Liver transplantation is a treatment that significantly improves the patients’ quality of life. However, we should be more ambitious and seek an improvement in their fitness through training protocols allowing them to fully return to daily activities.
English and Spanish-language articles on PubMed and the Cochrane Library were searched until 2014. Articles were reviewed by 2 of the authors to determine if they were suitable for inclusion.
It is shown a compilation of studies that included patients who have participated in aerobic, strength, or both combined training programmes, without implying a risk for the graft function. There is a lack of studies with high scientific evidence that establish a proper exercise programme methodology, supervised by specialists in physical activity and sports.
El trasplante hepático es un tratamiento que ha permitido mejorar de manera significativa la calidad de vida de los pacientes. Sin embargo, se debe ser más ambiciosos y buscar una mejora de su condición física a través de protocolos de entrenamiento que permitan una reincorporación total a las actividades de la vida diaria.
Se buscaron artículos en los idiomas español e inglés, en las bases de datos PubMed y Cochrane, hasta el año 2014. Todos los artículos fueron revisados por 2 autores para determinar si eran apropiados para su inclusión.
Se muestra una recopilación de estudios donde se consiguen mejoras en el estado físico de pacientes que han participado en programas de entrenamiento aeróbico, de fuerza, o en combinación de ambos, sin que esto suponga un riesgo para el injerto. No obstante, existe una falta de trabajos de alta evidencia científica, que establezcan una correcta programación del ejercicio, tutorizada por especialistas en la actividad física y el deporte.
In recent years, society in general has shown growing interest in health through physical activity and sport, while greater attention has been given to physical and psychological wellbeing. This is due to both the health effects of regular exercise as well as the relationship between lack of exercise and the development, continuity and severity of several chronic diseases.1 An active lifestyle has verifiable social importance due to the benefits seen in individuals and in society itself.2 Promoting physical exercise should be fundamental, not only in the prevention and promotion of wellbeing among the healthy population,3 but in groups with some sort of special need, such as liver transplant recipients.
Orthotopic liver transplantation (OLT) is considered the only treatment possible for patients with terminal liver disease.4 In spite of the immense surgical complexity, this operation has become a common procedure in our country. As reports indicate, survival is very high, with a one-year survival rate of around 80%.5 Although providing a cure for these patients is the essential achievement, we need to be more ambitious. While patients’ health-related quality of life (HRQOL) is improved,6,7 it may be insufficient for patients to completely recuperate their normal daily lives. To this end, some research groups are already including physical exercise programmes as a complementary therapy to standard treatment after transplantation.8–14
The objective of this article is to determine the physical condition of patients before transplantation, the changes that are caused by the intervention itself and whether exercise programmes adapted to their physical condition accelerate the physical recovery process, without negatively affecting liver function or the state of the graft.
MethodologyWe conducted a search of the medical literature in Spanish and English in the PubMed and Cochrane databases up to the year 2014. Search terms (in different combinations) included: liver cirrhosis, liver transplantation, physical exercise, physical activity, physical training programme, aerobic training, strength training, and physical therapy. All the articles were reviewed by at least 2 authors to determine whether they were appropriate for inclusion.
ResultsCorrelation Between Physical Condition and the Patient Prior to TransplantationBefore transplantation, patients experience a long period of weakness due to a decline in their physical condition by several variables. The level of disease in this phase seems to correlate with the level of physical condition.15 Patients with liver cirrhosis have affected proteostasis, resulting in problems in aerobic and muscle response16 related with the decrease in the quantity of adenosine triphosphate, phosphocreatine and total magnesium in the skeletal muscles, causing deficient strength in the extremities7–20 and in respiratory muscle capacity.21 These factors limit the functional capacity for daily activities, causing a decline in HRQOL and sociability.17
The parameter that has most often been associated with HRQOL is cardiorespiratory condition or aerobic capacity, represented by maximum oxygen uptake (VO2max).22 To assess the importance of this variable, it can be stated that a reduction of 10% from reference variables compared with healthy subjects of the same age is associated with an increased risk for mortality of 12% in the general population.23 In patients who are about to receive a transplant, VO2max is reduced by between 60% and 78%24–26 compared to reference values. This technique, in combination with others, defines what state the patient is in before transplantation and provides data that indicate his/her future after the procedure,27–29 which makes it an excellent predictor of post-transplantation disease and death. In fact, in a study about mortality during the first 100 days, Epstein et al.18 found that the patients with a lower aerobic capacity within the 15 months prior to the intervention had a higher risk for death a posteriori. Another predictive test of waiting list and post-transplantation mortality is the 6-min walk test, which is able to calculate the aerobic capacity of patients when it is not possible to do so under laboratory conditions. Each 100m increment in the test is associated with a decrease in post-transplant mortality by 52%.30 This test can be conducted in a hospital corridor measuring 20m in length.31
To diminish these negative effects on the physical condition of patients, Ritland et al.32,33 defend the need for physical exercise. To determine the effect of physical training in patients with hepatitis, the same author34 carried out a study with 9 participating patients in 3 test periods to define their VO2max: one at the start of training, another after 4–5 weeks, and the last after 10–12 weeks. After 4 weeks, the VO2max had significantly increased by 19%, and by 29% after 10–12 weeks. No complications arose related with the programme, and most patients perceived improvement in their functional capacity when performing daily activities.
Correlation Between Physical Condition and the Patient Post-transplantationAfter transplantation, patients are fatigued even one year after surgery.35 Fatigue is defined as an excessive sensation of tiredness, lack of energy and feeling of exhaustion that makes normal life difficult to enjoy.
Aadahl et al.36 and van den Berg-Emons et al.37measured the level of fatigue experienced by patients after transplantation. The nature of the fatigue was measured with the Multidimensional Fatigue Inventory (MFI-20)38 and the level of fatigue with the Fatigue Severity Scale (FSS).39 The first group of authors carried out a cross-sectional study of 130 patients. These suggested that the occupational status and survival time after transplantation are associated with physical function and fatigue, and that this is above all physical and not psychological, as stated by Talwakar.40 In contrast, the second group argued that the fatigue was not psychological or due to lack of motivation, but instead it was due to poor physical condition as the fatigue was experienced by those patients who did little daily physical exercise. Both argued that the lack of physical exercise does not improve fatigue over time.
As for the correlation between physical aerobic capacity and patient dependence with hospitalisation, Dharancy et al.41 conducted a study with 135 patients where those that had a serious decline in VO2max showed a tendency towards a longer mean hospital stay and need for oxygen. Thus, it is confirmed that VO2max measurement is an excellent tool for the evaluation of functional capacity, both before and after THO.42
Previous Exercise Programmes Applied in Liver Transplant RecipientsGiven the problem of weakness and fatigue experienced by patients after transplantation, several authors have supported the implementation of exercise programmes after transplantation (Table 1). Various authors8–14 have applied a 6-month exercise programme, all of whom have achieved substantial improvements in parameters such as VO2max, strength and quality of life. This latter measurement was assessed with self-perceived health questionnaires like the SF-36.
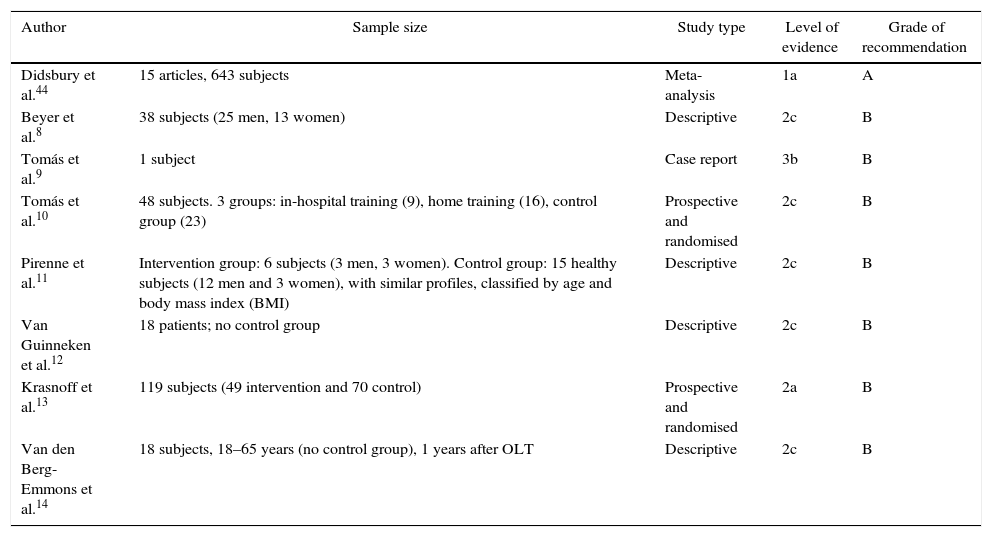
Levels of Evidence and Oxford Centre Grades of Recommendation43 of the Included Studies.
| Author | Sample size | Study type | Level of evidence | Grade of recommendation |
|---|---|---|---|---|
| Didsbury et al.44 | 15 articles, 643 subjects | Meta-analysis | 1a | A |
| Beyer et al.8 | 38 subjects (25 men, 13 women) | Descriptive | 2c | B |
| Tomás et al.9 | 1 subject | Case report | 3b | B |
| Tomás et al.10 | 48 subjects. 3 groups: in-hospital training (9), home training (16), control group (23) | Prospective and randomised | 2c | B |
| Pirenne et al.11 | Intervention group: 6 subjects (3 men, 3 women). Control group: 15 healthy subjects (12 men and 3 women), with similar profiles, classified by age and body mass index (BMI) | Descriptive | 2c | B |
| Van Guinneken et al.12 | 18 patients; no control group | Descriptive | 2c | B |
| Krasnoff et al.13 | 119 subjects (49 intervention and 70 control) | Prospective and randomised | 2a | B |
| Van den Berg-Emmons et al.14 | 18 subjects, 18–65 years (no control group), 1 years after OLT | Descriptive | 2c | B |
The study by Beyer et al.8 used a staged exercise programme in 38 patients. The first 3 weeks after transplantation, all patients were included in a programme that involved protective isolation in a semi-intensive care unit. During this period, the patients were treated with early postoperative mobilisation and performed daily exercise that increased in intensity, which included aerobic exercise (walking and bicycle ergometer) at a rhythm that was individually prescribed. Once this initial programme concluded, patients were organised in small groups and followed a programme of warm-up exercises, aerobic exercises on a stationary bicycle and strength, balance and flexibility training. The exercise load and intensity were individualised in accordance with each patient's functional capacity. It is not specified at what intensity the exercises should be done, which is a crucial methodological bias that frequently affects a relatively important number of research studies dealing with this subject.
After hospital discharge, patients were offered to continue one-hour training sessions twice a week for 6 months after surgery. They were given a training programme, whose content is not specified by the authors, which they were told to do at home 2 or 3 times per week. Furthermore, they were encouraged to participate in physical activities or non-contact sports after leaving the hospital. After 6 months of physical training, the patients had improved their VO2max by 43%, quadriceps strength by 60%–100% and functional performance by 22%–27%. One year after transplantation, patient quality of life, interpreted as self-perceived general health and measured with the SF-36 questionnaire, had improved and was qualified as good or excellent. All patients were independent for activities of daily living, and their levels of physical activity had increased.
The study by Tomás et al.9 applied a training programme with a patient who received a transplant due to familial amyloid polyneuropathy, from 6 to 12 months after the intervention. The programme consisted of moderate intensity aerobic exercise, that were not specified in the text, with a frequency of 3 one-hour weekly sessions. After 6 months, fatigue had dropped 31%, VO2max had increased by 21.6%, quadriceps strength had improved by 28.3%, and the patient walked 21.3% more in the 6-minute walk test.
In another article, Tomás et al.10 studied 48 patients, divided into 3 groups: the in-hospital training group (9 subjects), home training group (16 subjects) and the control group (23 subjects). Training involved 60-min sessions, 3 days a week, for 24 weeks. The in-hospital group started with a 10-min warm-up and finished with a 10-min cool-down. The main part combined aerobic and strength training. The aerobic training was done on the treadmill, bicycle or rowing machine, with an effort of 15 over 20 (moderate). The initial treadmill speed was set at 50% of the speed reached during the 6-minute walk test. Strength training used elastic resistance bands, free weights, dumbbells and the patients’ own body weight, done in 1–2 series of 8–12 repetitions, for each of the 8–10 exercises. In addition, they completed balance training with unstable platforms in order to improve proprioception.
The home training group did exercises with resistance bands and materials to improve grasping, such as rubber balls and bars. The organisation of the sessions was similar to the hospital training group. These patients were taught the exercises before the programme started and had a follow-up visit once a month.
The in-hospital training group achieved better results in weight, body mass index (BMI), lean mass and walking capacity than the group that trained at home, and this group in turn showed greater improvement over the control group. No significant differences were obtained (P>.05) for maximum strength of the quadriceps, upper extremities and dominant lower extremity. Nevertheless, by calculating the percentages that they show in their publication, it can be observed that the quadriceps strength increased by 35% in the in-hospital training group and 30% in the home group, while it decreased by 1% in the control group. Nonetheless, in upper extremity strength, the control group recovered the most strength. In lower extremity strength, the 2 training groups achieved an increase of 21%, while the control group only improved 8.5%. Also, 10 patients were measured 24 weeks after the end of the programme to determine whether the adaptations had been maintained, and the data corroborated the hypothesis.
In the study by Pirenne et al.,11 a group of 6 patients (3 men and 3 women), who had undergone surgery 2 years before, participated in a 6-month training programme. Once completed, they climbed Mt. Kilimanjaro to an altitude of 5895m in a total of 7 days. The physical capacity and level of susceptibility to acute mountain sickness was compared with another group of 15 healthy subjects (12 men and 3 women), with a similar profile and classified by age and BMI. The level of effort perceived and resting pulmonary parameters were compared prospectively with another group of 6 patients with similar VO2max and gender. The results found no significant differences for the following parameters (oxygen saturation, arterial tension, heart rate, acute mountain sickness and other medical problems) during the different stages of the ascent. This suggests that these patients, if they follow an appropriate training programme, can perform intense physical activity and tolerate altitude in similar conditions to healthy individuals.
The most complete studies in this field have been done by van Ginneken et al.,12 Krasnoff et al.13 and van den Berg-Emmons et al.14 The study by van Ginneken et al.12 analysed the effects of a training programme on the reduction of fatigue, daily function, participation in activities, HRQOL, anxiety and depression. The sample included 18 patients and there was no control group. These were included in a 12-week programme with supervised exercise, 2 times/week in one-hour sessions (aerobic and strength) and 4 sessions of activity at home following training instructions, in weeks 1, 4, 8 and 12, to stimulate physical activity. Sessions were organised in groups of 2–4 patients, and the daily sessions were done individually. The methodology for the strength activities were not detailed in the text. Parameters that were evaluated by questionnaires included pre- and post-programme functional level, level of participation, quality of life, anxiety and depression. The conclusion the authors reached was that a daily physical exercise and advice programme significantly influences health-related daily functionality (P=.007). The variable that improved was autonomy (P=.001), and the HRQOL variables that improved were physical functionality (P=.007) and vitality (P=.019) in patients who had received transplants. In spite of the improvements, there were no changes in the level of daily activity, physical limitations, social relationships, anxiety or depression. Furthermore, the programme showed no benefits in long-term fatigue. In any event, the authors argued that a 12-week programme is insufficient to change the sensation of patients about their improved general state of health.
Krasnoff et al.13 studied 119 patients and the combined effects of a physical exercise programme and dietary advice after OLT. The variables analysed were: VO2max, maximum quadriceps strength, body composition, nutritional intake and quality of life. These were examined 2, 6 and 12 months after the intervention and divided into 2 groups. Both groups did the following tests: (1) exertion test on a bicycle ergometer with a gas analyser to determine VO2max, muscle strength of the quadriceps by isokinetic dynamometre (Biodex 3), body composition with densitometry, SF-36 questionnaire (HRQOL), and Block 95 questionnaire (nutritional intake). Each patient received individualised instructions about training and the diet that they should follow at home. As for exercise, only cardiovascular exercise was to be done (walking, biking) at least 3 days a week, 30min per session, at an intensity that began at 60%–65% and increased to 75%–80% or between 13 and 15 out of 20 on the Borg scale of perceived exertion. This is a scale that goes from 6 to 20, where 6 is very light and 20 is maximum exertion. Strength exercises were not included. In this study, the group that did exercise showed an improvement of 24% in VO2max (P<.001), while that of the control group was not significant. Both groups obtained improved body composition, muscle strength, and quality of life, although they were not significant in the interaction between groups. These results demonstrate the beneficial changes of following an exercise programme and controlled diet. According to the authors, this new lifestyle should be initiated within 6 months after the transplantation.
Van den Berg-Emmons et al.14 conducted a study of 18 liver transplant recipients, who completed an exercise programme with 2 one-hour sessions per week for 12 weeks. The exercise programme included aerobic exertion and strength training. The former involved ergometer cycling for 30min, starting at an intensity of 40%–50% of the heart rate reserve, using the Karvonen method.45 After 12 weeks, patients were to pedal at 60% of the heart rate reserve. The strength training lasted 30min per session and focused on the large muscle groups. The intensity and number of repetitions increased over the 12-week period from a series of 10–15 repetitions at 30% maximum repetition (1 MR), to 3 series of 20 repetitions at 60% of 1 MR. In order to determine the changes between the start and end of the programme, aerobic capacity was measured by means of a maximum exertion test on a cycloergometer and the 6-minute walk test. Maximum strength was measured in the quadriceps and hamstring muscles with an isokinetic dynamometre (Biodex). The test was done 5 times at 60° per second. Body composition and fatigue were also evaluated. The results of this study were satisfactory. The VO2max increased by 10% (P<.05) and strength only increased in the hamstrings by 10% (P=.04). There were no changes in BMI, although the percentage of body fat decreased significantly (P=.49).
The key to this study was the percentage of compliance with the programme. As it was in-hospital, there was 93% assistance to the training sessions. The limitation of this study was the lack of a control group.
To improve the development of these programmes, more should be known about the state of the capacities and the effects of physical exercise in this patient population, but the documentation is limited. There are only a few studies that use rehabilitation and physical preparation as part of pre- and post-transplant treatment. The aforementioned studies only contemplate the effects of the programme on different physical variables, and they are limited in terms of number of patients, methodology and observance of long-term cardiovascular improvement.44
None of the studies has analysed how exercise affects other clinical parameters in these patients, such as renal function.
ConclusionsWhile many surgical teams have focused on the success of the surgery itself, adjustments to immunosuppressant therapy and normalisation of patients’ lives, few have developed physical re-adaptation programmes to improve the quality of life of patients for the rest of their lives.
It seems that the patients who most benefit from exercise are those who have a poor physical condition, meaning those who go from a sedentary to an active lifestyle. Thus, it could be interesting to begin with the programme as soon as possible (performance status grade 0–1), since improved physical condition even before the transplantation can result in a lower risk for post-transplant disease and death. To date, it has been observed that the aerobic exercise of walking at a mild to moderate intensity is beneficial. Nonetheless, there are few studies with quality methodologies that have developed capacities such as strength or functional aptitude. Therefore, according to related published articles, it has been proposed that the key to scheduling and properly executing a physical training plan entails applying the necessary dose for each patient, with an aerobic training content (walking, cycling, swimming, aquaerobics), strength training (resistance bands, machines, aquagym) and functional aptitudes (balance, agility, flexibility). A proper plan could include 2–3 one-hour training sessions per week with aerobic, strength and functional aptitude exercises, organised in combination at moderate intensity. As demonstrated, moderate intensity exercise does not negatively affect the function of the new graft. Therefore, when we consider that higher intensities produce greater improvements in the different capacities, a future study of interest should analyse a specific training programme with these characteristics.
Conflict of InterestsThis paper has received no financial support and the authors have no conflicts of interests.
Please cite this article as: Moya-Nájera D, Borreani S, Moya-Herraiz Á, Calatayud J, López-Andújar R, Colado JC. ¿Es perjudicial el ejercicio físico para el trasplantado de hígado? Revisión de la literatura. Cir Esp. 2016;94:4–10.