Mediastinal tumours in children are rare. Around 25% of them can be malignant. Thymoma is an uncommon neoplasm, and during adulthood it corresponds to 30% of anterior mediastinum tumours. The peak incidence is between 55 and 65 years.
Clinical caseA case of lymphocytic thymoma case is reported in a 4-year-old patient with no previous or associated symptomatology. There was only a volume increase on the anterior neck region. The neck radiography and neck and chest tomography confirmed an anterior mediastinal mass surrounding the aorta and vena cava, as well as multiple mediastinal lymph nodes.
ConclusionsEarly diagnosis and complete resection are the basis for management and prognosis.
Los tumores de mediastino en niños son poco frecuentes, el 25% de ellos corresponde a tumores malignos. El timoma es una neoplasia poco común, en adultos corresponde al 30% de los tumores de mediastino anterior y con un pico de incidencia es entre los 55-65 años.
Caso clínicoSe reporta el caso de un timoma linfocítico en una paciente de 4 años, sin sintomatología previa ni asociada, solo aumento de volumen en región anterior de cuello. Las radiografías y tomografías de cuello y tórax confirman una masa mediastinal anterior, que rodea a la aorta y la cava, así como múltiples adenomegalias mediastinales.
ConclusionesEl diagnóstico temprano y la resección completa son la base del tratamiento y del pronóstico.
Mediastinal tumours in children are rare. They represent 3% of total surgical chest interventions in children. 45–50% correspond to primary tumours; of these 40–45% are malignant tumours and a third of them present in children under 2 years of age.1–3 The main types of primary tumours are: thymic, neurogenic, lymphatic, or of germinal or mesenchymal cells; secondary tumours are due to lymphatic dissemination of intrathoracic organs towards the mediastinum.2 The main location is the posterior mediastinum and the majority are of neurogenic and benign origin.1
Since the majority of these tumours are asymptomatic, most are diagnosed as radiographic findings1 and on occasion present as fever, weight loss, general malaise, severe respiratory failure with dyspnoea, intercostal retractions, atelectasis or more aggressively as superior vena cava syndrome, dysphagia, dysphonia, laryngeal spasm caused by recurrent laryngeal nerve compromise, diaphragmatic paralysis caused by phrenic nerve involvement or Horner syndrome caused by lymph node and sympathetic nerve compromise.1,2 Once the tumour has been located, clinical study protocol includes: blood biometry, comprehensive metabolic panel, skin and soft tissue infection panel, and tumour markers. A lateral projection chest X-ray is required. The gold standard is simple and contrast CAT. On occasions magnetic resonance imaging, iodine-123 nuclear medicine imaging, iodine-131 testing for ectopic thyroid tissues, technetium-99 testing to examine for gastric mucosa in intestinal duplications or the use of metaiodobenzylguanidine for diagnosing neuroblastoma are carried out. In children the use of cervical and chest ultrasound is usually useful, or even fine needle biopsy. The latter may, however, be limiting for diagnosis and there may be a risk of bleeding.2,4,5 Standard treatment consists of surgery unless tumours are germinal or lymphatic. A cervical and sternal approach is used, with the performance of mediastinoscopy or video-assisted thoracoscopy.4,5
The thymus is a central lymphoid organ, corresponding to the anterior mediastinum, where the stem cells of the bony medulla are divided into mature T lymphocytes, with a tendency to degenerate at around 2 years of age. When this process continues they may grow or become malignant and are functional as both. Hyperplasia (70%) and thymomas (15%) with myasthenia gravis may occur, as the T lymphocytes are intolerant to anti-acetylcholine anti-bodies.6 The thymus may be a location for lung, breast or thyroid cancer metastasis. Thymoma is a low frequency tumour of uncertain patterns of behaviour. It corresponds to 26–50% of mediastinal tumours and to 82% of thymus tumours4,6,7; diagnosis is generally incidental and occurs through chest X-ray and a CAT scan to determine the location and extension of the tumour.5,6 With regards to thymoma classification, although no standard classification exists, the one proposed by Masaoka et al.3 is the most commonly used. This classification is divided into 4 categories in accordance with the stage of tumour infiltration into: I, capsule intact; II, invasion of the capsule; III, macroscopic invasion into neighbouring organs; IVa and IVb, pleural or pericardial dissemination and lymphogenous or haematogenous metastasis to distant sites respectively. The WHO classification is: epitelial A, mixed AB, lymph node B1, spindle-shaped B2 and thymic carcinoma.4,6,7 Thymic carcinoma is the neoplasm with the highest malignancy of the thymus. Symptoms tend to present earlier and are aggressive with pain, weight loss, superior vena cava syndrome, dyspnoea caused by pericardial effusion and compression of the airway. It is rare and presentation is atypical of its cells which are not similar in appearance to the original thymus ones. It represents around 5% of mediastinal tumours in adults and accounts for approximately 1–2% of mediastinal tumours in children.1,2,4,7–9 The treatment of choice for thymus tumour is surgical resection.4–6,9,10 which may consist of sternotomy, upper cervical sternotomy, thoracotomy, video-assisted thoracoscopy, mediastinoscopy and ideally total surgical resection. If the latter is not performed treatment is complemented with radiotherapy and/or chemotherapy depending on the tumour type, invasion and extension.4,6–10 Surgical complications may be pneumonia, haemothorax, pneumothorax, sternal dehiscence, mediastinitis, osteomyelitis10 and less frequently chylothorax, diaphragmatic paralysis, dysphonia and myasthenic crisis.
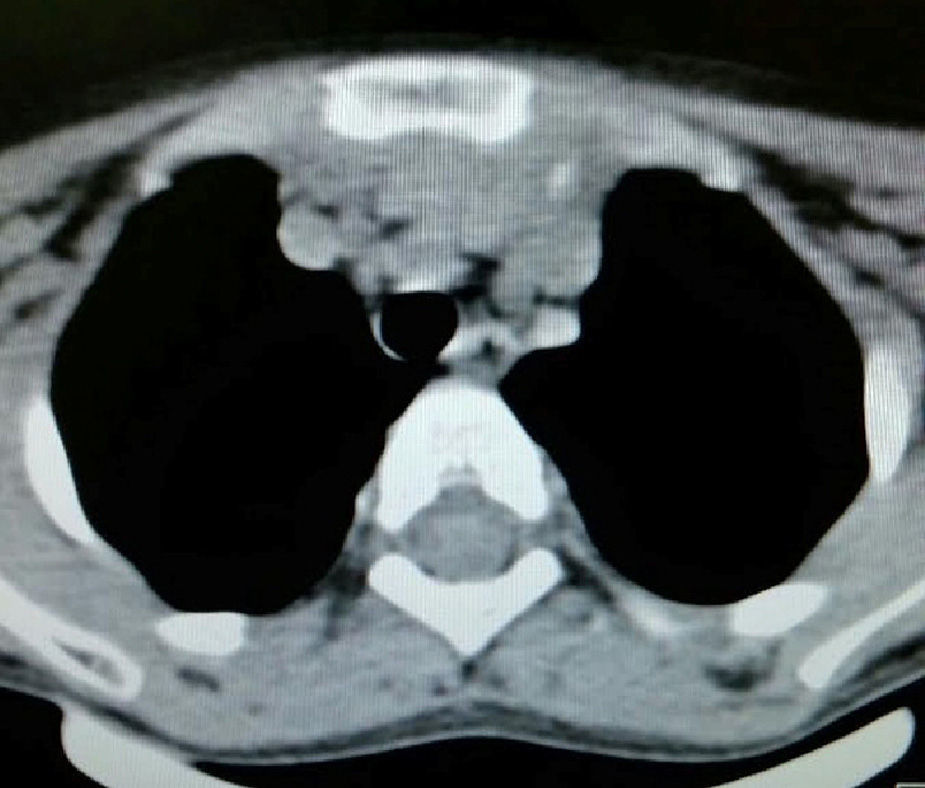
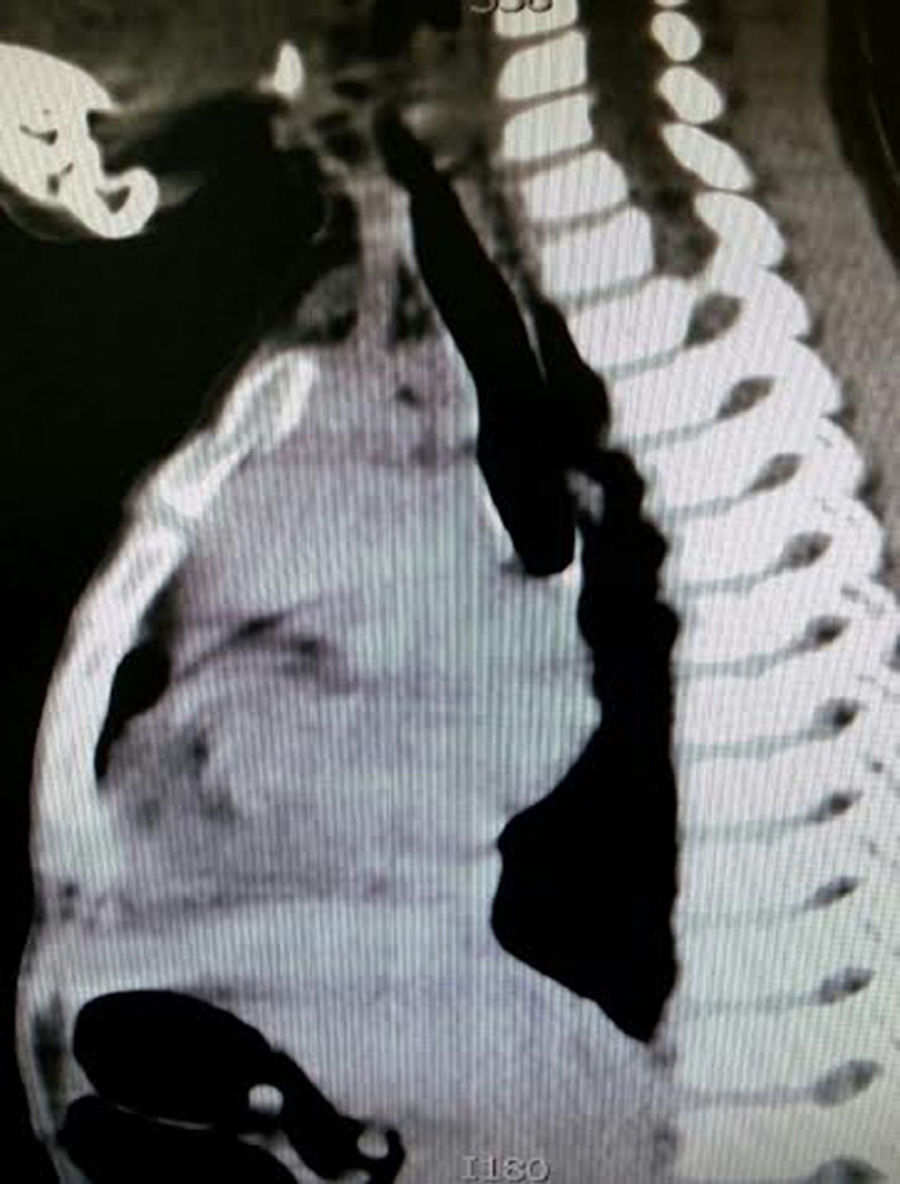
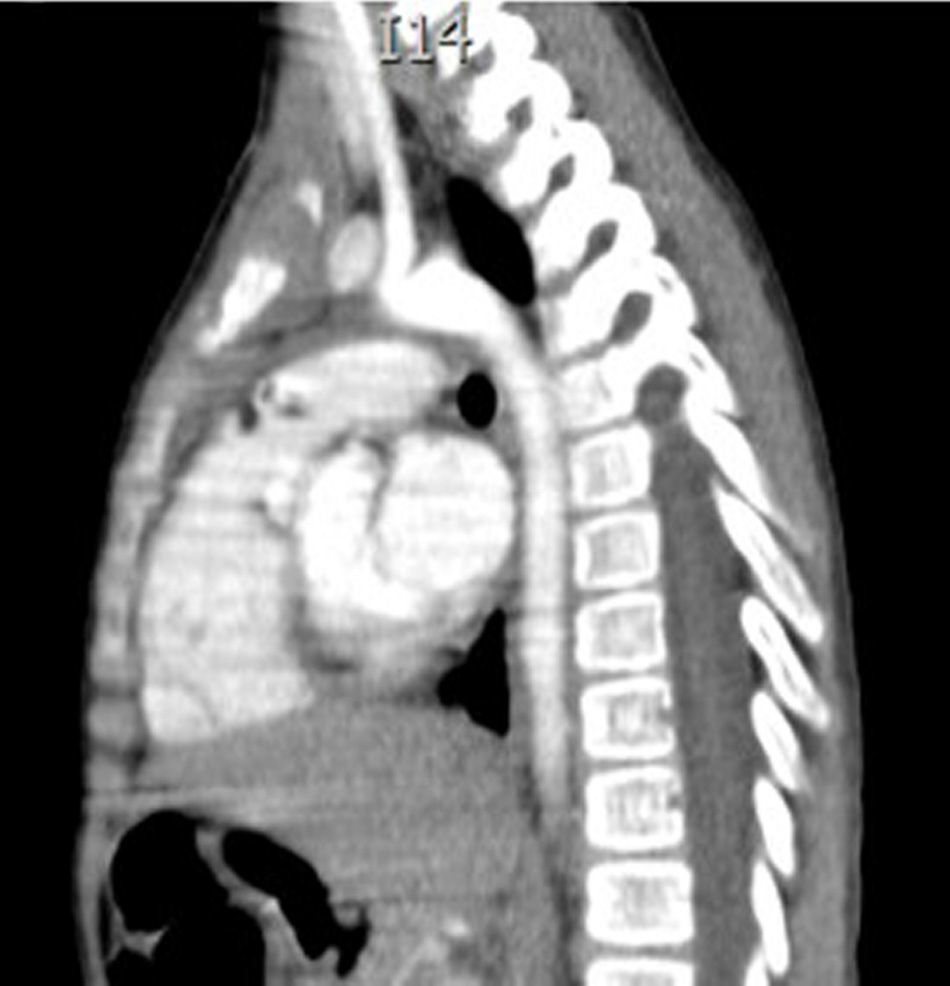
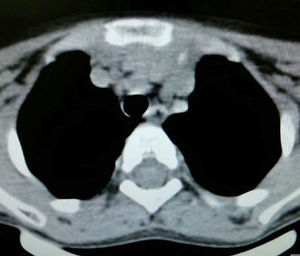
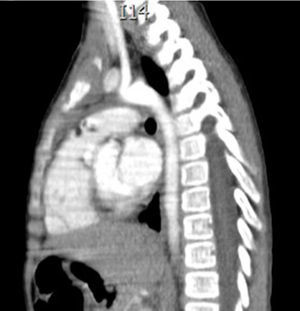
Clinical caseThis is the case of a 4-year-old female child, the mother's second child, and who had a normal pregnancy, and normal delivery at 39 weeks. APGAR was 8/9. The child weighed 3.100g at birth, development was normal and she was sent for consultation due to an increase in neck volume at the age of 2 months. There was no dysphnoea, dysphagia, cyanosis, paleness, fever, cough, weight loss or neuromuscular changes. On examination the patient was found to be eutrophic, with a heart beat of 100/min, breathing rate of 28/min, a temperature of 36.2°C, saturation at a mean of 97%, weight of 19kg, with no dyspnoea or dysphonia, with correct skin colouring, well hydrated, with no hyperaemia of the larynx. Her neck had an increased volume of approximately 1.5–2cm in supra sternal cavity area I, which was soft, pain free, not moveable, and had a smooth surface, of renitent consistency, with no changes of skin tone, no movement on swallowing. Her thyroid glands were without palpable mass, were mobile and pain free. Adenomegalies were soft, approximately 5–7mm in bilateral III–IV jugular lymph node chain, were pain free, smooth in surface, mobile, and not attached to any deep tissues. Her chest had no deformations, air entered and exited successfully, and there were no crackling or wheezing, no heart murmurs, tone and intensity were good with no additional puffing. The abdomen was soft, easily depressed, was pain free and without visceromegalias. Peristalsis was normal and active. Limbs were intact, with immediate capillary refill, and no cyanosis. Laboratory tests reported: leukocytes of 5600/μl, haemoglobin of 14.6g/dl, platelets of 324,000/μl, 47.6% of total reactive lymphocytes, 31% total neutrophils, alkaline phosphatise raised to 250UI/l (40–150), normal transaminase, amylase of 92U/l, lipase 19/l, cholesterol 169mg/dl, triglycerides 68mg/dl, uric acid 3.5mg/dl, normal coagulation ranges, total bilirubin 0.1mg/dl, albumin of 3.1g/dl, glucose 96mg/dl, ureic nitrogen of 10mg/dl, creatinine of 0.46mg/dl, calcium of 9.1mg/dl, sodium of 140mmol/l, potassium of 4mmol/l, chlorine of 110mmol/l, phosphate of 4.3mg/dl, magnesium of 2.4mg/dl, a normal general urine test, stimulating folic hormone of 13.5mlU/ml, luteinizing hormone of 0.36mlU/ml, prolactin of 25ng/ml, carcinoembryonic antigen of 2.1ng/ml all tested normal. Normal thyroid profile with T3T 15.7ng/dl, TSH 2.61μU/ml, T4L 0.97/ml, eosinophils in nasal mucosa tested negative, immunoglobulin G 887mg/dl (681–1648), immunoglobulin A 117mg/dl (84–484), immunoglobulin M 285mg/dl (48–312), immunoglobulin E 18.34mg/dl (under 60), negative co-parasitoscopic tests. Negative nasal and laryngeal exudation were found. Neck ultrasound reported a simple left thyroid cyst. Neck tomography showed a rounded, heterogenous tumour of 1cm in supra sternal cavity, and an irregular, heterogenous mass in the superior mediastinum, with partial compression of mediastinal structures, and stage II, III and VII stage cervical adenomegaly, approximately 0.8cm in size with no pleural effusion or pleural or parenchymal involvement (Figs. 1 and 2). Surgery was performed with general anaesthesia, in the Rosier position. A transverse cervical incision was made. The rounded thinly encapsulated tumour was located at the pre-thyroid supra sterna region. It was yellow in colour, and extended towards the anterior mediastinum surrounding the aorta and cava superior. Upper median sternotomy was therefore performed, and the normal thyroid glands and multiple adenomegalies at levels IV, V and VI were located. The tumour was resectioned and ganglionic emptying was performed at mediastinal levels IV, V and VI and cervical levels II and III approximately. A 19 Fr mediastinal probe was inserted and we proceeded with Penrose type drainage in the neck. Vicryl suture closure was of the sternotomy was carried out.
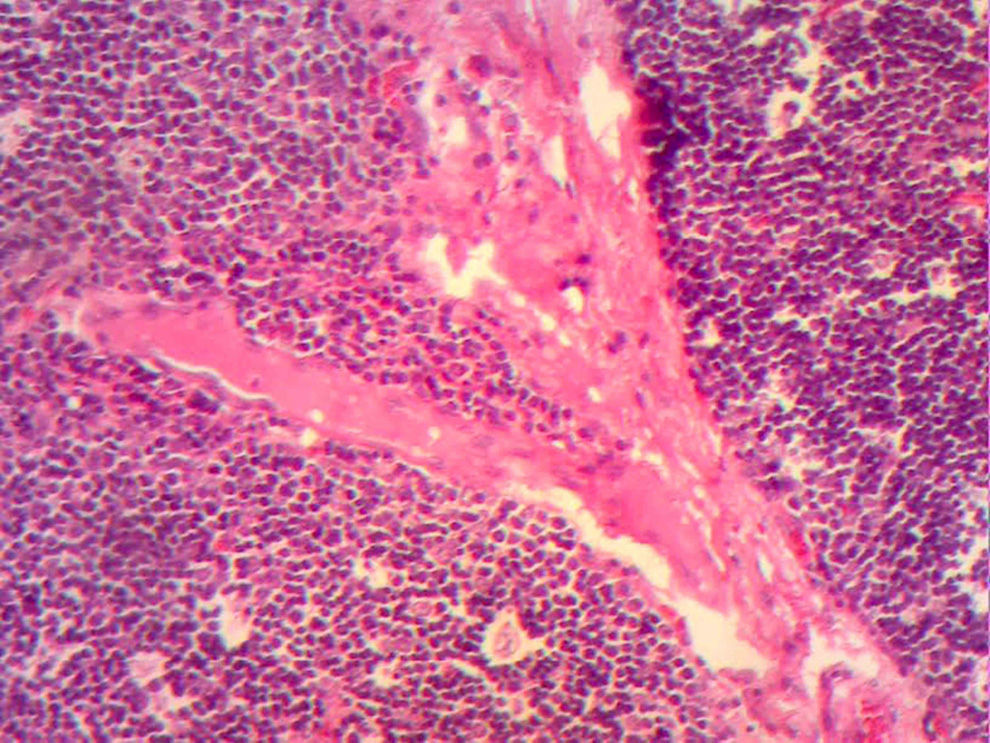
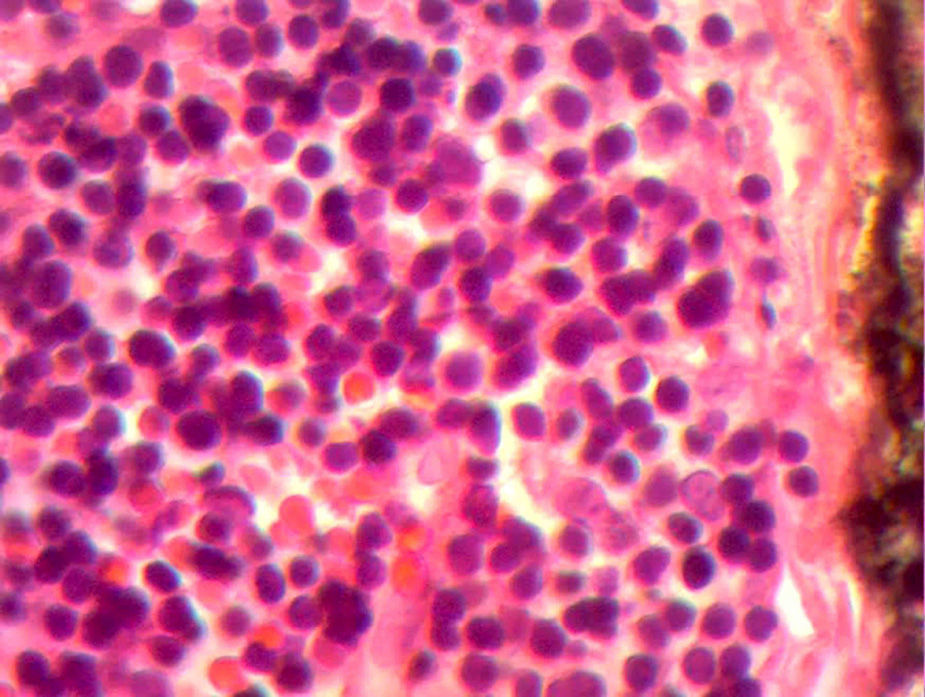
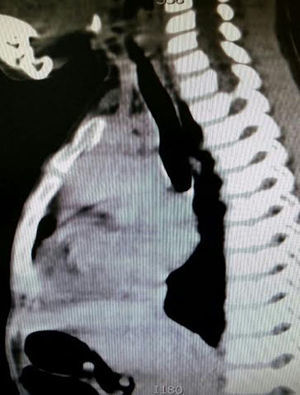
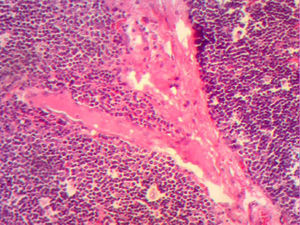
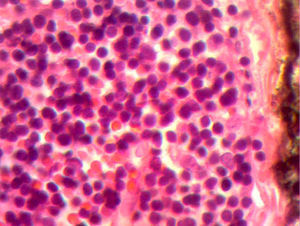
The control CAT scan showed correct lung expansion, with no effusion. The Penrose drainage was removed on day two. On day three the mediastinal probe was removed and the patient was discharged from the hospital. Histopathologic findings showed: inclusion cyst with queratin, neck and mediastinal lymph nodes with no neoplastic cells but with lymphoid hyperplasia. Lymphocyte thymoma was B1 on the WHO or Masaoka I classification scale (Figs. 3 and 4); findings were sent for assessment by the paediatric oncology department which considered that no coadjuvant management was required. The patient was asymptomatic 4 months after surgery, with no signs of miastenia gravis. The control scan showed the neck with no adenomegaly, the thyroid gland as normal, central mediastinum without mass, large vessels with no lesions, pulmonary parenchymas with no masses, and no pleural effusion (Fig. 5).
Histological image with HE staining where the loss of normal structure is noted, with no medullary residues and with a network of large neoplastic cells, with large vesicular nuclei, and prominent nucleoli in the network of vessels and septa, and with scattered immature lymphocytes around the blood vessels.
Thymomas are rare even in adults. In children the medical approach takes into account the anatomic site of the mediastinum and the patient's age, since those of neurogenic origin are more frequently found in children under 3 and those of lymphatic origin in school age children and teenagers2–4; they may be associated with miastenia gravis or not, although it is known that approximately 15–25% of patients with miastenia have thymoma and that of patients with malignant or benign thymic growth, 35–50% will develop miastenia gravis.6 Miastenia gravis is a disease of the motor nerve, characterised by fatigue and muscular weakness, its base is auto immune with the presence of anti-bodies against the acetylcholine receptors. It was first described in 1672 by Thomas Willis, but classified by Osserman into the childhood stage, neonatal, congenital, young and young adult under 40 stages and sub-classified into: stage I, ocular uni- or bilateral; stage IIa generalised; stage IIb, generalised and bulbous; stage III severe respiratory; and stage IV chronic respiratory, with stages IIa, IIb and III being those that benefit from thymectomy.2,4,6 It is 6 times more common in women. Apart from miastenia, in addition to thymomas and thymic carcinomas (Masaoka IV, WHO C) pure anaemia of red blood cells may present, as may hypogammaglobulinaemia, rheumatoid arthritis, systemic erythematosus lupus, pemphigus, polymyositis, Hashimoto thyroiditis, and nephrotic syndrome.5 In our study we presented the case of a 4 year old patient, classified as thymoma type B1 Masaoka I, with no symptoms associated with the tumour and only raised reactive lymphocytes. Diagnosis of the mediastinal tumour was made from a scan finding2,4,5 and confirmed by the histopathologic report. The miastenia up until that time has not developed, but considering the past history and the fact this was a female patient, it could be expected to present in the future.1,3,4,6 The thymoma must be considered as a malignant tumour.1,2,7–10 and disuse of the term benign thymoma, used for Masaoka type I tumours, is now recommended.
Thanks to the advance of surgical techniques, approaches and chemotherapy treatments2–4,6–10 for cancer management in children there are more favourable results regarding survival. The treatment of choice will depend on the histopathological report and the spread of the tumour. Surgery is initially chosen, except in the case of germinal and lymphatic tumours7,8 where radiotherapy is the treatment of choice, once diagnosis has been made. Ideal surgery is R0 (total) resection. In the case of R1 and R2 surgery must be accompanied by radio and/or chemotherapy.1,2,4,5 Chemotherapy for this type of tumour is based on cisplatin, cyclophosphamide, steroids and adriamycin. A 85–100% survival rate of 5 years has been reported in Masaoka I tumours, 70–90% in Masaoka type II tumours, 59–75% in Masaoka type IIIa tumours and a 40% and 10% rate in Masaoka IVa and IVb type tumours respectively.3,4,7,8,10 When the tumour is mediastinal, and particularly anterior mediastinal, it is essential to carry out a preoperative anaesthetic assessment since the tumour itself may be compressing the airway and vascular structures, which would pose a challenge for intubation. Furthermore, even if the patient does not present with pre-surgical compression, once the patient is relaxed, severe compression may occur and with it intubation complications.1,2
ConclusionsAlthough thymic tumours are rare in children, when a lower cervical tumour or anterior mediastinal tumour presents, the possibility of a mediastinal tumour should be considered even in the absence of associated symptoms, as this may avoid delay in diagnosis and thereby its treatment. When the tumour presents without symptoms this may be associated with a benign tumour. However, there are no studies which specify this and it is known that, as with any tumour, prognosis will depend on the stage at which the tumour is found, the degree of surgical resection, the degree of infiltration and lymph node metastasis, haematological changes and the presence or absence of miastenia gravis.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Nolasco-de la Rosa AL, Mosiñoz-Montes R, Nuñez-Trenado LA, Román-Guzmán E, Chávez-Villicaña CE, Naranjo-Hernández G. Timoma en edad pediátrica. Reporte de un caso y revisión de la bibliografía. Cir Cir. 2016;84:324–328.