oriental authors.13,24 Resection of laparoscopic ports is significant for disease staging, but there are no advantages for patients with confirmed implants which usually progress to recurrence in the peritoneum.11,19,26 In this study, only one patient presented infiltration in the laparoscopic ports. In accordance with a recent study, we consider that this practice must be used selectively since it only offers advantages in terms of disease staging.27 Although several authors have maintained that routine resection of the gallbladder could facilitate lymphadenectomy28 no benefits have been defined in the number of resected lymph nodes or long-term survival and greater morbidity has been associated with it.28 Cystic duct invasion has been correlated with residual gallbladder disease and a positive biopsy of the cystic margin during re-examination is an indication for gallbladder resection.25,28
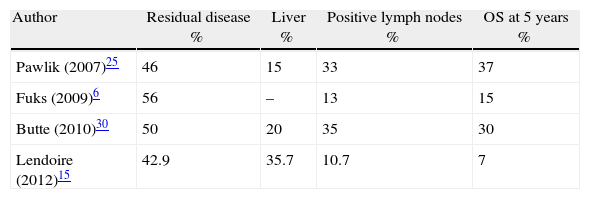
Compared with previous studies, the histopathological analysis of this series defined similar tumoural differentiation but a lower percentage of lymph node and perineural invasion.11,22 Although other authors have shown 0%–20% parenchyma infiltration on analysis of the hepatic resection specimen in incidental gallbladder cancer,21,29 in our analysis, it was found in 35% of hepatectomies. In a recent multicentre study of 115 patients treated with radical resection for incidental gall bladder cancer, hepatic infiltration presented in 15%, although we would note that the analysis presented a higher percentage of T2 tumours (69%).25 In a recent publication the importance of incidental gallbladder staging was analysed, showing similar figures to this analysis with 38% of hepatic invasion in the pathological study of resection specimens.4 The tumoural (T) level involvement was correlated with the presence of residual disease in the liver and lymph nodes.4 In Table 4 we can observe that comparison with 3 previous studies which evaluated the presence of residual disease post-resection demonstrated that the lower survival rate obtained in our series would be explained by the predominance of residual disease in the liver compared to the lymph nodes. Survival is clearly affected by the presence of residual disease in resected specimens whether or not the surgical margins are negative as in 100% of the bisegmentectomies of this analysis.6,26,30 Lymph node invasion appears to have a better prognosis than parenchyma invasion, although there would be a clear relationship with the radicality of N1 lymphadenectomy.4,25 Recently a relationship has been suggested between the number of retrieved lymph nodes and disease-free survival.4 With a median of 3 resected lymph nodes, comparable with other series, in this study only 3 patients (10.7%) presented lymph node invasion.25,31 Both adjuvant and neo-adjuvant chemotherapy should be complementary to the treatment of patients with residual disease after radical surgery but no benefits have yet been found.32 Prognostic factors of overall and disease-free survival in this analysis are similar to those defined in previous studies.4,12,15,19,33 In patients who undergo curative radical resection, prognostic factors principally relate to tumour spread. So that 3 interrelated factors such as T stage, TNM and the presence of residual disease in the resection specimen are the most relevant factors in different series.4,12,15,19,33
To conclude, tumoural stage (T) present in the cholecystectomy specimen and post resection TNM are factors that determine survival and recurrence in this study. Radical surgery of incidental gallbladder cancer is the only procedure for cure but its efficacy depends on the extent of the disease found during resection and in the deferred pathological study. Residual disease was predominantly in the liver (36%), it was correlated with T stage and was one of the most relevant prognostic factors in this study. Complementary treatments with adjuvant chemotherapy would be essential to improve radical surgery results in patients with incidental gallbladder cancer.
Conflict of InterestsThe authors have no conflict of interest to declare.
Please cite this article as: Gil L, Lendoire J, Duek F, Quarin C, Garay V, Raffin G, et al. Cirugía radical en el cáncer de vesícula incidental: valor del hallazgo de enfermedad residual en el estudio histopatológico diferido. Cir Esp. 2014;92:168–174.