Male breast cancer accounts for 1% of all breast cancers. Its low frequency leads to a lack of awareness, resulting in significant diagnostic delays. Additionally, this limits the available evidence, which primarily uses diagnostic-therapeutic algorithms based on women.
ObjectivesTo analyze the prevalence, clinical presentation, anatomical and pathological characteristics, and prognosis of male breast cancer using one of the largest series available. Secondarily, to compare our data with studies conducted in women.
Materials and methodsA multicenter, observational, descriptive, retrospective study was conducted in the autonomous community of Aragon, Spain, from 1995 to 2022 including men with a pathological diagnosis of breast cancer.
ResultsA total of 148 patients were included, with a prevalence of 1%. The most common clinical presentation was a palpable retroareolar mass. Invasive ductal carcinoma was the most frequent type (88.89%), and luminal B was the predominant subtype (47.76%). Surgery was the most utilized treatment; mastectomy was performed in 90.34% and AL in 46.89%. At diagnosis, 52.46% had extramammary involvement. The recurrence rate was 24.1%, and the mortality attributed to the disease was 14.6%.
ConclusionsThere is a high rate of metastatic involvement at diagnosis, a high percentage of mutilating surgeries, and a high number of recurrences compared to available studies on males. Additionally, a worse prognosis is observed compared to breast cancer in women, despite these tumors having a less aggressive molecular subtype. These findings highlight the importance of conducting studies focused on men to develop specific protocols.
el cáncer de mama en el varón representa el 1% de todos los cánceres de mama. Su baja frecuencia conlleva a la ausencia de conciencia que implica un retraso diagnóstico significativo. Además, limita la evidencia disponible utilizando algoritmos diagnóstico-terapéuticos basados en mujeres.
Objetivosanalizar la prevalencia, presentación clínica, características anatomo-patológicas y pronóstico del cáncer de mama en varones con una de las series más largas disponibles. Secundariamente, comparar nuestros datos con estudios realizados en mujeres.
Material y métodosSe realizó un estudio multicéntrico, observacional, descriptivo y retrospectivo en la CCAA de Aragón, abarcando desde 1995 a 2022, incluyendo varones con diagnóstico anatomopatológico de cáncer de mama.
Resultadosse incluyeron 148 pacientes, con una prevalencia del 1%. La presentación clínica más común fue una masa palpable retroareolar, siendo el carcinoma ductal infiltrante el tipo más frecuente (88.89%) y el subtipo luminal B el predominante (47.76%). La cirugía fue el tratamiento más utilizado, con mastectomía en el 90.34% y LA en el 46.89%. Al diagnóstico, el 52.46% presentaban afectación extramamaria. La tasa de recidiva fue del 24.1% y la mortalidad atribuida a la enfermedad fue del 14.6%.
Conclusionesse observa una alta tasa de afectación metastásica al diagnóstico, un elevado porcentaje de cirugías mutilantes y un alto número de recidivas en comparación con los estudios disponibles sobre varones. Además, se evidencia un peor pronóstico en comparación con el cáncer de mama en mujeres, a pesar de tratarse de tumores con un subtipo molecular de menor agresividad. Estos hallazgos subrayan la importancia de realizar estudios centrados en varones para desarrollar protocolos específicos adaptados a sus características.
Male breast cancer accounts for approximately 1% of all breast cancers. However, and despite limited data, annual incidence rates have been increasing (ranging from 5% to 15%). It should also be noted that there are variations between geographic areas and different ethnic groups, with a higher incidence among African-American men.1,2
As it is a rare disease, current data on optimal treatment and follow-up are still limited. Most algorithms are extrapolated from the numerous studies conducted in women, even though the follow-up data on male breast cancer survivors is particularly scarce. The evidence available regarding this subgroup of patients comes from small retrospective case series or databases designed for other purposes. Furthermore, several studies have indicated possible differences in the clinicopathological characteristics of male breast cancer compared to breast cancer in women. In this context, our study is important as it analyzes one of the longest series currently available of men with breast cancer, comparing the results obtained with female patients. Therefore, it contributes towards developing a specific approach to their characteristics in order to achieve optimal treatment and improve prognosis.3–6
The main risk factor (RF) for males to develop breast cancer is genetic mutations, especially in the BRCA1 and BRCA2 genes. Family history or situations that predispose these individuals to hormonal imbalances have also been shown to be contributing factors.2,5,7,8
These tumors typically present with the appearance of a palpable retroareolar mass. In men, this presentation is 5−10 years later than in women, at an average of 67 years, which also means that these patients have more comorbidities. Moreover, there is a delay of up to 10 months before the initial medical consultation after the onset of symptoms. According to the literature, axillary involvement is found at diagnosis in 43% and metastasis in 4%–5.7% of cases.4,7,9–13
Treatment is surgical in most patients. Modified radical mastectomy is the most commonly used technique, accompanied by adjuvant hormone therapy with a selective estrogen receptor modulator or aromatase inhibitors. The significant impact on quality of life due to the toxicity of hormone therapy can lead to early discontinuation of treatment in one out of 4 men.3,7,14–17
The lack of population education, the stigma created by this disease (traditionally considered a “women’s disease”), and the lack of suspicion even among healthcare professionals are responsible for late diagnosis and at more advanced stages. Therefore, men with this pathology have a worse prognosis than women, with lower disease-free survival and overall 5-year survival.4,8,13,18
MethodsWe present a multidisciplinary, multicenter, observational, descriptive and retrospective study, carried out in the Autonomous Community of Aragon, Spain, at hospitals where breast cancer surgery is performed. From the province of Zaragoza, these included: H. Universitario Miguel Servet, H. Clínico Lozano Blesa, H. Nuestra Señora de Gracia and H. Ernest Lluch in Calatayud. In Huesca, these were: H. San Jorge and H. de Barbastro. From the province of Teruel: H. Obispo Polanco and H. de Alcañiz.
This study included all male patients over 18 years of age with a pathological diagnosis compatible with breast cancer from January 1995 to December 2022 (a total of 27 years). For recruitment, the collaboration of the Archives and Documentation Department was requested to select the diagnoses according to the ICD-9 until 2015 and then ICD-10 until December 2022, using the code for male breast cancer. The search was completed by reviewing all male patients who had undergone a breast biopsy during the study period; this information was provided by the Pathological Anatomy Service. Initially, 427 patients were identified; however, after ruling out benign results, this number was reduced to 148 patients among all participating hospitals.
Regarding data collection, it is necessary to mention the limitation of obtaining certain data in a study of these characteristics (retrospective over a period of almost 3 decades, and multicenter with non-digitalized systems), which is reflected in the tables as “missing data”.
The demographic characteristics collected included the age at diagnosis as well as variables that, according to the literature, act as risk factors for the development of breast cancer in men. Family history included all gynecological or breast tumors in both men and women in first-degree relatives. Smoking and obesity were defined according to the criteria of the World Health Organization (WHO). Liver disease included both steatosis and cirrhosis. Regarding prostate disease, both benign prostatic hyperplasia and neoplasms were included. Estrogen alterations were defined as hormonal imbalances, and testicular disease referred to both orchitis and epididymitis or tumors. Other variables collected included the characteristics at diagnosis, reason for consultation, location, laterality, radiological size (on ultrasound or mammography), and staging. All clinicopathological parameters that influenced prognosis and subsequent treatment were taken into account, such as histological type, hormone receptors, HER2 and Ki67, cytokeratin 19, grade, Scarff Bloom classification, resulting molecular subtype and the definitive size in the histological analysis. This was followed by the treatment received, whether neoadjuvant chemotherapy was administered, type of surgical intervention, number of positive lymph nodes in the case of AL (divided into 3 groups according to prognostic interest), and whether adjuvant treatment was administered (chemotherapy, hormone therapy, radiotherapy or Anti-HER2). Lastly, the recurrence rate during subsequent follow-up was analyzed along with its location and whether the patient died from their breast cancer.
This study complies with the Declaration of Helsinki and clinical research bioethics. It was approved by the Research Ethics Committee of the Community of Aragon (CEICA) (04/05/2023, Minutes 07/2023, version 2.0 03/28/2023, protocol number: PI23/126).
Statistical analysis: The SPSS program version 25 was used to conduct a descriptive analysis. Contingency tables were created in order to calculate the absolute and relative frequencies of the variables of interest. The valid percentage was calculated on the total number of patients in whom that variable was collected, discarding the missing data. In the case of quantitative variables, these were grouped into intervals.
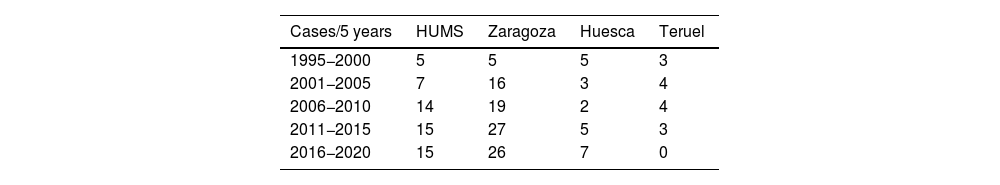
ResultsIn total, we analyzed a definitive sample of 148 male patients with a pathological diagnosis of breast cancer. In our series, male breast cancer represents 1% of the total number of breast cancers treated surgically, calculated based on men operated on during a one-year period at HUMS versus women, with an upward trend in the last decade in all provinces (Table 1).
The first variable that demonstrated an important therapeutic and prognostic impact was the age at diagnosis. Our patient sample had a mean age of 67.58 years with a standard deviation of 12.27, and 72.4% of our patients were over 61 years of age (43.3% between 61–76, and 29% >77 years old).
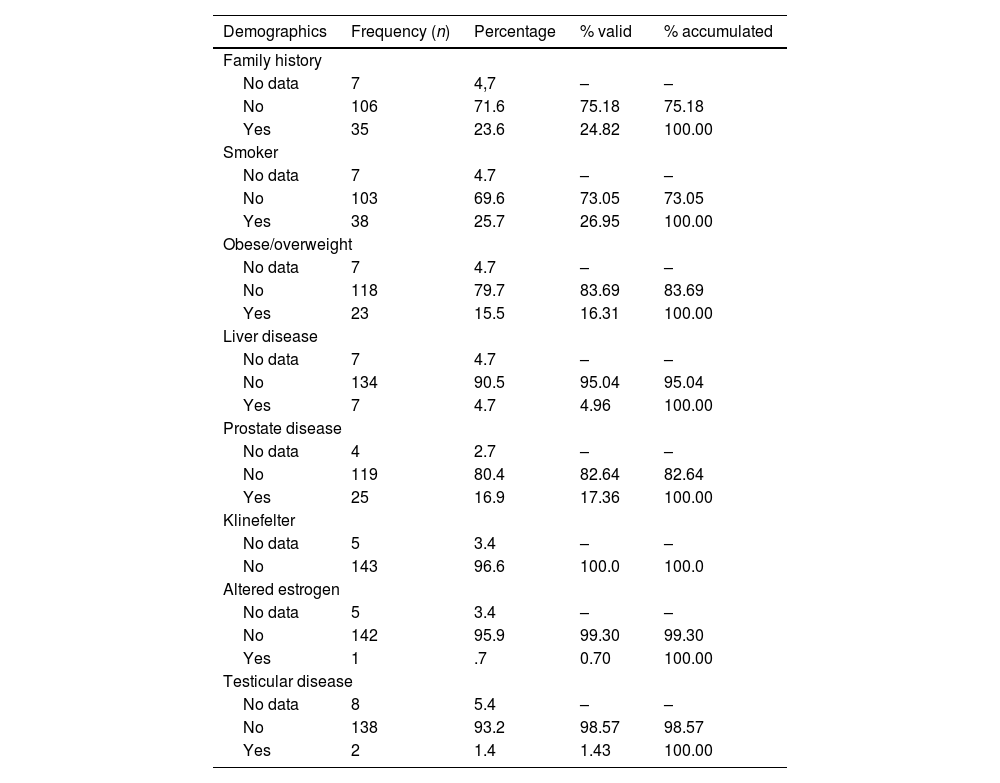
In terms of risk factors, 46.85% of our patients presented at least one, the most frequent being smoking (26.95%). A family history was recorded in 24.82% of men (Table 2).
Risk factors.
| Demographics | Frequency (n) | Percentage | % valid | % accumulated |
|---|---|---|---|---|
| Family history | ||||
| No data | 7 | 4,7 | – | – |
| No | 106 | 71.6 | 75.18 | 75.18 |
| Yes | 35 | 23.6 | 24.82 | 100.00 |
| Smoker | ||||
| No data | 7 | 4.7 | – | – |
| No | 103 | 69.6 | 73.05 | 73.05 |
| Yes | 38 | 25.7 | 26.95 | 100.00 |
| Obese/overweight | ||||
| No data | 7 | 4.7 | – | – |
| No | 118 | 79.7 | 83.69 | 83.69 |
| Yes | 23 | 15.5 | 16.31 | 100.00 |
| Liver disease | ||||
| No data | 7 | 4.7 | – | – |
| No | 134 | 90.5 | 95.04 | 95.04 |
| Yes | 7 | 4.7 | 4.96 | 100.00 |
| Prostate disease | ||||
| No data | 4 | 2.7 | – | – |
| No | 119 | 80.4 | 82.64 | 82.64 |
| Yes | 25 | 16.9 | 17.36 | 100.00 |
| Klinefelter | ||||
| No data | 5 | 3.4 | – | – |
| No | 143 | 96.6 | 100.0 | 100.0 |
| Altered estrogen | ||||
| No data | 5 | 3.4 | – | – |
| No | 142 | 95.9 | 99.30 | 99.30 |
| Yes | 1 | .7 | 0.70 | 100.00 |
| Testicular disease | ||||
| No data | 8 | 5.4 | – | – |
| No | 138 | 93.2 | 98.57 | 98.57 |
| Yes | 2 | 1.4 | 1.43 | 100.00 |
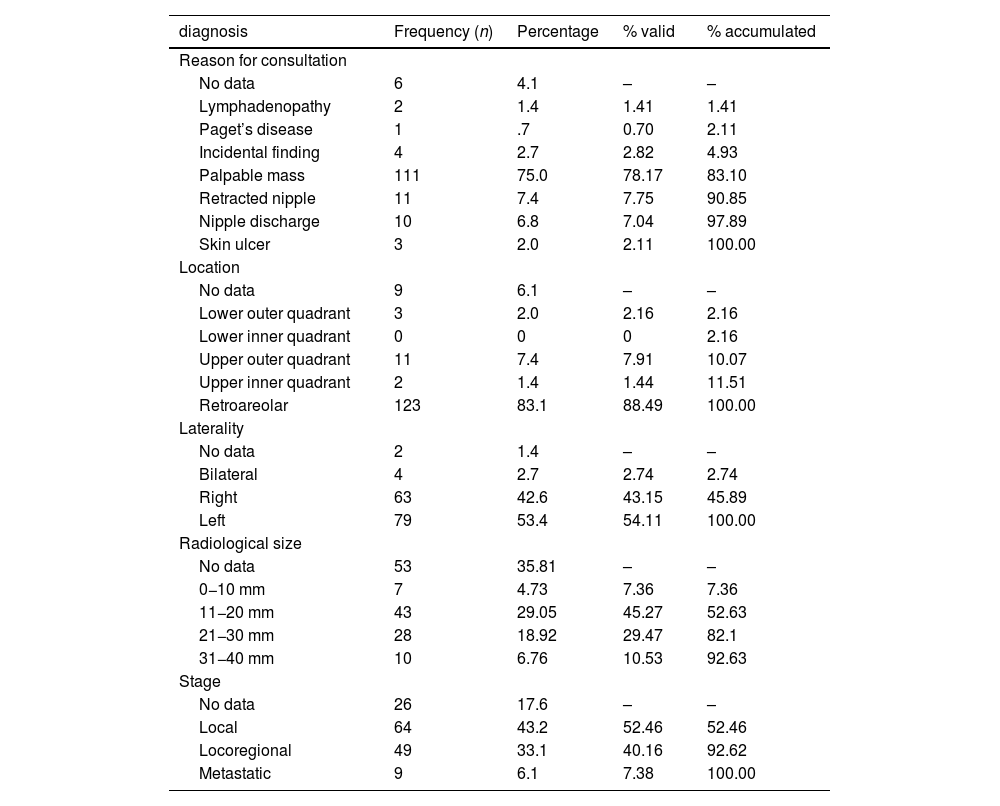
In most patients, the clinical presentation was the appearance of a palpable breast mass in a retroareolar location, with a delay in seeking medical attention ranging from 6−12 months (Table 3).
Characteristics at diagnosis.
| diagnosis | Frequency (n) | Percentage | % valid | % accumulated |
|---|---|---|---|---|
| Reason for consultation | ||||
| No data | 6 | 4.1 | – | – |
| Lymphadenopathy | 2 | 1.4 | 1.41 | 1.41 |
| Paget’s disease | 1 | .7 | 0.70 | 2.11 |
| Incidental finding | 4 | 2.7 | 2.82 | 4.93 |
| Palpable mass | 111 | 75.0 | 78.17 | 83.10 |
| Retracted nipple | 11 | 7.4 | 7.75 | 90.85 |
| Nipple discharge | 10 | 6.8 | 7.04 | 97.89 |
| Skin ulcer | 3 | 2.0 | 2.11 | 100.00 |
| Location | ||||
| No data | 9 | 6.1 | – | – |
| Lower outer quadrant | 3 | 2.0 | 2.16 | 2.16 |
| Lower inner quadrant | 0 | 0 | 0 | 2.16 |
| Upper outer quadrant | 11 | 7.4 | 7.91 | 10.07 |
| Upper inner quadrant | 2 | 1.4 | 1.44 | 11.51 |
| Retroareolar | 123 | 83.1 | 88.49 | 100.00 |
| Laterality | ||||
| No data | 2 | 1.4 | – | – |
| Bilateral | 4 | 2.7 | 2.74 | 2.74 |
| Right | 63 | 42.6 | 43.15 | 45.89 |
| Left | 79 | 53.4 | 54.11 | 100.00 |
| Radiological size | ||||
| No data | 53 | 35.81 | – | – |
| 0−10 mm | 7 | 4.73 | 7.36 | 7.36 |
| 11−20 mm | 43 | 29.05 | 45.27 | 52.63 |
| 21−30 mm | 28 | 18.92 | 29.47 | 82.1 |
| 31−40 mm | 10 | 6.76 | 10.53 | 92.63 |
| Stage | ||||
| No data | 26 | 17.6 | – | – |
| Local | 64 | 43.2 | 52.46 | 52.46 |
| Locoregional | 49 | 33.1 | 40.16 | 92.62 |
| Metastatic | 9 | 6.1 | 7.38 | 100.00 |
mm: millimeters.
After the clinical diagnosis, imaging tests were performed (mammography and/or breast and axillary ultrasound), and the most frequent finding was the appearance of a T ≥ 2 nodule in 40% of cases. Regarding staging, almost half of the patients presented extramammary involvement at diagnosis (47.38%) (Table 3).
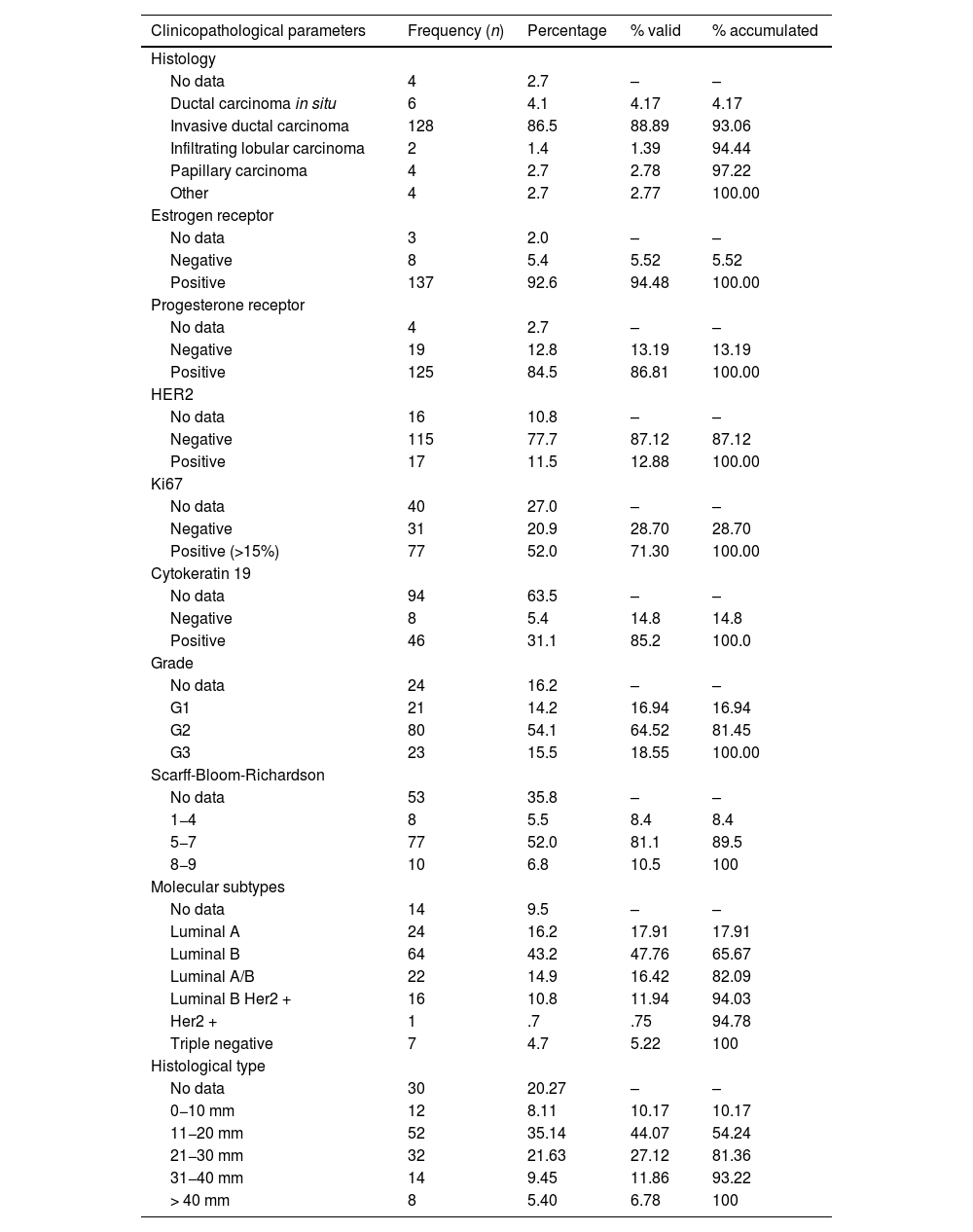
Diagnoses were confirmed by core needle biopsy (CNB), and infiltrating ductal carcinoma was the most frequent histological type (88.89% of cases). Regarding immunohistochemistry, 89.1% of tumors expressed hormone receptors, and Luminal B was the most frequent subtype (82.09%). Table 4 also includes the Luminal A/B subtype due to the difficulty in collecting data regarding Ki67.
Clinicopathological characteristics.
| Clinicopathological parameters | Frequency (n) | Percentage | % valid | % accumulated |
|---|---|---|---|---|
| Histology | ||||
| No data | 4 | 2.7 | – | – |
| Ductal carcinoma in situ | 6 | 4.1 | 4.17 | 4.17 |
| Invasive ductal carcinoma | 128 | 86.5 | 88.89 | 93.06 |
| Infiltrating lobular carcinoma | 2 | 1.4 | 1.39 | 94.44 |
| Papillary carcinoma | 4 | 2.7 | 2.78 | 97.22 |
| Other | 4 | 2.7 | 2.77 | 100.00 |
| Estrogen receptor | ||||
| No data | 3 | 2.0 | – | – |
| Negative | 8 | 5.4 | 5.52 | 5.52 |
| Positive | 137 | 92.6 | 94.48 | 100.00 |
| Progesterone receptor | ||||
| No data | 4 | 2.7 | – | – |
| Negative | 19 | 12.8 | 13.19 | 13.19 |
| Positive | 125 | 84.5 | 86.81 | 100.00 |
| HER2 | ||||
| No data | 16 | 10.8 | – | – |
| Negative | 115 | 77.7 | 87.12 | 87.12 |
| Positive | 17 | 11.5 | 12.88 | 100.00 |
| Ki67 | ||||
| No data | 40 | 27.0 | – | – |
| Negative | 31 | 20.9 | 28.70 | 28.70 |
| Positive (>15%) | 77 | 52.0 | 71.30 | 100.00 |
| Cytokeratin 19 | ||||
| No data | 94 | 63.5 | – | – |
| Negative | 8 | 5.4 | 14.8 | 14.8 |
| Positive | 46 | 31.1 | 85.2 | 100.0 |
| Grade | ||||
| No data | 24 | 16.2 | – | – |
| G1 | 21 | 14.2 | 16.94 | 16.94 |
| G2 | 80 | 54.1 | 64.52 | 81.45 |
| G3 | 23 | 15.5 | 18.55 | 100.00 |
| Scarff-Bloom-Richardson | ||||
| No data | 53 | 35.8 | – | – |
| 1−4 | 8 | 5.5 | 8.4 | 8.4 |
| 5−7 | 77 | 52.0 | 81.1 | 89.5 |
| 8−9 | 10 | 6.8 | 10.5 | 100 |
| Molecular subtypes | ||||
| No data | 14 | 9.5 | – | – |
| Luminal A | 24 | 16.2 | 17.91 | 17.91 |
| Luminal B | 64 | 43.2 | 47.76 | 65.67 |
| Luminal A/B | 22 | 14.9 | 16.42 | 82.09 |
| Luminal B Her2 + | 16 | 10.8 | 11.94 | 94.03 |
| Her2 + | 1 | .7 | .75 | 94.78 |
| Triple negative | 7 | 4.7 | 5.22 | 100 |
| Histological type | ||||
| No data | 30 | 20.27 | – | – |
| 0−10 mm | 12 | 8.11 | 10.17 | 10.17 |
| 11−20 mm | 52 | 35.14 | 44.07 | 54.24 |
| 21−30 mm | 32 | 21.63 | 27.12 | 81.36 |
| 31−40 mm | 14 | 9.45 | 11.86 | 93.22 |
| > 40 mm | 8 | 5.40 | 6.78 | 100 |
mm: millimeters.
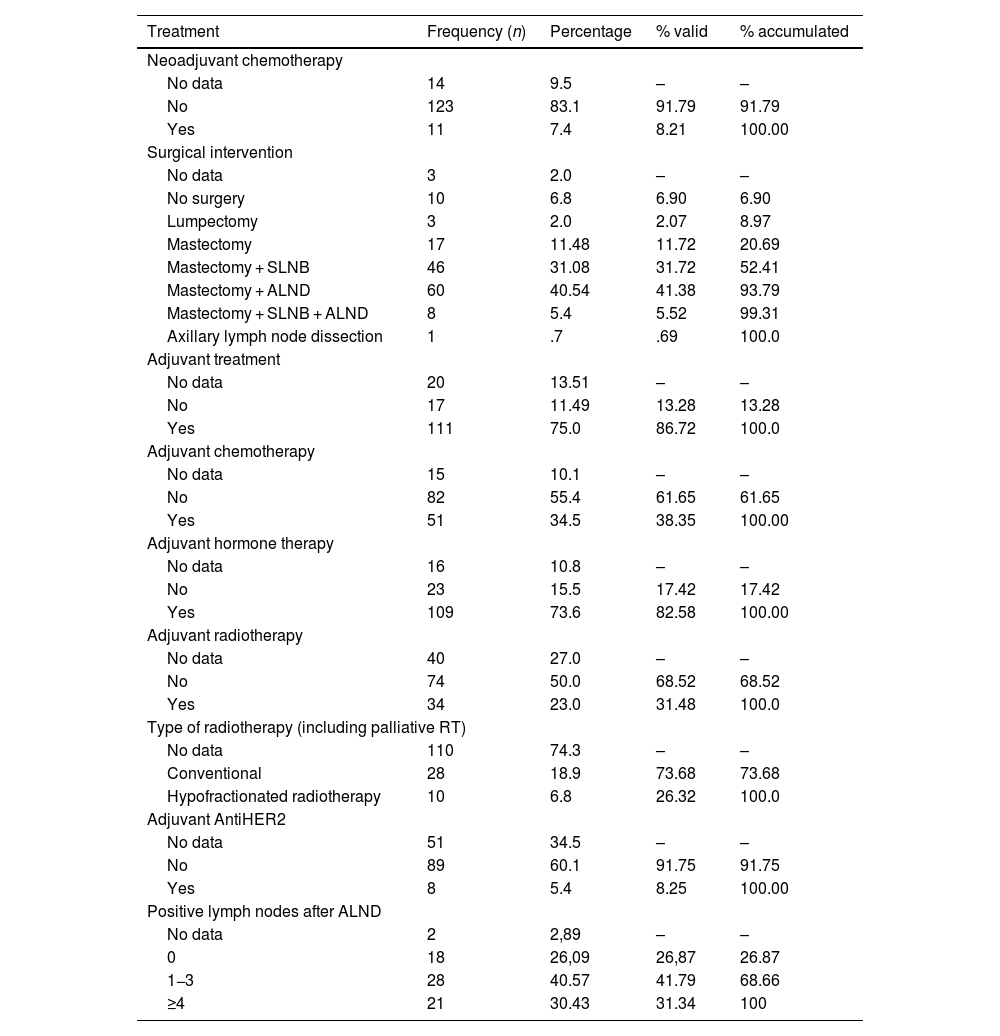
In terms of treatment, 135 patients underwent surgery (91.2%), mastectomy being the technique of choice. Among the 10 patients who did not undergo surgery, 6 were elderly and had metastasis at diagnosis, 2 had severe concomitant pathologies, and 2 had no clinical information to justify the decision. Axillary lymphadenectomy (AL) was performed in a total of 46.89% of cases; in 41.38%, this was initially due to clinical involvement, or because radiological and cytological tests were suspicious of malignancy. Lumpectomy or conservative surgery was performed in only 2.07%. After the pathological analysis of the lymph nodes from the AL, 31.34% had 4 or more affected lymph nodes, thus determining the indication for adjuvant axillary radiotherapy. After surgery, 82.72% of patients received hormone treatment (Table 5).
Treatment and prognosis.
| Treatment | Frequency (n) | Percentage | % valid | % accumulated |
|---|---|---|---|---|
| Neoadjuvant chemotherapy | ||||
| No data | 14 | 9.5 | – | – |
| No | 123 | 83.1 | 91.79 | 91.79 |
| Yes | 11 | 7.4 | 8.21 | 100.00 |
| Surgical intervention | ||||
| No data | 3 | 2.0 | – | – |
| No surgery | 10 | 6.8 | 6.90 | 6.90 |
| Lumpectomy | 3 | 2.0 | 2.07 | 8.97 |
| Mastectomy | 17 | 11.48 | 11.72 | 20.69 |
| Mastectomy + SLNB | 46 | 31.08 | 31.72 | 52.41 |
| Mastectomy + ALND | 60 | 40.54 | 41.38 | 93.79 |
| Mastectomy + SLNB + ALND | 8 | 5.4 | 5.52 | 99.31 |
| Axillary lymph node dissection | 1 | .7 | .69 | 100.0 |
| Adjuvant treatment | ||||
| No data | 20 | 13.51 | – | – |
| No | 17 | 11.49 | 13.28 | 13.28 |
| Yes | 111 | 75.0 | 86.72 | 100.0 |
| Adjuvant chemotherapy | ||||
| No data | 15 | 10.1 | – | – |
| No | 82 | 55.4 | 61.65 | 61.65 |
| Yes | 51 | 34.5 | 38.35 | 100.00 |
| Adjuvant hormone therapy | ||||
| No data | 16 | 10.8 | – | – |
| No | 23 | 15.5 | 17.42 | 17.42 |
| Yes | 109 | 73.6 | 82.58 | 100.00 |
| Adjuvant radiotherapy | ||||
| No data | 40 | 27.0 | – | – |
| No | 74 | 50.0 | 68.52 | 68.52 |
| Yes | 34 | 23.0 | 31.48 | 100.0 |
| Type of radiotherapy (including palliative RT) | ||||
| No data | 110 | 74.3 | – | – |
| Conventional | 28 | 18.9 | 73.68 | 73.68 |
| Hypofractionated radiotherapy | 10 | 6.8 | 26.32 | 100.0 |
| Adjuvant AntiHER2 | ||||
| No data | 51 | 34.5 | – | – |
| No | 89 | 60.1 | 91.75 | 91.75 |
| Yes | 8 | 5.4 | 8.25 | 100.00 |
| Positive lymph nodes after ALND | ||||
| No data | 2 | 2,89 | – | – |
| 0 | 18 | 26,09 | 26,87 | 26.87 |
| 1−3 | 28 | 40.57 | 41.79 | 68.66 |
| ≥4 | 21 | 30.43 | 31.34 | 100 |
| Prognosis | Frequency (n) | Percentage | % valid | % accumulated |
|---|---|---|---|---|
| Recurrence | ||||
| No data | 15 | 10.1 | – | – |
| No | 101 | 68.2 | 75.9 | 75.9 |
| Yes | 32 | 21.6 | 24.1 | 100.0 |
| Location of recurrence | ||||
| No data | 116 | 78.38 | – | – |
| Local | 2 | 1.35 | 6.25 | 6.25 |
| Locoregional | 3 | 2.0 | 9.37 | 15.62 |
| Distance | 27 | 18.2 | 84.38 | 100 |
| Mortality | ||||
| No data | 11 | 7.4 | – | – |
| No death | 72 | 48.6 | 52.55 | 52.55 |
| Death | 45 | 30.4 | 32.85 | 84.4 |
| Death due to breast cancer | 20 | 13.5 | 14.60 | 100 |
SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection; RT: radiotherapy.
The recurrence rate in our series was 24.1%, and distant metastases were most frequent (84.38%). The location of the metastases was mainly in the bone, followed by the lung and pleura, liver, brain and pericardium. Finally, regarding mortality, 14.6% of men in our series died from breast cancer (Table 5).
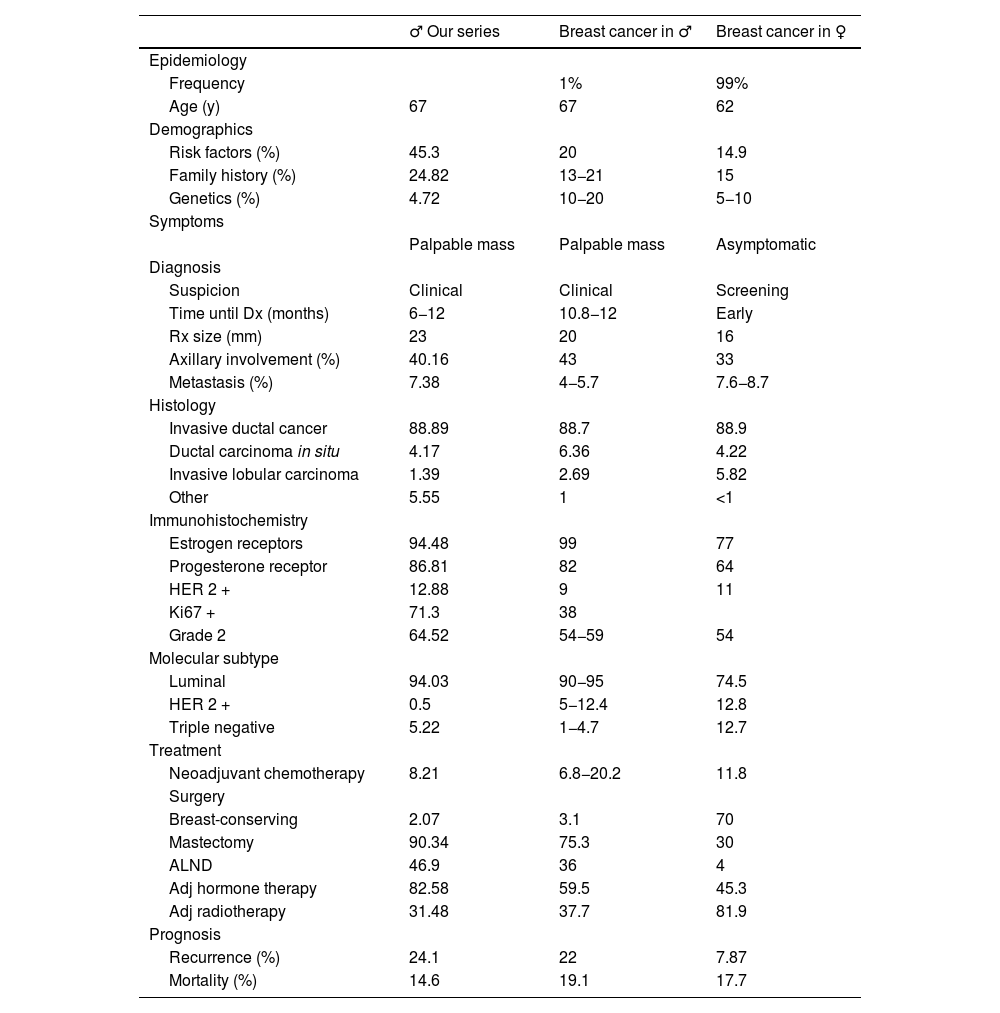
DiscussionThe incidence of breast cancer in men is low. According to the literature, it represents around 1% of all breast cancers, which is confirmed in our series. Studies have shown that annual incidence rates of breast cancer are increasing, and this has also occurred in our region of Spain, especially over the last decade (Table 1).1,2
In our series, the average patient age at diagnosis was 67 years of age, a result that agrees with other reports. It is therefore a disease of advanced age in men, whereas the peak age among woman is 62 years (Table 6).2,5,6
Comparison of our series, literature in males, and studies in females.
| ♂ Our series | Breast cancer in ♂ | Breast cancer in ♀ | |
|---|---|---|---|
| Epidemiology | |||
| Frequency | 1% | 99% | |
| Age (y) | 67 | 67 | 62 |
| Demographics | |||
| Risk factors (%) | 45.3 | 20 | 14.9 |
| Family history (%) | 24.82 | 13−21 | 15 |
| Genetics (%) | 4.72 | 10−20 | 5−10 |
| Symptoms | |||
| Palpable mass | Palpable mass | Asymptomatic | |
| Diagnosis | |||
| Suspicion | Clinical | Clinical | Screening |
| Time until Dx (months) | 6−12 | 10.8−12 | Early |
| Rx size (mm) | 23 | 20 | 16 |
| Axillary involvement (%) | 40.16 | 43 | 33 |
| Metastasis (%) | 7.38 | 4−5.7 | 7.6−8.7 |
| Histology | |||
| Invasive ductal cancer | 88.89 | 88.7 | 88.9 |
| Ductal carcinoma in situ | 4.17 | 6.36 | 4.22 |
| Invasive lobular carcinoma | 1.39 | 2.69 | 5.82 |
| Other | 5.55 | 1 | <1 |
| Immunohistochemistry | |||
| Estrogen receptors | 94.48 | 99 | 77 |
| Progesterone receptor | 86.81 | 82 | 64 |
| HER 2 + | 12.88 | 9 | 11 |
| Ki67 + | 71.3 | 38 | |
| Grade 2 | 64.52 | 54−59 | 54 |
| Molecular subtype | |||
| Luminal | 94.03 | 90−95 | 74.5 |
| HER 2 + | 0.5 | 5−12.4 | 12.8 |
| Triple negative | 5.22 | 1−4.7 | 12.7 |
| Treatment | |||
| Neoadjuvant chemotherapy | 8.21 | 6.8−20.2 | 11.8 |
| Surgery | |||
| Breast-conserving | 2.07 | 3.1 | 70 |
| Mastectomy | 90.34 | 75.3 | 30 |
| ALND | 46.9 | 36 | 4 |
| Adj hormone therapy | 82.58 | 59.5 | 45.3 |
| Adj radiotherapy | 31.48 | 37.7 | 81.9 |
| Prognosis | |||
| Recurrence (%) | 24.1 | 22 | 7.87 |
| Mortality (%) | 14.6 | 19.1 | 17.7 |
Dx: diagnosis; Rx: radiological; ALND: axillary lymph node dissection; mm: millimeters.
Various authors have suggested a greater influence of risk factors among males to develop breast cancer. It has been reported that 20% have some predisposing factor, which is a higher rate than in women (14.9%). In our study, the figure was much higher, at 45.3%. This is justified because other authors do not consider family history a risk factor, as it is not strictly a personal medical history. Smoking was the most frequent RF in our series, justified by its high prevalence in the general population. Second was family history, with a percentage in our study that agrees with the literature for men, which was higher than in women in both instances (Table 6).1–3,5,9,10,19
According to the recommendations of American guidelines (NCCN and ASCO), genetic testing should be done for all men diagnosed with breast cancer. The European guidelines, however, are much more restrictive and only performed on patients with associated risk factors. Therefore, and given the long period of time, the percentage of these tests performed in our series is very low. Furthermore, only 4.72% were positive for some type of mutation, which is a very low percentage compared to the literature available, which is from 10%–20%.19,26
As described in the literature, the typical presentation of breast tumors in men is a palpable mass in a retroareolar location, which was found in 78.17% of the patients in our series. Nipple retraction and discharge were the next most frequent, although their rates do not reach 20% overall. Most patient clinical histories report a mass that had been developing over several months but no consultation with their doctor until it was accompanied by other symptoms, which took more than a year in certain cases. This is similar to reports by other authors (Table 6).4,7,9–12
The diagnosis of breast cancer in men is made after consultation due to a clinical finding. This is dissimilar to women, in whom the diagnosis is made in early stages due to screening programs (non-palpable and clinically asymptomatic lesions). Therefore, almost 50% of male patients are diagnosed at an advanced stage with extramammary involvement. In our series, there was greater metastatic involvement than other descriptions to date (Table 6).4,20
In our series, the rate of invasive ductal carcinoma was 88.89% of the tumors, which concurs with other series published in the literature. The majority were ER + and PR+ (more so than among women), HER-2 negative, and Ki67 positive. We should mention the high percentage of elevated Ki67 in our series (71.3% versus 38% described in the literature), with no justifiable cause found by our Pathological Anatomy Department (Table 6).4,5,7,9
Based on immunohistochemistry, the luminal subtype represented the majority, which was 94.03% in our series and compatible with the literature. The HER2-positive and triple-negative subtypes were poorly represented (<10%) compared to the female sex, which reached 30% of cases (Table 6).4,9,21
Radical surgery is the treatment of choice in men, and mastectomy was performed in 90.34% of cases in our series, which is higher than the 70% described in the literature as the technique of choice. However, despite being less common even in recent years, the NCCN guidelines present conservative surgery and radiotherapy as a safe and feasible treatment technique, with an equivalent survival rate, better aesthetic results and less psychological impact, which should not be underestimated in men. LA is performed both in our series and in the literature in almost 50%, representing a much higher percentage than that performed in women. The aforementioned delay in diagnosis and more advanced stages explain this percentage (Table 6).3,7,14–17
After surgery, 86.72% of patients received adjuvant treatment. The most frequent treatment was hormone therapy (Tamoxifen for 5 years) in 82.58% of cases, which is higher than the data from other series. Radiotherapy and/or chemotherapy were added in stages with locoregional extension in percentages compatible with the literature. In our series, radiotherapy was administered in 31.48% of cases. However, the radiotherapy rate is much higher among women (81.9% of patients), which is justified by the rise in conservative surgeries among this patient group (Table 6).4,7,9,19,22
Only 8.21% of patients received neoadjuvant chemotherapy in our series. This percentage was much lower than in women and is striking with a disease that presents at a more advanced stage. The indication for organ preservation is not used in men, and the percentage of tumors with positive hormone receptors is very high, while that of other subtypes that are more sensitive to chemotherapy is low (Table 6).4
The poor prognosis of breast cancer in men is reflected by the high rate of recurrence, which in our series rose to 24.1%, higher than the 22% described in the literature (Table 6).23
In our series, 14.6% of patients died from their breast cancer, although this figure is lower than the literature, which reports a mortality rate of 19.1%. In women, this rate is around 17%, representing the first cause of death from cancer in women, justified by the high prevalence of this tumor (Table 6).23–25
To conclude, breast cancer in men is a rare disease, which underlines the need for more thorough studies focused on this patient population. This study, with its large sample size and long follow-up period, contributes significantly to the knowledge about this disease.
Among the findings, there was higher metastatic involvement at diagnosis, a high number of mutilating surgeries, and a high recurrence rate compared to the available literature on breast cancer in men. In addition, when we compared our results with the data on breast cancer in women, a worse prognosis was evident in men, despite the fact that their neoplasms present less aggressive molecular subtypes. This worse prognosis is largely justified by late diagnosis.
To improve these results, it is essential to intensify population education, implement screening in cases with risk factors and promote ongoing training among healthcare professionals. It is also crucial to work towards reducing the stigma associated with this disease in men, consider the patient's quality of life and support both patients and their families in all areas, especially after aggressive surgeries.
These efforts can contribute towards earlier diagnosis and more effective management of breast cancer in men, thereby improving the prognosis.
FundingThis study has received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.