Infections caused by carbapenemase-producing Enterobacteriaceae (CPE) are dramatically increasing worldwide, with an important impact on surgical patients. Our aim was to assess the clinical profile, outcomes, treatment, mortality and costs of CPE-related surgical site infection (SSI) in patients with abdominal surgery.
MethodsReview of CPE-related SSI in patients with abdominal surgery from January 2013 to December 2018. Patient factors and interventions present previously to the SSI identification were recorded, and a mortality analysis was also performed in patients with abdominal surgery and CPE-related organ/space SSI.
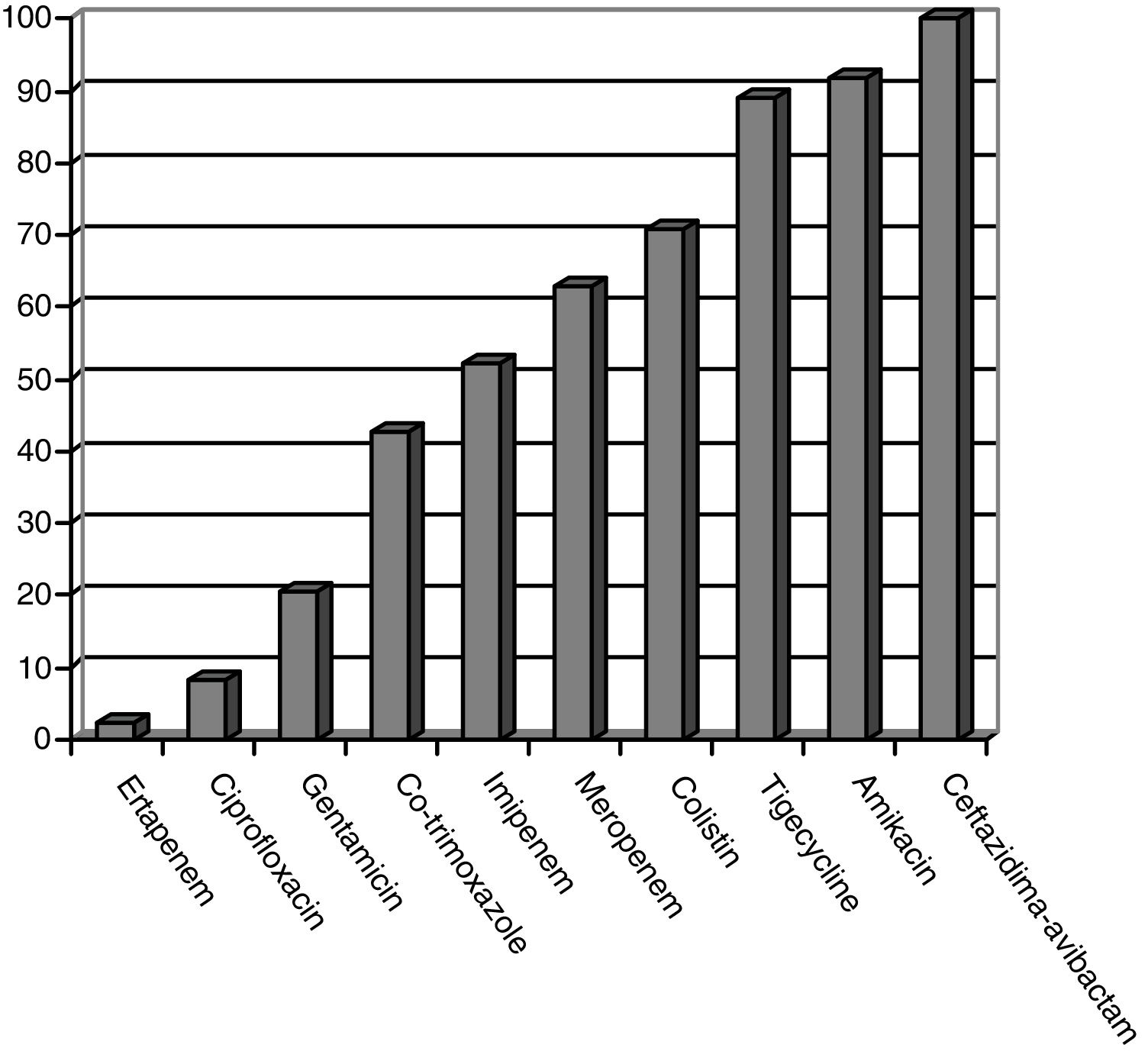
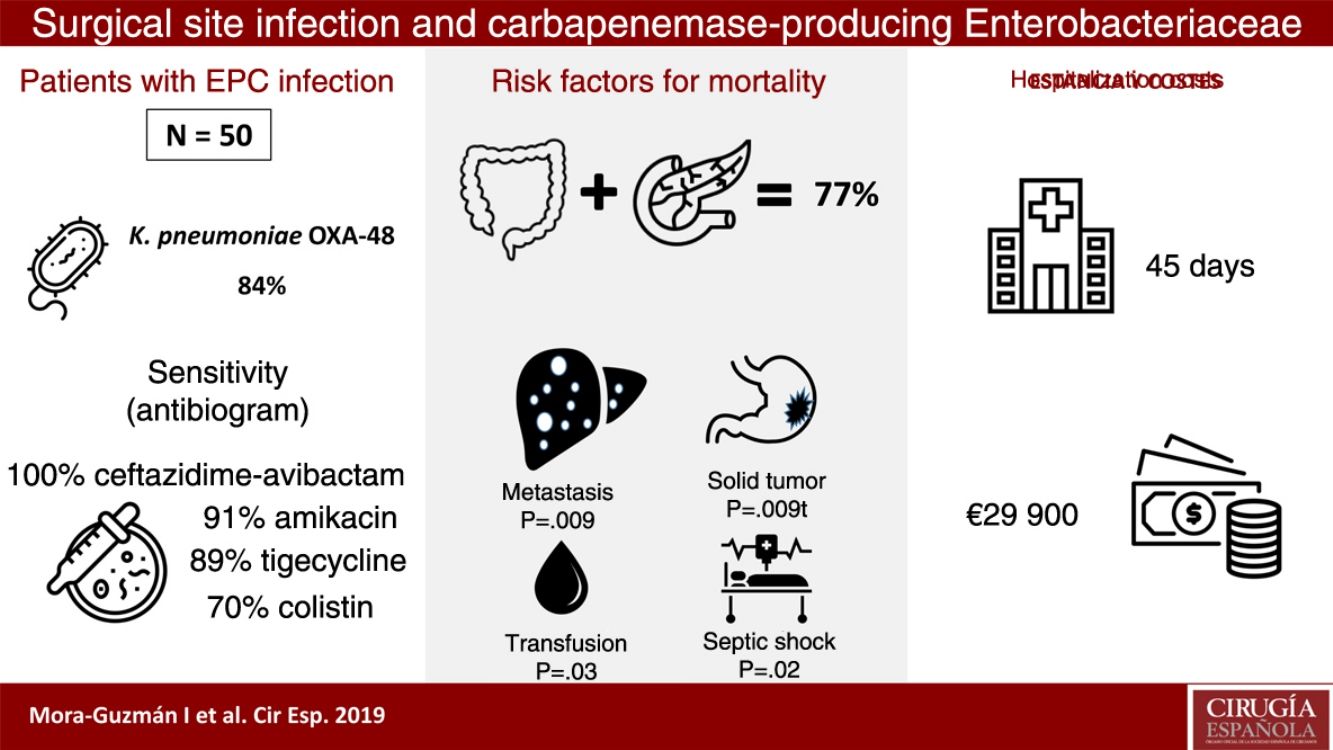
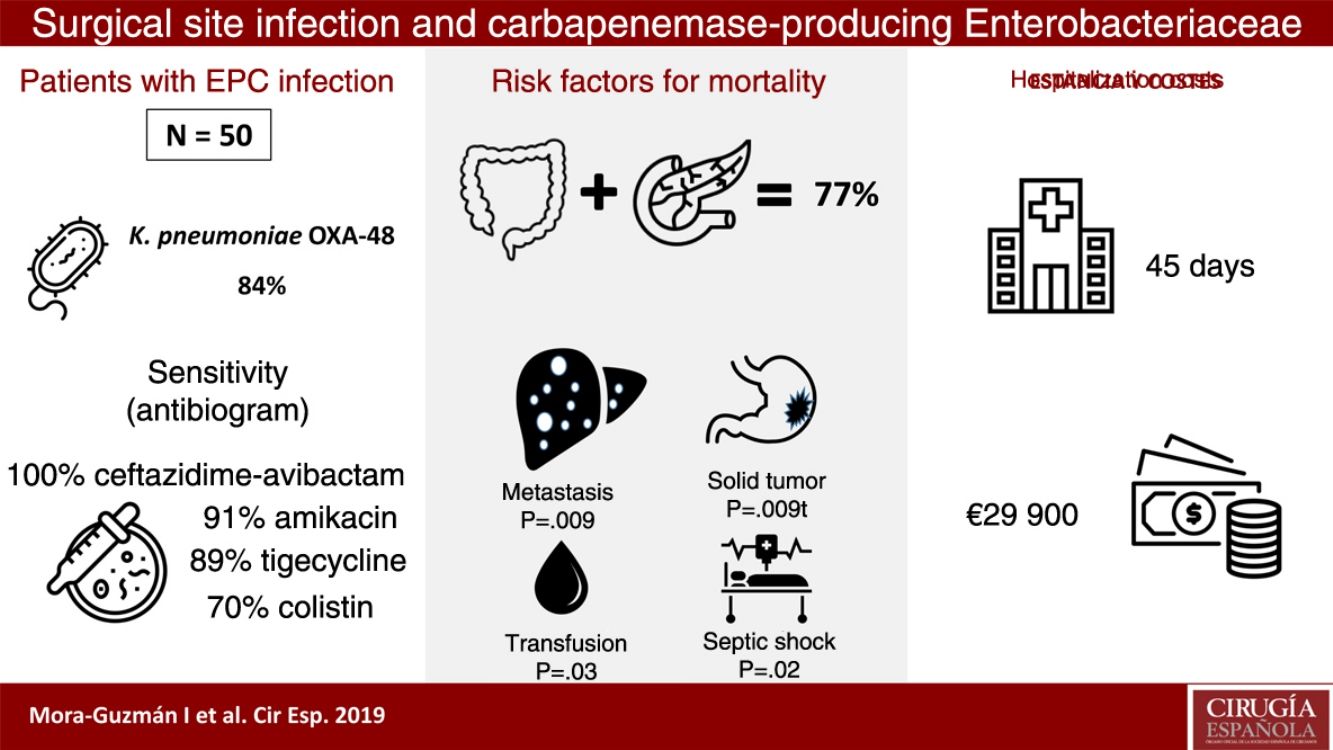
ResultsFifty patients were included: superficial incisional SSI 50%, deep incisional SSI 28%, organ/space SSI (or intra-abdominal infection) 70%. Klebsiella pneumoniae OXA-48 was present in 84%, and the most frequent were colorectal surgery (40%) and pancreatic surgery (20%). The antimicrobial susceptibility was: ceftazidime–avibactam 100%, amikacin 91.7%, tigecycline 89.1%, colistin 70.8%, meropenem 62.8%, and imipenem 52.1%. An appropriate definitive antimicrobial treatment was administered in 86%, using a combined scheme in 76%. Global 30-day mortality rate for intra-abdominal infection was 20%, and mortality-related factors were: solid tumor (P=.009), solid metastasis (P=.009), septic shock (P=.02), and blood transfusions (P=.03). Median global stay was 45 (IQR 26–67) days. Median global cost of hospitalization was €29946 (IQR 15405–47749).
ConclusionsThe clinical profile of patients with CPE-related SSI associates several comorbidities, interventions, prolonged stay and elevated costs. Mortality-related factors in intra-abdominal infection are solid tumor, metastasis, septic shock or blood transfusions.
Las infecciones producidas por enterobacterias productoras de carbapenemasas (EPC) están aumentando drásticamente a nivel mundial, con especial relevancia en pacientes quirúrgicos. El objetivo de este estudio fue analizar el perfil clínico, las complicaciones, el tratamiento, la mortalidad y los costes en pacientes con infección de sitio quirúrgico (ISQ) asociada a EPC tras cirugía abdominal.
MétodosPacientes con ISQ asociada a EPC tras cirugía abdominal entre enero de 2013 y diciembre de 2018. Se incluyeron aquellos factores y procedimientos previos a la identificación de ISQ, y se realizó un análisis de mortalidad para identificar factores de riesgo en aquellos pacientes con ISQ órgano-cavitaria por EPC tras cirugía abdominal.
ResultadosCincuenta pacientes fueron incluidos: ISQ incisional superficial 50%, ISQ incisional profunda 28%, ISQ órgano-cavitaria (o infección intraabdominal) 70%. Se identificó Klebsiella pneumoniae OXA-48 en el 84%, siendo más frecuentes la cirugía colorrectal (40%) y la pancreática (20%). La sensibilidad antimicrobiana fue: ceftazidima-avibactam 100%, amikacina 91,7%, tigeciclina 89,1%, colistina 70,8%, meropenem 62,8%, imipenem 52,1%. Se utilizó antibioterapia dirigida adecuada en el 86%, incluyendo terapia combinada en el 76%. La mortalidad global a 30 días de la infección intraabdominal fue de un 20%, siendo factores predictores: neoplasia sólida (p=0,009), metástasis sólida (p=0,009), shock séptico (p=0,02), transfusión de hemoderivados (p=0,03). La mediana global de estancia fue de 45 días (RIC 26-67). La mediana del coste global del ingreso fue 29.946€ (RIC 15.405-47.749).
ConclusionesEl perfil del paciente con ISQ causada por EPC incluye múltiples comorbilidades, procedimientos, larga estancia y altos costes asociados. Son predictores de mortalidad en infección intraabdominal la presencia de neoplasia, metástasis, shock séptico o transfusión.
The progressive increase in bacterial resistance to antibiotics (ATB) and the lack of development of any new antimicrobial agents have inevitably led to a reduction in the treatment options for infections associated with multidrug-resistance (MDR).1,2 In recent years, there has been a drastic increase worldwide in infections by carbapenemase-producing Enterobacteriaceae (CPE), and numerous countries have been declared endemic for certain strains.1–4 There is such concern about this situation that the World Health Organization has recently listed CPE among the antibiotic-resistant bacteria (ARB) with a level one (critical) priority for research and development of new ATB.5 Nosocomial infections caused by CPE have higher associated morbidity and mortality rates, longer hospital stays and elevated healthcare costs.1,6 Some recommended therapeutic options have obtained good results in studies using a combined ATB therapy in different clinical settings.6,7
Several publications have analyzed the epidemiology, resistance patterns, risk factors for acquisition, and mortality of infections associated with CPE.2,5,8–12 Currently, most published studies include patients with bacteremia, intra-abdominal infection (IAI) or patients admitted to an Intensive Care Unit (ICU), sometimes including individuals with a certain heterogeneity in terms of the type of infection associated with CPE. Currently, there are few research studies that study the clinical profile and risk factors for infections associated with MDR in surgical patients.13 Some studies have been published on CPE that analyze surgical patients in the ICU12,14 or immunocompromised patients after transplantation,15 showing that prior long-term prescription of ATB is a crucial factor. Performing a surgical intervention involves an increase in the morbidity of hospitalized patients, with an increased risk of acquiring an infection caused by resistant bacteria and the associated increase in hospital stay and costs.16 Early identification of risk factors and the clinical profile of patients with abdominal surgery could be essential to optimize the rational use of ATB and improve treatment strategies for surgical site infections (SSI).17 Given the progressive increase in nosocomial infections associated with CPE, and as surgical procedures and IAI are possible associated risk factors,2,10,12 there are sufficient reasons to justify conducting research in these patients with SSI.
The objective of this study was to identify the clinical profile of patients with SSI associated with CPE after abdominal surgery, resistance patterns, mortality risk factors, and associated costs.
MethodsThe study population consisted of consecutive patients who underwent abdominal surgery with SSI caused by CPE acquired during hospitalization in the General and Digestive Surgery (GDS) service between January 2013 and December 2018. The inclusion criteria were: adults over 18 years of age, presence of at least one positive culture more than 48h after admission, and associated with clinical signs of SSI. In order to conduct a complete follow-up of the evolution of each patient after the detection of SSI, we excluded those patients with isolated CPE who were not admitted to the GDS or had been transferred to other wards, patients with incomplete microbiological data, and patients colonized by CPE with no clinical manifestations.
Data were collected in a prospective database that included follow-up data of patients until hospital discharge or death. Patients were included only once, recording CPE isolates in multiple locations in the same individual.
The main outcome variables of the study were 30-day mortality and predictors of IAI-related mortality associated with CPE after abdominal surgery. Secondary outcome variables included: patient factors and comorbidities, SSI location, type of carbapenemase, resistance profile, ATB treatment used, hospital stay and total cost of hospitalization.
Clinical variables, microbiological data, treatment received, and complications were recorded after reviewing patient medical files. Demographic data and comorbidities were included, including the Charlson index.18 The risk factors for CPE infection present before SSI were: hospitalization during the previous 12 months, ATB >48h (previous 30 days), abdominal surgery (previous 30 days), dialysis (previous 30 days) and endoscopic procedures (previous 30 days). Procedures and interventions performed during hospitalization prior to SSI identification were collected, including: dialysis, blood product transfusion, intubation/mechanical ventilation, tracheotomy, central venous catheter, nasogastric tube, urinary catheter, abdominal drainage, parenteral nutrition, and admission >48h in ICU. Surgical variables collected included: anesthetic risk classification of the American Society of Anesthesiologists, specific origin of the disease (liver, bile ducts, pancreas, stomach, colon, rectum, etc.), urgent/scheduled surgery, approach (open/minimally invasive), surgical wound classification according to the Centers for Disease Control (CDC), reoperation, and complications (Clavien-Dindo classification).19 The microbiological and infection-related variables were: date and location of sample collection, main infection site, septic shock, isolated CPE species and classification,20 CPE resistance patterns, and isolation of concomitant resistant bacteria (extended spectrum beta-lactamases [ESBL]). Empirical ATB treatment and antibiogram-directed CPE therapy (including the use of combination therapy) were also recorded.
Hospital stay and 30-day mortality rate were recorded, including the total cost of hospitalization (in euros) for each patient. A mortality analysis was performed in order to identify possible risk factors in patients with organ-cavity infection due to CPE after abdominal surgery.
The protocol for this study was approved by our hospital's Clinical Research Ethics Committee.
The following definitions were specified prior to analysis of the database:
Nosocomial infection was defined as an infection that takes place at least 48h after hospital admission, or an infection that already existed within the previous 2 weeks and was related with a previous hospital admission.21 Septic shock was classified as sepsis with persistent hypotension despite adequate volume replacement and associated organ failure.22 The most likely infection focus according to microbiological data in each patient was defined based on the clinical evaluation by 2 physicians, in accordance with CDC definitions.21 Antibiotic therapy for CPE was considered appropriate if it showed in vitro activity with administration for a minimum duration of 48h.23 Treatment was defined as monotherapy or combination therapy depending on the number of active antimicrobials used.
As for the microbiological study, the samples were collected and incubated according to the site: exudate from the surgical wound, intra-abdominal abscess, blood culture, catheter, urine culture, and respiratory exudate.
The microbiological processing of the samples was done following the standard methodology of our laboratory. CPE identification and antibiogram determination were done with the MicroScan WalkAway® system (Beckman Coulter, Pasadena, CA, USA), following the manufacturer's recommendations. The study included all the strains identified with a minimum inhibitory concentration >0.125mg/L for ertapenem and meropenem, and a minimum inhibitory concentration >1mg/L for imipenem, following the recommendations of the European Committee on Antimicrobial Susceptibility Testing.24 As for the CPE strains isolated in 2013 and 2014, the phenotypic identification was done with CARBA NP25 colorimetric methods, and genotyping was done at the National Center for Microbiology (Instituto de Salud Carlos III). In the CPE identified from 2015 on, the phenotype study was performed with the OXA-48 Card Letitest immunochromatographic test (Coris BioConcept, Gembloux, Belgium).26 When the test was negative, real-time molecular biology techniques were used with polymerase chain reaction (Xpert® Carba-R, Cepheid, Sunnyvale, CA, USA).27
Statistical AnalysisResults were expressed as percentages for categorical variables and as mean and standard deviation for continuous variables, using the median and interquartile range (IQR) for the variables with asymmetric distribution. The Kolmogorov–Smirnov test was used to study the normality of the variables. The Chi-square test or Fisher's exact test was used to compare the categorical variables. For the study of continuous variables, the Student's t test and the Mann–Whitney U test were used, depending on whether there was normality of variables or not, respectively. Statistically significant differences were considered bilaterally with P values <.05. The statistical analysis was performed using SPSS® v. 25.0 for Windows (SPSS Inc., Chicago, IL, USA).
ResultsDuring a 6-year period (2013–2018), 50 consecutive patients were studied with SSI associated with CPE after abdominal surgery. We observed that 50% of the patients presented superficial incisional SSI, 28% deep incisional SSI and 70% organ-cavity SSI (or IAI). Mean age was 66.5±12.9 years, and the median Charlson comorbidity index was 4 (IQR 1–6); 54% of patients were male. The most common comorbidities were: previous malignancy (48%), immunosuppression (38%), diabetes mellitus (28%) or cardiopathy (22%). During the previous 12 months, 60% of the patients had been hospitalized at least once, and 100% had been treated with ATB during the 30 days prior to SSI (carbapenems in 68% of patients). Other highly prevalent factors during admission were: central venous catheter (64%), parenteral nutrition (56%), transfusion (48%), endoscopy (42%), and prolonged ICU admission (42%). The median time from admission to CPE isolation was 16 days (IQR 10–30).
Taking into account the location and underlying pathology, the most frequent surgery in patients with SSI associated with CPE was colorectal surgery (40%), followed by pancreatic surgery (20%). Regarding the degree of contamination of the surgery, the most common was clean-contaminated surgery (52%), followed by contaminated surgery (30%). In the series as a whole, the rate of major complications (Clavien-Dindo ≥3) was 66%, with a 30-day mortality rate of 14% (7 patients). All cases of mortality had presented organ-cavity SSI; therefore, the 30-day mortality rate in the organ-cavity SSI (or IAI) subgroup was 20%. The percentages of reoperation and readmission in our series were 34 and 24%, respectively.
Klebsiella pneumoniae strains were the most frequently identified CPE (42 cases; 84%). Enterobacter cloacae was isolated in 4 patients (8%), Escherichia coli in 3 (6%) and Morganella morganii in one (2%). The OXA-48 class was present in 49 cases (98%), and VIM in one patient with E. cloacae. Two CPE were identified simultaneously in one patient: OXA-48 K. pneumoniae and OXA-48 E. coli. There was previous infection associated with ESBL in 9 cases (18%).
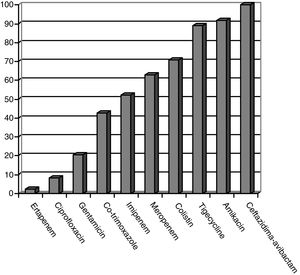
CPE sensitivity patterns according to the antibiogram are shown in Fig. 1. The CPE were very susceptible to ceftazidime–avibactam (100%), amikacin (91.7%), tigecycline (89.1%) and colistin (70.8%), with minimal inhibitory concentrations, but with acceptable susceptibility to meropenem (62.8%) and imipenem (52.1%). There was poor susceptibility to ciprofloxacin (8.3%) and ertapenem (2.3%).
Appropriate empirical antibiotic therapy (determined by in vitro activity against CPE) was used in 7 patients (14%), and antibiotic therapy directed against CPE was appropriately prescribed in 43 (86%). As for the prescribed antibiotic therapy directed against CPE, there were no significant differences in terms of mortality: in patients with appropriate treatment (43), 6 patients died (14%); and in patients with inappropriate treatment (7), one patient died (14.3%).
Targeted combination antibiotic therapy was prescribed in 38 patients (76%), using a combination of 2 active ATB in 35 patients (70%). Mortality percentages were similar, regardless of whether combination therapy was used: 6 patients (15.8%) with combined ATB and one patient (8.3%) without. In 23 cases (46%), the combined therapy included a carbapenem. Median duration of directed ATB was 14 days (IQR 9–20).
Out of the 35 patients with organ-cavity SSI (IAI), the majority underwent surgery for colorectal or biliopancreatic disease (77.1%), and 34.3% required reoperation.
In cases with IAI, OXA-48 carbapenemase-producing K. pneumoniae was also the most prevalent CPE (29 cases; 82.8%). E. cloacae was identified in 3 patients (8.6%) (2 OXA-48 [5.7%] and one VIM). OXA-48 E. coli was found in 2 (5.7%) and OXA-48 M. morganii in 1 (2.8%). Previous ESBL infection was identified in 8 cases (22.8%). The ATB with the best sensitivity as treatment for IAI associated with CPE were: ceftazidime–avibactam (100%), amikacin (90.9%), tigecycline (89.6%), colistin (78.9%) and meropenem (56.7%). When we analyzed the prescribed ATB treatment, 88.6% of patients had appropriate targeted ATB therapy, and using combined therapy in 77.1%. Regarding the CPE-directed antibiotic therapy, 6 out of 31 patients (19.4%) with appropriate antibiotic therapy died, and one out of 4 patients (25%) with inappropriate antibiotic therapy died, showing no statistically significant differences (P=1). Also, no significant differences were observed when mortality was analyzed according to the use of monotherapy or combination antibiotics.
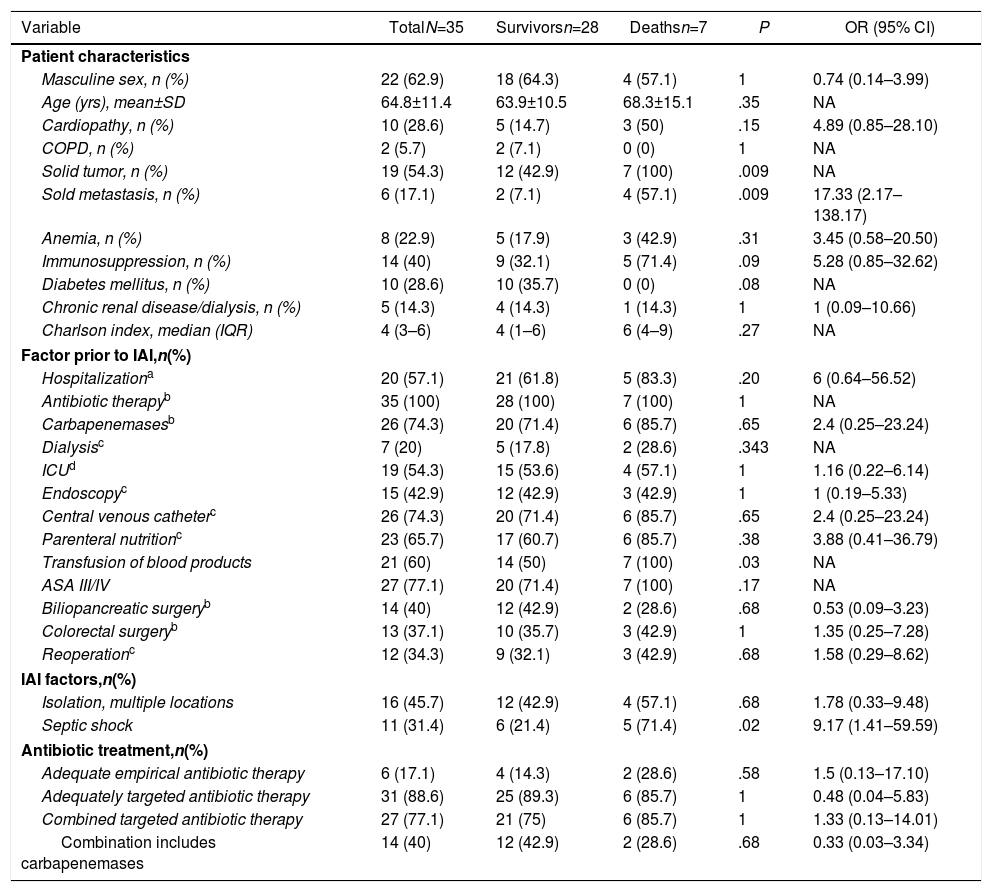
In the IAI mortality analysis (Table 1), significant differences were identified in the following variables as risk factors: solid tumor (P=.009), solid metastasis (P=.009), septic shock (P=.02) and transfusion of blood products (P=.03).
Analysis of Factors Associated With Mortality in Patients With Intra-abdominal Infection by Carbapenemase-producing Enterobacteriaceae After Abdominal Surgery.
| Variable | TotalN=35 | Survivorsn=28 | Deathsn=7 | P | OR (95% CI) |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Masculine sex, n (%) | 22 (62.9) | 18 (64.3) | 4 (57.1) | 1 | 0.74 (0.14–3.99) |
| Age (yrs), mean±SD | 64.8±11.4 | 63.9±10.5 | 68.3±15.1 | .35 | NA |
| Cardiopathy, n (%) | 10 (28.6) | 5 (14.7) | 3 (50) | .15 | 4.89 (0.85–28.10) |
| COPD, n (%) | 2 (5.7) | 2 (7.1) | 0 (0) | 1 | NA |
| Solid tumor, n (%) | 19 (54.3) | 12 (42.9) | 7 (100) | .009 | NA |
| Sold metastasis, n (%) | 6 (17.1) | 2 (7.1) | 4 (57.1) | .009 | 17.33 (2.17–138.17) |
| Anemia, n (%) | 8 (22.9) | 5 (17.9) | 3 (42.9) | .31 | 3.45 (0.58–20.50) |
| Immunosuppression, n (%) | 14 (40) | 9 (32.1) | 5 (71.4) | .09 | 5.28 (0.85–32.62) |
| Diabetes mellitus, n (%) | 10 (28.6) | 10 (35.7) | 0 (0) | .08 | NA |
| Chronic renal disease/dialysis, n (%) | 5 (14.3) | 4 (14.3) | 1 (14.3) | 1 | 1 (0.09–10.66) |
| Charlson index, median (IQR) | 4 (3–6) | 4 (1–6) | 6 (4–9) | .27 | NA |
| Factor prior to IAI,n(%) | |||||
| Hospitalizationa | 20 (57.1) | 21 (61.8) | 5 (83.3) | .20 | 6 (0.64–56.52) |
| Antibiotic therapyb | 35 (100) | 28 (100) | 7 (100) | 1 | NA |
| Carbapenemasesb | 26 (74.3) | 20 (71.4) | 6 (85.7) | .65 | 2.4 (0.25–23.24) |
| Dialysisc | 7 (20) | 5 (17.8) | 2 (28.6) | .343 | NA |
| ICUd | 19 (54.3) | 15 (53.6) | 4 (57.1) | 1 | 1.16 (0.22–6.14) |
| Endoscopyc | 15 (42.9) | 12 (42.9) | 3 (42.9) | 1 | 1 (0.19–5.33) |
| Central venous catheterc | 26 (74.3) | 20 (71.4) | 6 (85.7) | .65 | 2.4 (0.25–23.24) |
| Parenteral nutritionc | 23 (65.7) | 17 (60.7) | 6 (85.7) | .38 | 3.88 (0.41–36.79) |
| Transfusion of blood products | 21 (60) | 14 (50) | 7 (100) | .03 | NA |
| ASA III/IV | 27 (77.1) | 20 (71.4) | 7 (100) | .17 | NA |
| Biliopancreatic surgeryb | 14 (40) | 12 (42.9) | 2 (28.6) | .68 | 0.53 (0.09–3.23) |
| Colorectal surgeryb | 13 (37.1) | 10 (35.7) | 3 (42.9) | 1 | 1.35 (0.25–7.28) |
| Reoperationc | 12 (34.3) | 9 (32.1) | 3 (42.9) | .68 | 1.58 (0.29–8.62) |
| IAI factors,n(%) | |||||
| Isolation, multiple locations | 16 (45.7) | 12 (42.9) | 4 (57.1) | .68 | 1.78 (0.33–9.48) |
| Septic shock | 11 (31.4) | 6 (21.4) | 5 (71.4) | .02 | 9.17 (1.41–59.59) |
| Antibiotic treatment,n(%) | |||||
| Adequate empirical antibiotic therapy | 6 (17.1) | 4 (14.3) | 2 (28.6) | .58 | 1.5 (0.13–17.10) |
| Adequately targeted antibiotic therapy | 31 (88.6) | 25 (89.3) | 6 (85.7) | 1 | 0.48 (0.04–5.83) |
| Combined targeted antibiotic therapy | 27 (77.1) | 21 (75) | 6 (85.7) | 1 | 1.33 (0.13–14.01) |
| Combination includes carbapenemases | 14 (40) | 12 (42.9) | 2 (28.6) | .68 | 0.33 (0.03–3.34) |
ASA: American Society of Anesthesiologists; SD: standard deviation; COPD: chronic obstructive pulmonary disease; 95% CI: 95% confidence interval; IAI: intra-abdominal infection; NA: not applicable; OR: odds ratio; IQR: interquartile range.
The median hospital stay was 45 days (IQR 26–67), with the median stay after CPE isolation being 25 days (IQR 12–51). The median specific stay in cases with IAI associated with CPE was 48 days (IQR 30–66).
The median total cost of hospitalization per patient was €29946 (IQR €15405–€47749), and the median cost of hospitalization in cases with IAI associated with CPE was €30813 (IQR €16072–€51853). These figures more than double the total average cost of hospitalization for a patient with IAI not associated with CPE who underwent colorectal or pancreatic surgery, which is €14710 (IQR €8808–€19651), which is a significant difference in costs (P<.001).
DiscussionThis study includes a detailed description of the clinical profile, complications and mortality of patients with SSI due to CPE after abdominal surgery, with special emphasis on IAI. There is a high incidence of nosocomial infections in surgical patients, and the association between MDR bacterial infections and patients with specific characteristics has been described, which should be considered.13,28 Currently, many countries have been declared endemic for certain strains of CPE, including several European countries. In Spain, OXA-48 producing K. pneumoniae strains are the most frequently isolated,1,12 with a percentage higher than 80% in our experience. The clinical profile of the patients described in our series includes several risk factors, all of which have been previously described1,2,11,29; the high percentages of patients with previous hospitalization, prolonged ICU hospitalization, transfusions or endoscopy are significant. These findings correlate with findings from a recent study about patients admitted to a surgical ICU at a tertiary hospital,12 finding a strong association between ESBL production and CPE carriers. Independent factors for having prior antibiotic therapy included abdominal surgery and prior digestive or biliary endoscopy.
In our series, 70% of the patients with SSI presented IAI, including 7 individuals who died after the detection of CPE, all in the subgroup of patients with IAI (20% mortality rate). IAI is an important cause of morbidity and mortality in surgical patients, and mortality rates above 9% have been described in multicenter studies for complicated IAI, with special attention to urgent surgery.30 This fact is frequently linked to prolonged admissions, with a high percentage of ICU stay and, consequently, the use of broad-spectrum, long-lasting ATB (commonly including carbapenems). Most studies describe high mortality rates for infections associated with CPE, along with other variables taken into account, such as clinical setting, hospital, or reference area. A recent review with meta-analysis reports an overall mortality of 41% in patients with infection caused by carbapenemase-producing K. pneumoniae (including patients with bacteremia, urinary tract infection and, occasionally, IAI).31 The risk factors for mortality in IAI found in our series were the presence of neoplasm and solid metastases, septic shock, and transfusion, which are all variables that have been previously described.2,6,9,10,23 Regarding the risk of mortality, validated predictive models have been developed in patients with bacteremia due to CPE according to the presence of risk factors (INCREMENT model).32 In our study, no significant differences were observed in mortality in IAI regarding the use or not of combined targeted therapy versus CPE, although several previous studies have described more favorable results with the combination of active ATB.8–10,23 Although a recent study has described the possibility of targeted treatment with monotherapy in low-risk patients according to mortality models,33 combined therapy is the present recommendation according to current guidelines on the management of IAI.7,34 In recent years, ceftazidime–avibactam has been used as a monotherapy ATB treatment, having already been included in IAI management guidelines.7 Recent publications have detailed the safety and promising results of this drug when used as monotherapy.35,36 Despite this, the urgent need has been declared for the development of new ATB against CPE,5 and different antimicrobials are currently under investigation.37
The days of hospital stay and the costs associated with CPE infections pose an additional problem that is demonstrated in our results. ATB resistance is currently responsible for a high number of deaths and healthcare costs, the main cause being the inappropriate prescription of ATB.16,38 To curb this situation, different measures have been proposed, such as ATB optimization programs or international collaborative alliances for optimal prescription control,39 also including IAI.17 In this way, today's surgeons should not only be familiar with the prescription of ATB, but also comply with the optimal measures reported to have the greatest effectiveness for preventing SSI,40 while keeping in mind the possible appearance of resistances, with all that this entails.41 Based on available scientific evidence, ATB surveillance initiatives or programs for optimizing the use of antibiotics (PROA), also known as antimicrobial stewardship programs, are based on multidisciplinary management protocols that effectively reduce the incidence of SSI and resistance after implementation follow-up.38,39
Lastly, we should remember that the best treatment also includes the best possible prevention. Therefore, measures must be taken to identify patients who are potentially carriers of CPE. Several international organizations, such as the World Health Organization or the European CDC (ECDC), have published action protocols with specific measures for any hospitalized patient who is potentially an asymptomatic CPE carrier. The ECDC42 stepwise algorithm currently recommends the use of specific measures for any hospitalized patient, using preventive isolation (with contact isolation measures) and CPE screening in patients with any high-risk factor for being a carrier. The high-risk factors established by the ECDC are: known CPE history, epidemiological connection with an identified patient with CPE, hospitalization for 24h or more in the previous year in a healthcare-related institution, dependent on dialysis in the previous year, or treatment with chemotherapy for cancer in the previous year.
Our study presents some limitations to consider. First, it includes a series with a low number of patients, a fact that could affect the identification of statistically significant differences. As a single-center study with a limited number of GDS patients, the results are drawn from a population with very specific characteristics and perhaps cannot be extrapolated to other populations or hospitals with different clinical or microbiological profiles. Lastly, this study is an observational study, based on data for patient characteristics and on the decision-making criteria of each surgeon. Thus, the detailed results and conclusions should be considered with due caution.
In short, SSI associated with CPE, and especially IAI after abdominal surgery, are relevant concerns to be considered by modern surgeons. In our patients, these infections have high percentages of associated complications, procedures, necessary treatments, hospital stay and healthcare costs, which should make us aware of the need to implement necessary prevention and ATB optimization measures.
Conflict of InterestsThe authors declare that there is no conflict of interests regarding this study.
Please cite this article as: Mora-Guzmán I, Rubio-Perez I, Maqueda González R, Domingo Garcia D, Martín-Pérez E. Infección de sitio quirúrgico asociada a enterobacterias productoras de carbapenemasas. Un desafío para el cirujano actual. Cir Esp. 2020;98:342–349.