Colorectal cancer (CRC) is the second most common neoplasm worldwide. 15%–20% of cases present synchronous liver metastases (SLM).1 Resection of CRC and SLM offers a real possibility for a cure but is only feasible in a minority of patients.2 In the past, 2 strategies were used for with SLM of CRC: 1) the traditional approach of CRC surgery followed by SLM surgery; and 2) simultaneous CRC and SLM surgery.2
In 2006, Mentha et al. proposed a new therapeutic algorithm for patients with asymptomatic CRC and SLM that were initially either unresectable or difficult to resect.3 This strategy, called primary liver surgery, reverse strategy, or liver-first Approach (LFA), consists of initial administration of chemotherapy, followed by resection of SLM, chemo/radiotherapy, and then removal of the primary tumor.3–5 The complete treatment includes 2 major surgeries plus chemotherapy/radiotherapy and has high morbidity, with major Complications (MC) rates of 15%–20%.3–6
The literature comparing the 3 mentioned techniques has found no differences in MC, mortality, or 5-year survival rates.5 However, LFA seems to be a better option for patients with a high burden of liver disease, providing excellent short-term results and longer survival.4,7–9
The most common method to determine quality of care is by using the Clavien-Dindo classification and/or Comprehensive Complication Index (CCI®) to measure MC.10 Few studies have focused on postoperative complications after LFA and they are usually retrospective. We present the complication rate and associated factors observed in patients from the Spanish National Registry of Reverse Surgery (RENACI, Registro Nacional de Cirugía Inversa).
This is a prospective, descriptive, observational study of consecutive patients with CRC and SLM recruited at participating hospitals. The study period was from January 6, 2019 to August 8, 2020. The protocol was approved by the Ethics Committee and registered in Clinical Trials (NCT04683783). All patients gave written informed consent. The ethical principles of the Declaration of Helsinki and Good Clinical Practice were followed. The inclusion criteria were patients ≥ 18 years of age with ASA I-III who had undergone LFA.
The treatment strategy was: neoadjuvant chemotherapy followed by CT evaluation; when there was partial response or stabilization of the liver disease and R0 resection was feasible with sufficient liver volume, LFA was performed; this was followed by radiotherapy or chemotherapy/radiotherapy on the rectal tumors (depending on the stage), and lastly surgery on the primary tumor. In the colon, surgery after LFA was adapted to the location.
We determined preoperative, intraoperative, and postoperative variables as well as the characteristics of CRC and SLM. Complications were measured after 90 days in accordance with the Clavien-Dindo classification,10 and MC were defined as grade ≥ IIIa. Failure to rescue was defined as deceased patients who presented MC divided by the total number of patients with MC.
Quantitative data were expressed as median and interquartile range (IQR), and qualitative data as frequencies or percentages. To study the inter-group differences, the non-parametric Kruskall-Wallis test was used in the case of quantitative variables, and the Pearson chi-squared test was applied for qualitative variables. When the value in any of the categories was less than 5, the Fisher test was used.
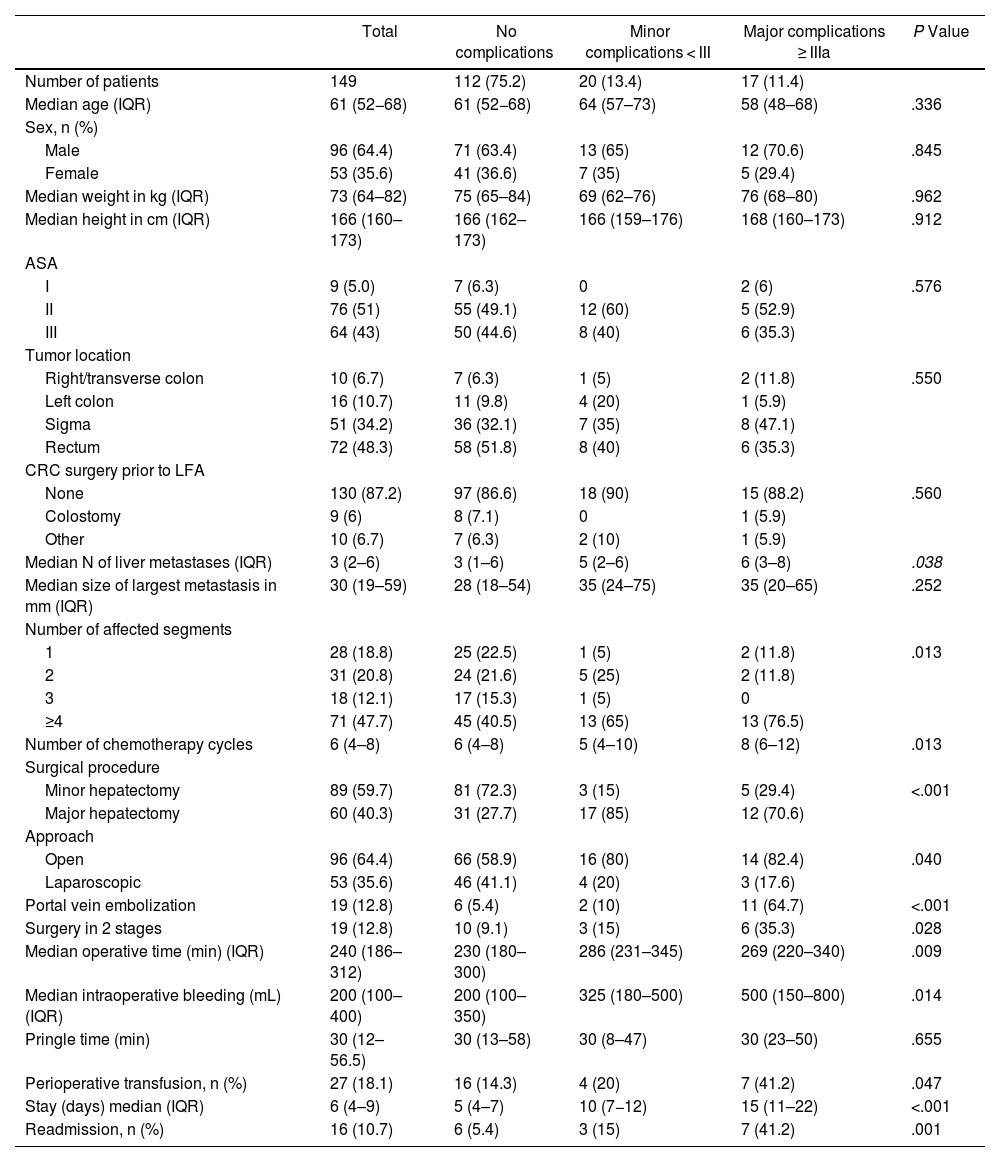
In total, 40 hospitals participated in the study, and 149 patients underwent surgery (Table 1). Median age was 61 years (IQR: 52−68); 64.4% were men; 94% were ASA II-III. Regarding location, 48.3% of the tumors were located in the rectum. Median number of SLM was 3 (IQR: 2−6); 47.7% of patients had more than 4 lesions. The size of the largest lesion was 30 mm (IQR: 19–59). In terms of surgical type, 60% of the surgeries performed were minor hepatectomies; 36% of the total LFA were performed using a minimally invasive approach, and 12.8% were two-stage procedures. Transfusion was necessary in 18.1% of patients. No complications were observed in 75.2% of patients, although 16.1% had minor complications and 8.7% MC. Median ICC was 22.6 (20.9–28.0). Specifically, liver complications were: hemorrhage in 17 patients (11.3%) (ISGLS: 15 A and 2 B), liver failure in 21 patients (14%) (ISGLS: 15 A, 5 B and 1 C), and biliary fistula in 13 (8.6%) (ISGLS: 5 A, 7 B and 1 C). Four patients (2.7%) underwent reoperation. The failure-to-rescue rate was 5.9%, mortality was 0.7%, and hospital stay was 6 days (IQR: 4–9). Colorectal surgery was performed after LFA in 89.3% of patients 2.14 months later [IQR: 1.35–3.02]. It was not possible to perform colorectal surgery in 16 (10.7%) patients for the following reasons: 9 due to complications after liver surgery, 6 due to progression of liver disease, and one due to postoperative death. Right/transverse colon tumors were operated on after a median of 85 days (IQR: 53–92), left colon after 65 days (40–76), sigmoid colon after 64 days (37–95), and rectum after 71 days (43–92). There were no significant differences among groups (P = .681, Kruskal–Wallis non-parametric test).
Comparison between patients with minor and major complications.
| Total | No complications | Minor complications < III | Major complications ≥ IIIa | P Value | |
|---|---|---|---|---|---|
| Number of patients | 149 | 112 (75.2) | 20 (13.4) | 17 (11.4) | |
| Median age (IQR) | 61 (52−68) | 61 (52−68) | 64 (57–73) | 58 (48–68) | .336 |
| Sex, n (%) | |||||
| Male | 96 (64.4) | 71 (63.4) | 13 (65) | 12 (70.6) | .845 |
| Female | 53 (35.6) | 41 (36.6) | 7 (35) | 5 (29.4) | |
| Median weight in kg (IQR) | 73 (64–82) | 75 (65–84) | 69 (62–76) | 76 (68–80) | .962 |
| Median height in cm (IQR) | 166 (160–173) | 166 (162–173) | 166 (159–176) | 168 (160–173) | .912 |
| ASA | |||||
| I | 9 (5.0) | 7 (6.3) | 0 | 2 (6) | .576 |
| II | 76 (51) | 55 (49.1) | 12 (60) | 5 (52.9) | |
| III | 64 (43) | 50 (44.6) | 8 (40) | 6 (35.3) | |
| Tumor location | |||||
| Right/transverse colon | 10 (6.7) | 7 (6.3) | 1 (5) | 2 (11.8) | .550 |
| Left colon | 16 (10.7) | 11 (9.8) | 4 (20) | 1 (5.9) | |
| Sigma | 51 (34.2) | 36 (32.1) | 7 (35) | 8 (47.1) | |
| Rectum | 72 (48.3) | 58 (51.8) | 8 (40) | 6 (35.3) | |
| CRC surgery prior to LFA | |||||
| None | 130 (87.2) | 97 (86.6) | 18 (90) | 15 (88.2) | .560 |
| Colostomy | 9 (6) | 8 (7.1) | 0 | 1 (5.9) | |
| Other | 10 (6.7) | 7 (6.3) | 2 (10) | 1 (5.9) | |
| Median N of liver metastases (IQR) | 3 (2–6) | 3 (1–6) | 5 (2–6) | 6 (3–8) | .038 |
| Median size of largest metastasis in mm (IQR) | 30 (19–59) | 28 (18–54) | 35 (24–75) | 35 (20–65) | .252 |
| Number of affected segments | |||||
| 1 | 28 (18.8) | 25 (22.5) | 1 (5) | 2 (11.8) | .013 |
| 2 | 31 (20.8) | 24 (21.6) | 5 (25) | 2 (11.8) | |
| 3 | 18 (12.1) | 17 (15.3) | 1 (5) | 0 | |
| ≥4 | 71 (47.7) | 45 (40.5) | 13 (65) | 13 (76.5) | |
| Number of chemotherapy cycles | 6 (4–8) | 6 (4–8) | 5 (4–10) | 8 (6–12) | .013 |
| Surgical procedure | |||||
| Minor hepatectomy | 89 (59.7) | 81 (72.3) | 3 (15) | 5 (29.4) | <.001 |
| Major hepatectomy | 60 (40.3) | 31 (27.7) | 17 (85) | 12 (70.6) | |
| Approach | |||||
| Open | 96 (64.4) | 66 (58.9) | 16 (80) | 14 (82.4) | .040 |
| Laparoscopic | 53 (35.6) | 46 (41.1) | 4 (20) | 3 (17.6) | |
| Portal vein embolization | 19 (12.8) | 6 (5.4) | 2 (10) | 11 (64.7) | <.001 |
| Surgery in 2 stages | 19 (12.8) | 10 (9.1) | 3 (15) | 6 (35.3) | .028 |
| Median operative time (min) (IQR) | 240 (186–312) | 230 (180–300) | 286 (231–345) | 269 (220–340) | .009 |
| Median intraoperative bleeding (mL) (IQR) | 200 (100–400) | 200 (100–350) | 325 (180–500) | 500 (150–800) | .014 |
| Pringle time (min) | 30 (12–56.5) | 30 (13–58) | 30 (8–47) | 30 (23–50) | .655 |
| Perioperative transfusion, n (%) | 27 (18.1) | 16 (14.3) | 4 (20) | 7 (41.2) | .047 |
| Stay (days) median (IQR) | 6 (4–9) | 5 (4–7) | 10 (7−12) | 15 (11–22) | <.001 |
| Readmission, n (%) | 16 (10.7) | 6 (5.4) | 3 (15) | 7 (41.2) | .001 |
IQR: interquartile range; CRC: colorectal cancer; PLS: primary liver surgery.
When we compared the groups with no complications vs minor complications vs MC, the following parameters were statistically significant: number of affected segments, number of metastases, number of CTx cycles (but not the use of antiangiogenics or the regimen used), type of hepatectomy (minor/major), approach, previous portal embolization, two-stage surgery, operative time, intraoperative bleeding and perioperative transfusion. The presence of complications led to an increase in length of hospital stay and readmissions.
There are multiple therapeutic options for the treatment of patients with CRC and SLM, and no differences in MC and postoperative mortality have been found between the 3 options.2,4,9 Although mortality after hepatectomy has decreased considerably, morbidity remains high.11 Risk factors that increase morbidity and mortality after liver surgery have been defined and classified into 2 large groups: patient-related factors and procedure-related factors.11 Complications affect quality of life, increase costs, and have a negative impact on survival.9,11
Studies about LFA have focused on oncological results and/or verifying whether the whole treatment was completed. Also, although morbidity data have been included (total complications [31.1%–81.6%], MC [21.1%], and mortality of LFA [0.6%–2.3%]),2–9 the factors associated with morbidity and mortality have not been studied.
The total number of complications in our series (25%) is lower than other publications, possibly due to an increase in minimally invasive approaches and fewer major hepatectomies. In our series, complicated patients had more affected segments and more metastases, required prior portal embolization, and were administered more CTx cycles. In terms of surgical factors, major hepatectomies, an open approach, two-stage surgery, longer operating times, greater intraoperative bleeding, and patients who required perioperative transfusion presented more complications. Although these factors could be considered logical, they had not been determined until now. While our sample size is small and causal associations cannot be established, we believe that patients who present these aforementioned factors require special care during the postoperative period.
Conflicts of interestThe authors declare that they have no conflicts of interest.
FundingThis study has been funded by the AEC Multicenter Studies Grant.
RENACI projectMario Serradilla-Martín1,*, Celia Villodre2, Laia Falgueras-Verdaguer3, Natalia Zambudio-Carroll4, José T. Castell-Gómez5, Juan L. Blas-Laina6, Vicente Borrego-Estella7, Carlos Domingo-del-Pozo8, Gabriel García-Plaza9, Francisco J. González-Rodríguez10, Eva M. Montalvá-Orón11, Ángel Moya-Herraiz12, Sandra Paterna-López13, Miguel A. Suárez-Muñoz14, Maialen Alkorta-Zuloaga15, Gerardo Blanco-Fernández16, Enrique Dabán-Collado17, Miguel A. Gómez-Bravo18, José I. Miota-de-Llamas19, Fernando Rotellar20, Belinda Sánchez-Pérez21, Santiago Sánchez-Cabús22, David Pacheco-Sánchez23, Juan C. Rodríguez-Sanjuan24, María A. Varona-Bosque25, Lucía Carrión-Álvarez26, Sofía de la Serna-Esteban27, Cristina Dopazo28, Elena Martín-Pérez29, David Martínez-Cecilia30, María J. Castro-Santiago31, Dimitri Dorcaratto32, Marta L. Gutiérrez-Díaz33, José M. Asencio-Pascual34, Fernando Burdío-Pinilla35, Roberto Carracedo-Iglesias36, Alfredo Escartín-Arias37, Benedetto Ielpo38, Gonzalo Rodríguez-Laiz2, Andrés Valdivieso-López39, Emilio De-Vicente-López40, Vicente Alonso-Orduña41 and José M. Ramia42
1 Department of Surgery, Hospital Universitario Virgen de las Nieves, Granada. Department of Surgery, School of Medicine, University of Granada, Granada (Spain); mserradilla@ugr.es
2 Department of Surgery, Hospital General Universitario Dr. Balmis. ISABIAL. Alicante (Spain); cvillodre@umh.es
3 Department of Surgery, Hospital Universitario Dr. Josep Trueta, Girona (Spain); lfalgueras.girona.ics@gencat.cat
4 Department of Surgery, Hospital Universitario Virgen de las Nieves, Granada (Spain); natalia.zambudio.sspa@juantadeandalucia.es
5 Department of Surgery, Hospital Universitario La Paz, Madrid (Spain); jtcastell@quironsalud.es
6 Department of Surgery, Hospital Royo Villanova, Zaragoza (Spain); jlblas@salud.aragon.es
7 Department of Surgery, Hospital Universitario Lozano Blesa, Zaragoza (Spain); vmborrego@salud.aragon.es
8 Department of Surgery, Hospital Universitario Dr. Peset, Valencia (Spain); domingo_cardel@gva.es
9 Department of Surgery, Hospital Universitario Insular, Las Palmas de Gran Canaria (Spain); ggarpla@gobiernodecanarias.org
10 Department of Surgery, Hospital Clínico Universitario de Santiago, Santiago de Compostela (Spain); francisco.javier.gonzalez.rodriguez2@sergas.es
11 Department of Surgery, Hospital Universitario y Politécnico La Fe, IIS La Fe, Ciberehd ISCIII, Valencia (Spain); montalva_eva@gva.es
12 Department of Surgery, Hospital Universitario de Castellón, Castellón (Spain); moya_ang@gva.es
13 Department of Surgery, Hospital Universitario Miguel Servet, Zaragoza (Spain); spaterna@salud.aragon.es
14 Department of Surgery, Hospital Universitario Virgen de la Victoria, Málaga (Spain); mangel.suarez.sspa@juantadeandalucia.es
15 Department of Surgery, Hospital Universitario Donostia, San Sebastián (Spain); maialen.alkortazuloaga@osakidetza.eus
16 Department of Surgery, Hospital Universitario de Badajoz, Badajoz (Spain); gerardoblanco@unex.es
17 Department of Surgery, Hospital Universitario San Cecilio, Granada (Spain); enriquej.daban.sspa@juntadeandalucia.es
18 Department of Surgery, Hospital Universitario Virgen del Rocío, Sevilla (Spain); mangel.gomez.sspa@juntadeandalucia.es
19 Department of Surgery, Hospital Universitario de Albacete, Albacete (Spain); jimiotad@sescam.jccm.es
20 Department of Surgery, Clínica Universidad de Navarra, Pamplona (Spain); frotellar@unav.es
21 Department of Surgery, Hospital Regional Universitario de Málaga, Málaga (Spain); belinda.sanchez.sspa@juntadeandalucia.es
22 Department of Surgery, Hospital Universitario de la Santa Creu i Sant Pau, Barcelona (Spain); ssanchezca@santpau.cat
23 Department of Surgery, Hospital Universitario Río Hortega, Valladolid (Spain); dpachecosa@saludcastillayleon.es
24 Department of Surgery, Hospital Universitario Marqués de Valdecilla, Santander (Spain); juancarlos.rodriguezs@scsalud.es
25 Department of Surgery, Hospital Universitario Nuestra Señora de la Candelaria, Santa Cruz de Tenerife (Spain); mvarbosa@gobiernodecanarias.org
26 Department of Surgery, Hospital Universitario de Fuenlabrada, Madrid (Spain); lucia.carrion@salud.madrid.org
27 Department of Surgery, Hospital Clínico Universitario, Madrid (Spain); sofiacristinadela.serna@salud.madrid.org
28 Department of Surgery, Hospital Universitario Vall d’Hebron, Barcelona (Spain); cristina.dopazo@vallhebron.cat
29 Department of Surgery, Hospital Universitario La Princesa, Madrid (Spain); elena.perez@uam.es
30 Department of Surgery, Hospital Universitario Virgen de la Salud, Toledo (Spain). Department of Surgery, Hospital Universitario La Princesa, Madrid (Spain); dmcecilia@salud.madrid.org
31 Department of Surgery, Hospital Universitario Puerta del Mar, Cádiz (Spain); mariaj.castro.santiago.sspa@juntadeandalucia.es
32 Department of Surgery, Hospital Clínico Universitario, Valencia (Spain); dorcaratto_dim@gva.es
33 Department of Surgery, Hospital Quirón, Zaragoza (Spain); martalgutierrezdi@salud.aragon.es
34 Department of Surgery, Hospital Universitario Gregorio Marañón, Madrid (Spain); josemanuel.asencio@salud.madrid.org
35 Department of Surgery, Hospital Universitario del Mar, Barcelona (Spain); fburdio@psmar.cat
36 Department of Surgery, Hospital Universitario Álvaro Cunqueiro, Vigo (Spain); roberto.carracedo.iglesias@sergas.es
37 Department of Surgery, Hospital Universitario Arnau de Vilanova, Lleida (Spain); aescartin.lleida.ics@gencat.cat
38 Department of Surgery, Hospital Universitario de León, León (Spain). Department of Surgery, Hospital Universitario del Mar, Barcelona (Spain); bielpo@psmar.cat
39 Department of Surgery, Hospital Universitario de Cruces, Barakaldo (Spain); acanvalecha@telefonica.net
40 Department of Surgery, Hospital Universitario HM Sanchinarro, Madrid (Spain); correo@emiliovicente.es
41 Department of Medical Oncology, Hospital Universitario Miguel Servet, Zaragoza (Spain); valonsoo@salud.aragon.es
42 Department of Surgery, Hospital General Universitario Dr. Balmis. ISABIAL. Universidad Miguel Hernandez. Alicante (Spain); ramia_jos@gva.es