Today's options for biliary bypass procedures, for difficult choledocholithiasis, range from open surgery to laparo-endoscopic hybrid procedures. The aim of this study was to analyze the outcomes of patients with difficult choledocholithiasis treated with laparoscopic choledochoduodenostomy.
MethodsWe performed a prospective observational study from March 2011 to June 2016. We included patients with difficult common bile duct stones (recurrent or unresolved by ERCP) in which a biliary bypass procedure was required. We performed a laparoscopic bile duct exploration with choledochoduodenostomy and intraoperative cholangioscopy.
ResultsA total of 19 patients were included. We found female predominance (78.9%), advanced mean age (72.4±12 years) and multiple comorbidities. Most patients with previous episodes of choledocholitiasis or cholangitis, mode 1 (min-max: 1–7). Mean common bile duct diameter 24.9±7mm. Mean operative time 218.5±74min, estimated blood loss 150 (30–600)mL, resume of oral intake 3.2±1 days, postoperative length of stay 4.9±2 days. We found a median of 18 (12–32) months of follow-up. All patients with normalization of liver enzymes during follow-up. One patient presented with sump syndrome and one patient died due to nosocomial pneumonia.
ConclusionsLaparoscopic choledochoduodenostomy with intraoperative cholangioscopy seems to be safe and effective treatment for patients with difficult common bile duct stones no resolved by endoscopic procedures. This procedure is a good option for patients with advanced age and multiple comorbidities. We offer all the advantages of minimally invasive surgery to these patients.
Las opciones actuales para realizar procedimientos permanentes de derivación biliodigestiva, secundarios a coledocolitiasis compleja, van desde la cirugía abierta hasta el empleo de procedimientos híbridos laparoendoscópicos. El objetivo del estudio fue realizar un análisis de los resultados obtenidos en pacientes con coledocolitiasis compleja tratados con colédoco-duodeno anastomosis laparoscópica.
MétodosSe realizó un estudio observacional longitudinal en el período comprendido de marzo de 2011 a junio de 2016. Se incluyeron pacientes con coledocolitiasis compleja no resuelta por CPRE o resueltas por CPRE pero con dilatación masiva de vía biliar y evidencia de colestasis persistente, los cuales fueron seleccionados para procedimiento quirúrgico con colédoco-duodeno anastomosis.
ResultadosSe incluyeron un total de 19 pacientes. La mayoría mujeres (78,9%), con edad media avanzada (72,4±12 años) y con múltiples comorbilidades. Todos con al menos un episodio (mín-máx: 1-7) de coledocolitiasis o colangitis previos. El diámetro del colédoco fue de 24,9±7mm. El tiempo quirúrgico fue de 218,5±74min, sangrado de 150 (30-600)mL, inicio de dieta en 3,2±1 días y estancia hospitalaria postoperatoria de 4,9±2 días. Se encontró una mediana de 18 (12-32) meses de seguimiento. Posterior al procedimiento se observó normalización de las pruebas de funcionamiento hepático. Un paciente presentó síndrome del sumidero y un paciente falleció por neumonía nosocomial.
ConclusionesLa colédoco-duodeno anastomosis laparoscópica asistida con coledocoscopia representa una técnica segura y eficaz para el tratamiento de pacientes con coledocolitiasis compleja con indicación de derivación biliodigestiva, ofreciendo los beneficios de la cirugía de mínima invasión.
Choledochoduodenostomy is a surgical procedure described for the first time at the end of the 20th century by Riedel.1
The main indications for anastomosis between the common bile duct and the duodenum are: older patients, unresolved choledocholithiasis after multiple attempts of endoscopic retrograde cholangiopancreatography (ERCP) or during bile duct exploration, patients with persistent cholestasis secondary to massive dilatation of the bile duct, multiple giant choledocholithiasis, benign stenosis of the distal bile duct, ampullary stenosis, juxtapapillary diverticulum and common bile duct larger than 20mm.1–4
Current options for patients requiring biliary diversion procedures range from traditional open surgery to endoscopic procedures and advanced laparoscopic surgery.5 Further development of these last two techniques has resulted in their merging into hybrid laparoendoscopic procedures.
The first laparoscopic choledochoduodenostomy (LCDD) was performed by Franklin and Balli in 1991.6 Since then, few groups around the world have adopted the technique and reported their results.
The aim of the present study is to analyze the results obtained in patients with complex choledocholithiasis who were candidates for a common bile duct diversion procedure at our hospital.
MethodsA prospective longitudinal observational study was carried out between March 1, 2011 and June 30, 2016 at the “Dr. Manuel Gea González” General Hospital in Mexico City. The study was previously approved by the hospital's Ethics and Research Committee (registry: 04-55-2012). All included patients signed informed consent forms and agreed to participate in the study.
The study included adult patients with a diagnosis of choledocholithiasis that remained unresolved after ERCP or recurrent choledocholithiasis (≥2 episodes) and one or more of the following characteristics: advanced age (≥70 years), multiple comorbidities, recurrent cholangitis (≥2 episodes), presence of juxtapapillary diverticulum, and bile duct dilatation (≥20mm) with persistent cholestasis.
The variables analyzed were divided into general demographic variables as well as intraoperative, transoperative, postoperative and follow-up variables.
Surgical ProcedureAll patients were operated on by the same surgeon, and the same surgical technique was used. The surgical approach consists of a hybrid laparoendoscopic technique,4 with the patient placed in the French position. Four trocars are used in total: 3 12mm trocars (supraumbilical/optical port, left paramedian and subxiphoid), as well as one right subcostal 5mm trocar. The procedure begins with diagnostic laparoscopy. The first and second portions of the duodenum and hepatoduodenal ligament are identified. In those patients without cholecystectomy (gallbladder in situ), the cystic artery and duct are dissected. In all patients, the Kocher maneuver is performed. Subsequently, intraoperative cholangiography is done with a 20-gauge needle, or transcystically (patients with gallbladder), with the injection of a water-soluble contrast agent.
The next step is to perform bile duct exploration using anterior longitudinal choledochotomy of the supraduodenal common bile duct (with the necessary extension for the removal of lithiasis) in those patients with choledocholithiasis that could not be resolved with ERCP. Choledochoscopy with a 9.8mm (Olympus GIF-H 180®) gastroscope is performed through the left paramedian port for the proximal portion of the biliary tree and through the subxiphoid port for the distal portion. Stents placed by ERCP are extracted through the choledochotomy, and the active extraction of stones is done under direct vision using a Dormia basket, endoscopic balloons and laparoscopic graspers, together with copious irrigation with sterile solution. In patients with gallbladder in situ, this was used as a means of traction for better exposure of the bile duct, and cholecystectomy was completed as a final step.
The anastomosis is created prior to longitudinal duodenotomy (shorter length than choledochotomy). The triangulation (diamond-shaped) technique of the anastomosis is completed with simple sutures and Gea extracorporeal knots7 of 3-0 or 4-0 absorbable monofilament, depending on the case. The end result is a side-to-side choledochoduodenal anastomosis on one plane with a minimum diameter of 2cm. The diameter of the anastomosis is calculated by using the 9.8mm diameter gastroscope as a reference.
After the anastomosis, intraoperative digestive endoscopy is performed transorally with choledochoscopy to verify the caliber of the LCDD and check for any leakage with a hydropneumatic test.8 A closed drainage system is placed in the hepatorenal space and close to, but not in contact with, the anastomosis.
The start of oral intake is determined by the recovery of intestinal function. Drain tubes are removed prior to discharge. Follow-up visits are scheduled for one week, one month and 6months post-op, with annual appointments thereafter. We request upper endoscopy and liver enzyme profiles one month post-operatively and then annually.
Statistical AnalysisThe data were collected in a Microsoft Excel® 2010 database (version 14.0) and analyzed using descriptive statistical methods: measures of central tendency (mean, median or mode) and dispersion (standard deviation, minimum/maximum values and interquartile range), as well as percentages.
For the comparison between the direct bilirubin, gamma-glutamyl transpeptidase and alkaline phosphatase levels at baseline and follow-up, we used the T-test for mean differences; P<.05 was considered statistically significant. The data were analyzed with SPSS v18.0 (SPSS, Inc., Chicago, IL, USA).
ResultsThe study included a total of 19 consecutive patients with a diagnosis of complex choledocholithiasis requiring side-to-side LCDD for the resolution of their biliary disease.
Patients were predominantly women (n=15; 78.9%), with an advanced mean age (72.4±12years) and a mean body mass index of 27.4±5kg/m2. In terms of the American Society of Anesthesiologists (ASA) rating, 9 patients were ASA II, 7 ASA III and 3 ASA IV. The most frequent comorbidity was arterial hypertension (n=11), followed by diabetes mellitus (n=6). We also observed that there were patients with a history of cerebral vascular disease (n=1), ischemic heart disease (n=2), chronic obstructive pulmonary disease (n=1) and chronic liver disease (n=1).
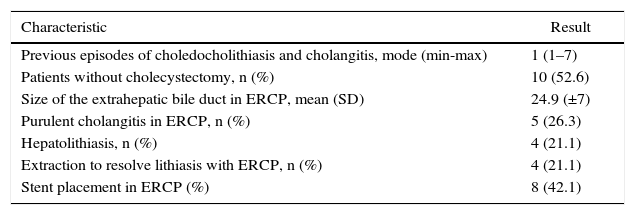
Regarding the number of previous episodes of choledocholithiasis and/or cholangitis, all patients had at least one episode (min-max: 1–7). In 4 patients, gallstones were able to be extracted by ERCP, although patients had massive dilatation of the biliary tract and persistent cholestasis. Three patients were diagnosed endoscopically with juxtapapillary diverticulum, and 9 were classified as complex choledocholithiasis that was not resolved with ERCP. Special cases included the finding of a giant staghorn calculus in a patient at the confluence of the hepatic ducts; in another, a cholecystoduodenal fistula was found, while another patient with choledocholithiasis had a history of antrectomy with gastrojejunal anastomosis due to a perforated peptic ulcer. The mean diameter of the extrahepatic bile duct was 24.9±7.7mm by ERCP. The study group had an average preoperative albumin level of 2.9±1g/dL. The remaining biliary disease characteristics are summarized in Table 1.
Characteristics of Bile Duct Pathologies of the Study Population, Preoperative Values.
| Characteristic | Result |
|---|---|
| Previous episodes of choledocholithiasis and cholangitis, mode (min-max) | 1 (1–7) |
| Patients without cholecystectomy, n (%) | 10 (52.6) |
| Size of the extrahepatic bile duct in ERCP, mean (SD) | 24.9 (±7) |
| Purulent cholangitis in ERCP, n (%) | 5 (26.3) |
| Hepatolithiasis, n (%) | 4 (21.1) |
| Extraction to resolve lithiasis with ERCP, n (%) | 4 (21.1) |
| Stent placement in ERCP (%) | 8 (42.1) |
ERCP: endoscopic retrograde cholangiopancreatography; SD: standard deviation.
In all cases, choledochoscopy allowed for the size of the anastomosis to be calculated, and the hydropneumatic leak test was not positive in any of the patients.
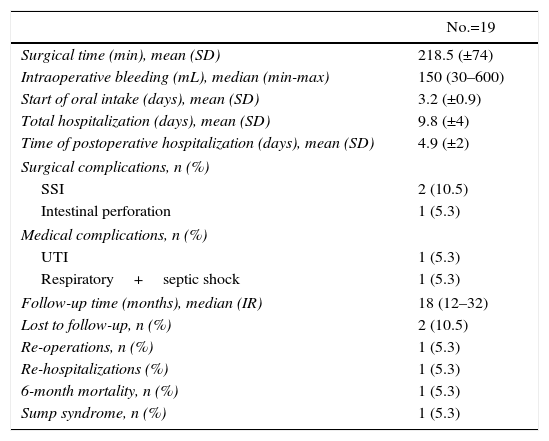
In 16 patients, firm intra-abdominal adhesions were found due to previous surgeries. No procedure was converted to open surgery. The mean surgical time was 218±74min and the estimated average blood loss was 150mL (min-max: 30–600) (Table 2). Oral intake was initiated at a mean of 3.2±1 days post-op, and mean postoperative hospitalization was 4.9±2 days. Three postoperative complications were recorded: 2 superficial surgical site infections and 1 patient with inadvertent intestinal perforation. The last patient was the only case that required surgical reoperation.
Immediate Surgical Results and Follow-Up in Patients With LCDD.
| No.=19 | |
|---|---|
| Surgical time (min), mean (SD) | 218.5 (±74) |
| Intraoperative bleeding (mL), median (min-max) | 150 (30–600) |
| Start of oral intake (days), mean (SD) | 3.2 (±0.9) |
| Total hospitalization (days), mean (SD) | 9.8 (±4) |
| Time of postoperative hospitalization (days), mean (SD) | 4.9 (±2) |
| Surgical complications, n (%) | |
| SSI | 2 (10.5) |
| Intestinal perforation | 1 (5.3) |
| Medical complications, n (%) | |
| UTI | 1 (5.3) |
| Respiratory+septic shock | 1 (5.3) |
| Follow-up time (months), median (IR) | 18 (12–32) |
| Lost to follow-up, n (%) | 2 (10.5) |
| Re-operations, n (%) | 1 (5.3) |
| Re-hospitalizations (%) | 1 (5.3) |
| 6-month mortality, n (%) | 1 (5.3) |
| Sump syndrome, n (%) | 1 (5.3) |
SD: standard deviation; SSI: surgical site infection; UTI: urinary tract infection; IR: interquartile range.
Follow-up time was a median of 18 months (interquartile range: 12–32 months). Only 3 patients had a follow-up of less than one year (6-month follow-up), 2 of which were lost to follow-up and the remaining patient was the last to be treated surgically. A maximum follow-up period of 60 months was observed in the first patient of the series (see Table 2).
One case of sump syndrome presented 3 months after LCDD, requiring re-hospitalization and endoscopic treatment (extension of sphincterotomy and lavage), which provided resolution. One patient died as a result of septic shock associated with respiratory complications (hospital-acquired pneumonia), unrelated with the procedure.
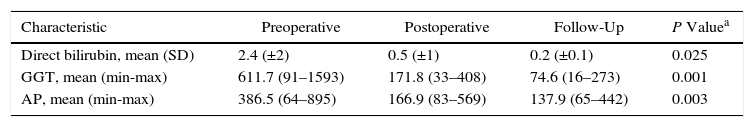
Table 3 summarizes the values found for direct bilirubin, gamma-glutamyl transpeptidase and alkaline phosphatase. We found a significant decrease from the initial levels and the values at the last follow-up visit: direct bilirubin, 2.4 (±2) vs 0.2 (±0.1), P=.025; gamma-glutamyl transpeptidase, 611.7 (91–1593) vs 74.6 (16–273), P=.001; alkaline phosphatase, 386.5 (64–895) vs 137.9 (65–442), P=.000.
Hepatic Enzyme Levels: Preoperative, Postoperative and Outpatient Follow-Up.
| Characteristic | Preoperative | Postoperative | Follow-Up | P Valuea |
|---|---|---|---|---|
| Direct bilirubin, mean (SD) | 2.4 (±2) | 0.5 (±1) | 0.2 (±0.1) | 0.025 |
| GGT, mean (min-max) | 611.7 (91–1593) | 171.8 (33–408) | 74.6 (16–273) | 0.001 |
| AP, mean (min-max) | 386.5 (64–895) | 166.9 (83–569) | 137.9 (65–442) | 0.003 |
SD: standard deviation; GGT: gamma-glutamyl transpeptidase; AP: alkaline phosphatase.
Follow-up endoscopy was performed 1 month and 12 months after LCDD. All endoscopies reported clear anastomosis with easy access to the bile duct (using transoral choledochoscopy) and absence of sump syndrome (except in the previously described patient).
DiscussionDespite advances in endoscopic and surgical therapy, there are a number of patients who require a permanent biliodigestive procedure to treat their disease. Even though it is required in a small number of cases (less than 1% at our hospital),4 it is the only alternative solution.2,3,9 The reported rate of non-successful ERCP range from 4.4% to 10%.3 ERCP is not feasible in certain cases, such as cases with Roux-en-Y gastric bypass.3 At our hospital, the vast majority of patients with choledocholithiasis are resolved with endoscopy (ERCP), so few patients require laparoscopic biliary tract exploration. In patients who need a permanent procedure, LCDD is preferred.
Minimally invasive biliodigestive bypass procedures, including LCDD, offer several advantages: less postoperative pain, shorter hospitalization, fewer wound-related complications, lower treatment costs, rapid return to daily activities and better cosmetic results.4,10 This is reflected in lower morbidity and mortality compared to open procedures.5
Carrying out cholecystectomy, choledochoscopy-assisted bile duct exploration and choledochoduodenal anastomosis in the same laparoscopic operation has become an attractive alternative to traditional open procedures in this type of patients.4,10
The main complications of choledochoduodenostomy are: recurrent cholangitis, anastomotic stenosis, alkaline gastritis and sump syndrome.2,3,11
We consider the use of intraoperative choledochoscopy to be an important factor for the success of LCDD as it is able to corroborate the adequate diameter of the anastomosis and confirm the absence of leakage in order to reduce the number of immediate and long-term complications, such as leaks, fistulas and stenosis.8
Much has been debated regarding the reflux of duodenal content and bacterobilia following LCDD as predisposing factors for recurrent cholangitis; however, recurrent cholangitis is thought to be secondary to anastomotic stenosis.11 The incidence of this complication ranges from 3.11 to 4.2%.2,12 Among patients with recurrent cholangitis, one-third have sump syndrome.3 In our series, we have had no recorded cases of recurrent cholangitis.
Sump syndrome is due to the retention of detritus, food particles, bacteria or gallstones in the portion of the common bile duct distal to the LCDD, which predisposes patients to episodes of recurrent cholangitis and hepatic abscesses. Although the actual incidence of this syndrome is unknown, it is reported at 0%–9.6%.1–3,13 Ample endoscopic sphincterotomy has been described as treatment for sump syndrome.11 We feel that an important factor for the prevention of this syndrome is to perform a wide sphincterotomy at the time of the initial ERCP or during the surgical procedure with rendezvous ERCP.2,4
The possibility of recurrent choledocholithiasis should be borne in mind in this type of patients. In this situation, LCDD facilitates endoscopic re-exploration of the bile duct, unlike what happens with the anatomical changes in patients with Roux-en-Y hepaticojejunal anastomosis.4 Another advantage of LCDD is that it can be done with less risk in patients with multiple intra-abdominal adhesions, unlike the dissection required for the Roux-en-Y.4 It is worth noting that 84.2% of our patients had multiple intra-abdominal adhesions, so LCDD was a quick and technically feasible procedure.
In elderly patients at high risk, LCDD provides good long-term results as a permanent biliary drainage procedure, especially in cases of complex choledocholithiasis or persistent cholestasis.2
LCDD stenosis is a complication described in the long term in up to 20% of cases, according to Demirel et al.14 In our series, all patients had follow-up endoscopies (transoral choledochoscopy) to corroborate the permeability of the anastomosis over time. This aspect is relevant as it provides access for future endoscopic instrumentation through the anastomosis, if necessary.
The main factors that adversely influence mortality following LCDD were: advanced age, hypoalbuminemia, hyperbilirubinemia, comorbidities and the presence of sepsis.11 The patients in our study were elderly (72.4±12 years) with multiple comorbidities. Ten patients were classified as ASA III-IV and had preoperative albumin levels of 2.9±1μg/dL.
The first LCDD series was published by Tinoco et al.15 in 1999, which included 25 patients and had a mean operative time of 115min and hospital stay of 4.2 days. Excellent perioperative results were seen in 24 patients, although one death was registered due to mesenteric ischemia. The study lacked a long-term follow-up.
In 2011, Chander et al.16 published their LCDD results from a 10-year period. The study included 27 patients (21 women) with a mean age of 45.7±13.5 years and common bile duct diameter of 19.6±4.4mm. Mean surgical time was 156.3±25.4min, blood loss 143.3±85.5mL, and hospital stay 6.4±3.8days. They reported a follow-up of 9 years, with no recurrence of symptoms. The mean age was lower than that of our patients (45.7 vs 72.4), our surgical time was slightly longer (218.5 vs 156.3min), the estimated blood loss was similar (150 vs 143.3ml) and the hospital stay was similar in this series (6.4 vs 4.9 days). Despite these differences, the resolution of the biliary pathology was achieved in our series with good short- and long-term results.
Critical factors for the success of the procedure are adequate training in advanced laparoscopic and endoscopic techniques, especially in laparoscopic suturing techniques.2,3,10 Our hospital aims to be a national reference for advanced hepatobiliary procedures by developing and training in hybrid laparoendoscopic techniques.
We can therefore conclude that choledochoduodenostomy is a safe and effective technique for the treatment of complex choledocholithiasis requiring a permanent bypass procedure in selected patients (patients with multiple ERCP attempts, massive biliary tract dilatation with persistent cholestasis, senior patients with several comorbidities, intraabdominal adhesions). The advancement of hybrid laparoendoscopic techniques by means of choledochoscopy-assisted bile duct exploration and side-to-side choledochoduodenostomy extends the benefits of minimally invasive techniques to complex cases, as described.
FundingNo funding was received for the completion of this study.
AuthorshipAdolfo Cuendis-Velázquez: study design, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Mario E. Trejo-Ávila: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Enrique Rosales-Castañeda: data collection, analysis and interpretation of the results, article composition.
Eduardo Cárdenas-Lailson: study design, analysis and interpretation of the results, critical review and approval of the final version.
Sujey Romero-Loera: study design, analysis and interpretation of the results, critical review and approval of the final version.
Martin E. Rojano-Rodríguez: study design, analysis and interpretation of the results, critical review and approval of the final version.
Carlos A. Sanjuan-Martínez study design, analysis and interpretation of the results, critical review and approval of the final version.
Mucio Moreno-Portillo: study design, analysis and interpretation of the results, critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank the surgical team at the GEA Hospital.
Please cite this article as: Cuendis-Velázquez A, Trejo-Ávila ME, Rosales-Castañeda E, Cárdenas-Lailson E, Rojano-Rodríguez ME, Romero-Loera S, et al. Colédoco-duodeno anastomosis laparoscópica. Cir Esp. 2017;95:397–402.