Primary breast lymphomas, a rare subtype of non-Hodgkin's lymphoma, represent 0.04–0.5% of all breast cancers, 0.38–0.7% of all lymphomas, and 1.7–2.2% of extranodal lymphomas. The treatment choice is based on chemotherapy containing anthracycline and rituximab. Surgery is limited to being less invasive and only for diagnostic purposes. Radiotherapy has an important role as consolidation therapy, particularly in patients with negative nodes.
Clinical caseA 70 year old woman with a breast nodule in the left upper outer quadrant, with slow growth, expansive, painless, and accompanied by skin changes, malaise, weight loss, fatigue, chill, and sweating. There was tissue replacement by the mammary gland tumour, skin changes due to invasion, and a 5cm axillary lymphadenopathy. The mammography showed skin thickening and a dense pattern of 80% of breast tissue replacement, and the lymphadenopathy with loss of radiolucent centre and soft tissue invasion. The biopsy confirmed a diffuse high grade large cell lymphoma. She received an rituximab (R-CHOP) chemotherapy scheme and radiotherapy with tangential and supraclavicular and axillary fields. After completing the chemotherapy, the patient is on follow-up, and at 15 months she is alive without disease activity.
ConclusionsPrimary lymphoma of the breast is a rare entity. Multimodal treatment with combined chemo-radiotherapy is the cornerstone. Surgery is reserved only for diagnostic purposes.
Los linfomas primarios de la glándula mamaria son un raro subtipo de linfoma no Hodgkin que representan del 0.04 al 0.5% de los tumores malignos mamarios, del 0.38 al 0.7% de todos los linfomas y del 1.7 al 2.2% de los linfomas extranodales. El tratamiento de elección está basado en la quimioterapia que contenga antraciclinas y rituximab. La cirugía está limitada a ser lo menos invasiva, y únicamente con propósitos diagnósticos; la radioterapia tiene un importante rol como terapia de consolidación, particularmente en pacientes con ganglios negativos.
Caso clínicoMujer de 70 años con un nódulo mamario izquierdo en el cuadrante superoexterno de crecimiento lento, expansivo, indoloro, acompañado de cambios cutáneos, con ataque al estado general, pérdida de peso, fatiga, calosfrío y diaforesis. Tiene sustitución tumoral de la glándula mamaria, con cambios por invasión en la piel; axila con adenopatía de 5 cm. En la mastografía se observa engrosamiento de la piel y un patrón denso que sustituye el 80% del tejido mamario, así como adenopatías con pérdida del centro radiolúcido e invasión en tejidos blandos. Una biopsia corrobora linfoma no Hodgkin difuso de células grandes de alto grado. Recibe quimioterapia con esquema tipo Rituximab (R-CHOP) y radioterapia con campos tangenciales y axilo/supraclaviculares. Una vez finalizado el tratamiento, la paciente queda en vigilancia médica por el Servicio de Oncología y, a 15 meses, se encuentra viva sin actividad de la enfermedad.
ConclusionesLos linfomas primarios de la glándula mamaria son una entidad rara. El tratamiento suele ser multimodal, donde la quimiorradioterapia combinada es la piedra angular; la cirugía está reservada con fines de diagnóstico.
Although primary lymphomas of the mammary gland are rare, they are a well-defined subtype of non-Hodgkin's lymphoma and represent from 0.04% to 0.5% of malign tumours of the mammary gland, from 1.7% to 2.2% of extranodal non-Hodgkin's lymphomas and from 0.38% to 0.7% of all non-Hodgkin's lymphomas.1–3
The term “mammary gland lymphoma” refers to primary lymphomas in the mammary gland, in the absence of other previously detected lymphoma locations.1
Wiseman and Liao4 established 4 diagnostic criteria for this entity in 1972. These consist, firstly, of appropriate pathological evaluation of the essential disease in diagnosis; secondly, in the association of nearby mammary tissue with lymphoma infiltration in the mammary gland; thirdly, there is the criterion of exclusion, which corresponds to patients with concurrent disseminated disease or those with a previous diagnosis of extra-mammary lymphoma; finally, the fourth criterion refers to the invasion of homolateral ganglia, which is considered acceptable on condition that both lesions developed simultaneously.
The treatment of choice after diagnosis and staging should be based on the use of chemotherapy with regimes containing anthracyclines and rituximab (R-CHOP). Surgery is restricted to being the least invasive, and it has the sole purpose of diagnosis. Radiotherapy plays an important role as a consolidation therapy, particularly in patients with negative ganglia.1–3
The case we present is that of a patient with non-Hodgkin's lymphoma of the left mammary gland. It was managed with biopsy of the lesion to confirm the disease and chemotherapy based on the monoclonal antibody rituximab together with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) for 8 cycles, followed by radiotherapy of the mammary gland and the axillary/supraclavicular field, as well as maintenance rituximab during 12 months; after 15 months of follow-up the patient is alive and without evidence of active disease.
Clinical caseA 70 year-old woman referred to the Oncology Department of Hospital de Ginecopediatría No. 48 of the Instituto Mexicano del Seguro Social, with the diagnosis of cancer of the left breast.
She is diabetic and in treatment with oral hypoglycemics; she also has hypertension, for which she takes enalapril.
The process commenced 4 months before she visited her doctor, when she detected mammary nodule in her left breast. The initial lesion was located in the superoexternal quadrant of the left mammary gland, and growth was slow, expansive and painless; it was accompanied by local cutaneous changes and malaise, with weight loss, fatigue, chill and sweating. She therefore visited her Family Medicine Unit, where the diagnosis was mastitis and antibiotic treatment was prescribed. As there was no improvement in the mammary lesion or the cutaneous lesions she was referred to the Oncology Department of Hospital de Ginecopediatría No. 48.
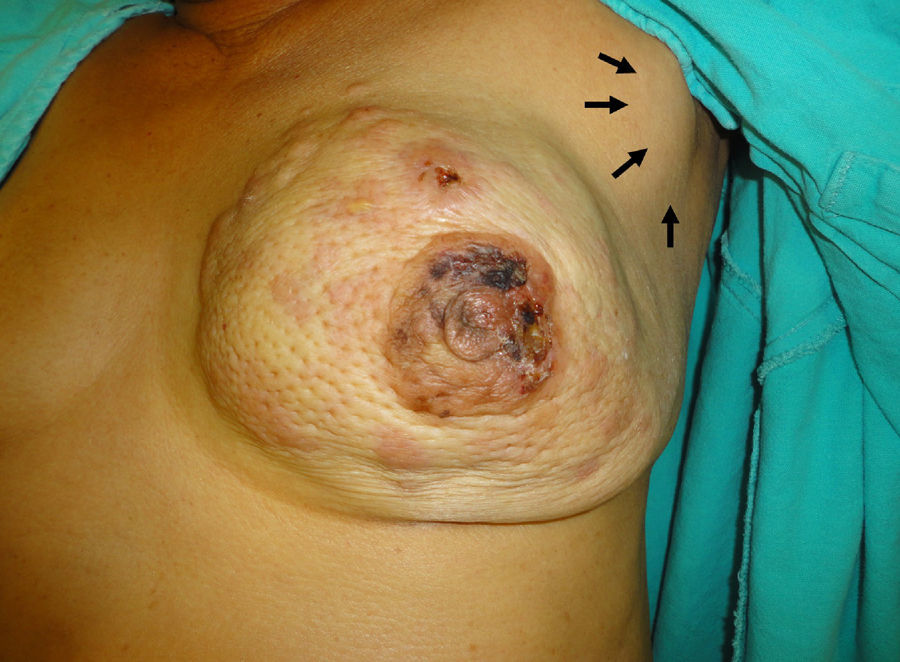
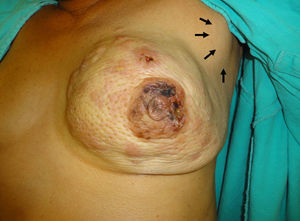
She presents with asymmetry due to tumour substitution of the left mammary gland, which has increased in volume with a tumour that includes all 4 quadrants, unattached to the pectoral muscle; cutaneous changes due to invasion, orange skin, cutaneous metastasis, retraction of the nipple- areola complex with invasion, areas of ischaemia in the surface of the areola, at expression of the nipple no material at all is expressed. The ipsilateral axilla has a conglomerate 5cm in diameter in level II, mobile. Supraclavicular without cervical adenopathies, the rest of the examination without relevant changes (Fig. 1).
Clinical image of the left mammary gland clearly showing asymmetry due to tumour substitution with increase in volume in all 4 quadrants, cutaneous changes due to invasion, orange skin, skin metastasis, retraction of the areola-nipple with invasion of areas of ischaemia in the surface of the areola, while a ganglion conglomerate can be seen in the axilla (arrows).
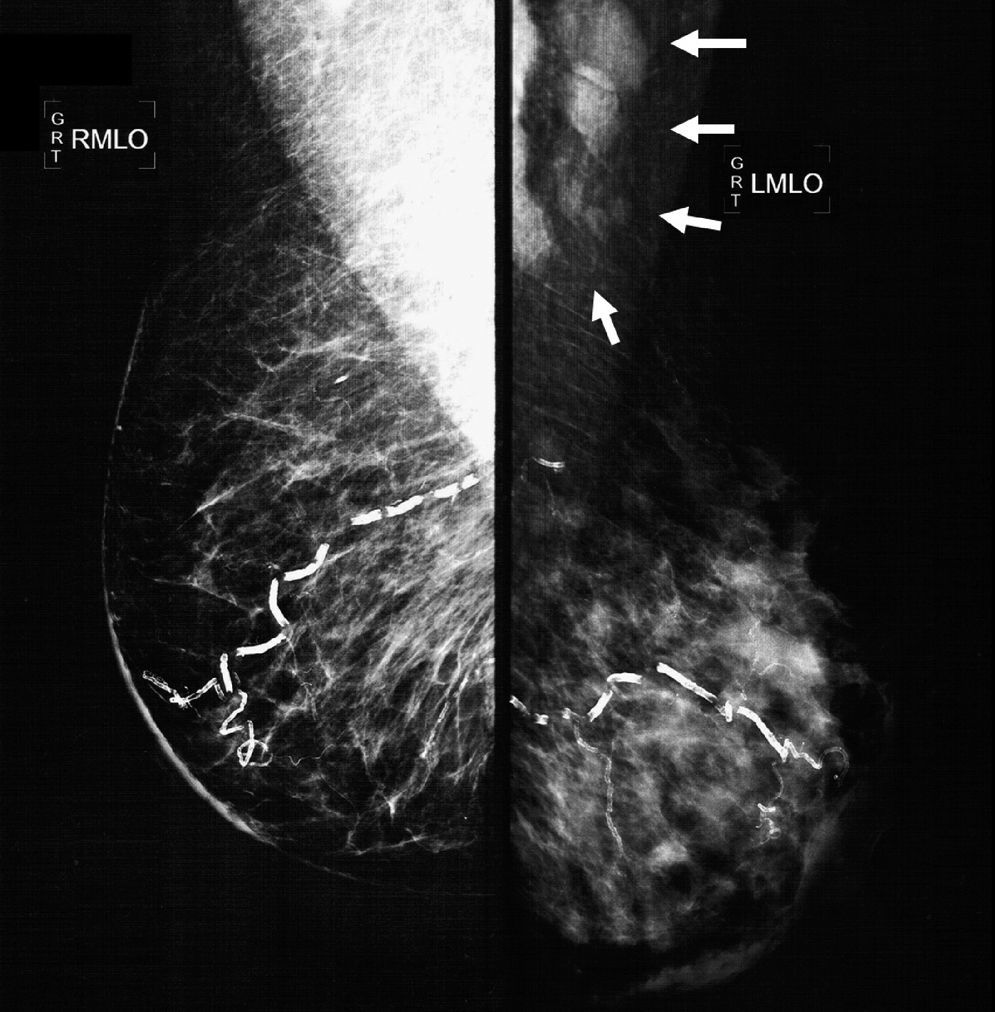
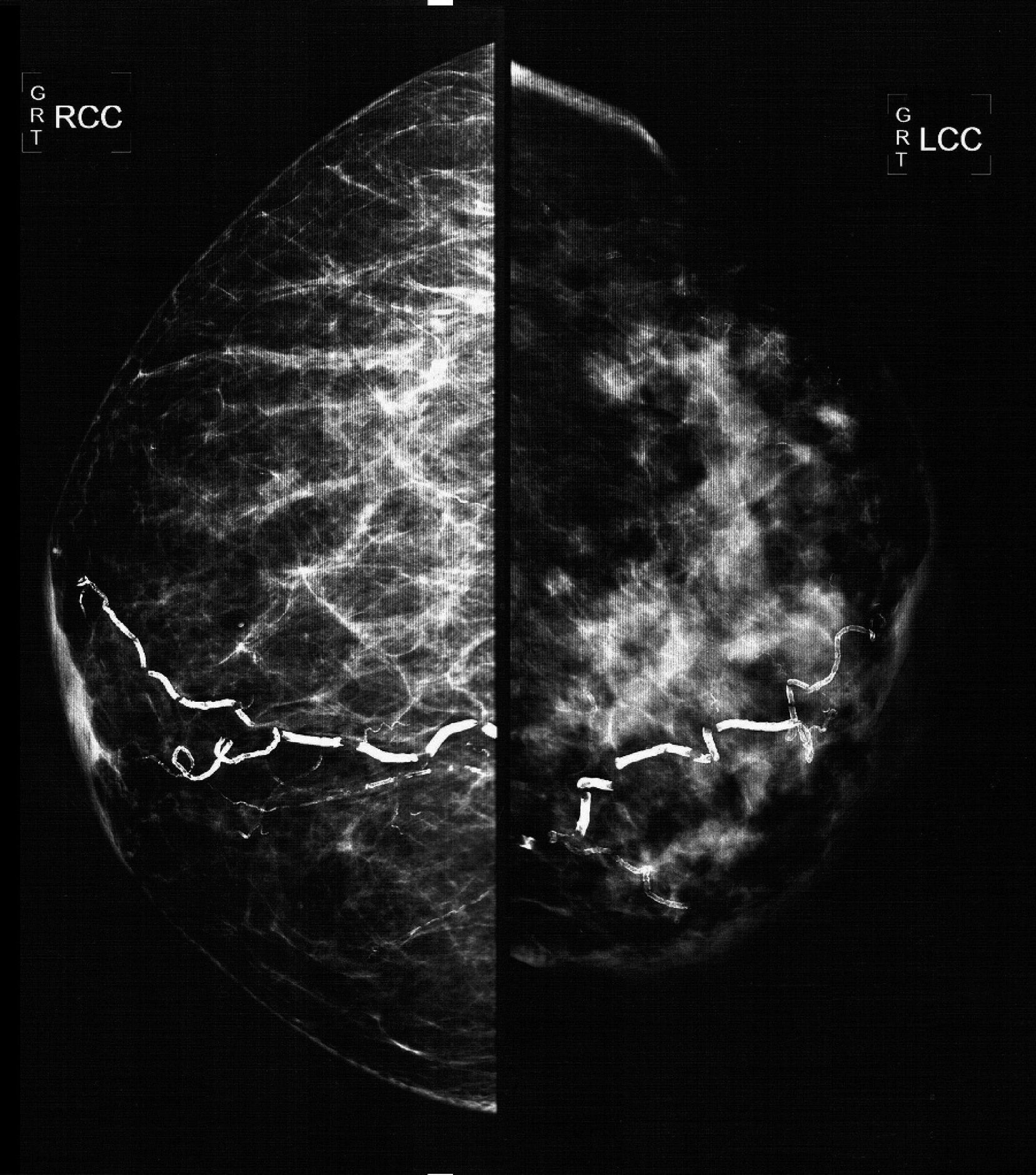
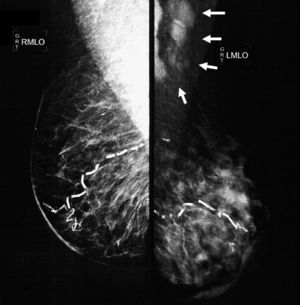
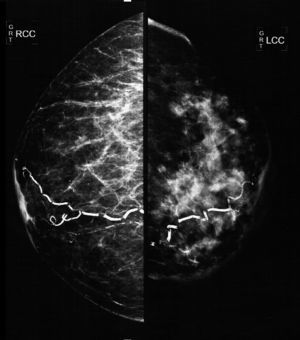
The mammographic study revealed: diffuse thickening of the skin of the left mammary gland, with a dense pattern that substituted 80% of the mammary tissue and extended in an irregular way to the pre- and retromammary fatty layer; the vascular trajectories are partially calcified. Multiple dense adenopathies are identified in the axillary regions, with loss of the radiolucid centre and invasion of the adjacent soft tissues (Figs. 2 and 3).
Oblique-lateral mammography showing diffuse thickening of the skin of the left mammary gland, a dense pattern that substitutes 80% of the breast tissue and extends irregularly to the pre- and retromammary fat layers. The vascular trajectories are partially calcified. In the left axillary region multiple dense adenopathies can be seen with loss of the radiolucid centre and invasion of the adjacent soft tissues (arrows).
Complementary ultrasound scan confirmed the diffuse thickening of the skin; the glandular tissue showed solid and irregular areas that were vascularised concentrically in lines A and B, which were interconnected by areas of diffuse infiltration. The axilla contained multiple adenopathies of up to 3.3cm, with loss of the fatty hilum.
The clinical diagnosis of probable breast cancer was established. Due to the local extension of the lesion it was staged as cT4b vs. cT4d because of the presence of skin metastasis or lymphatic infiltration in the skin of the mammary gland, and cN2 due to an ipsilateral axillary conglomerate, clinical stage IIIB. A biopsy was taken from the mammary gland with a skin core to document skin infiltration. At this moment tumour extension studies were requested; the histopathological report on the biopsy corroborated the infiltration by a diffuse large cell non-Hodgkin's lymphoma with a high histological grade.
The immunohistochemical profile of the lesion gave the result of CD20 positive, CD10 positive, BCL-2 positive, Ki-67 positive in 100% of tumour cells, CD5, cyclins and MUM-1 negative. A diffuse non-Hodgkin's lymphoma of immunophenoype B was confirmed, with a 100% proliferation index.
Computerised thoracic-abdominal-pelvic tomography reported no tumour activity in other organs apart from the mammary lesion and axillary adenopathy. Simple X-ray of the thorax is normal. Lactic dehydrogenase is almost double the normal value, at 1146U, beta-2 microglobulin is normal at 3.34mg; the other laboratory parameters are normal. Bone marrow aspirate shows no infiltration whatsoever.
This was staged as stage IIE non-Hodgkin's lymphoma, due to the local involvement of the mammary gland as well as the ipsilateral axillary infiltration.
She was given a total of 8 cycles of R-CHOP induction chemotherapy, one cycle every 3 weeks. At the end of this treatment she was given rituximab as single-drug maintenance therapy, with one cycle every 8 weeks during 12 months. At the end of the R-CHOP treatment she was given 50.4Gy radiotherapy with tangential axillary/supraclavicular fields in 25 fractions.
After the second treatment cycle the cutaneous changes in the mammary gland reduced, as did the axillary adenopathy: levels of lactic dehydrogenase in serum returned to their normal value.
A follow-up mammographic study at 6 months showed changes associated with mammary fibrosis, with no evidence of disease activity.
Once treatment had ended the patient remains under surveillance: at 15 months she is alive and free of disease activity.
DiscussionPrimary lymphomas of the mammary gland are unusual entities that share clinical and radiological similarities which commonly appear in breast cancer.
The diagnostic criteria for primary lymphoma of the mammary gland were established by Wiseman and Liao4 in 1972. They were subsequently revised by Hugh et al.5 in 1990, and consist of an appropriate pathological evaluation of the disease; in the close association of mammary tissue with the lyphomatose infiltrate in the mammary gland; in the absence of concurrent disseminated disease, although homolateral invasion of the ganglia is considered to be acceptable on condition that both lesions developed simultaneously. Hugh et al.5 considered those patients in which the mammary gland was the site where the disease presented or where it was the most voluminous to fulfil the criteria, even when in extension studies invasion of the ganglia at a distance was found (stage III) or when there was invasion of the bone marrow. Their criteria also exclude all those patients with a previous diagnosis of extramammary lymphoma.
The clinical signs of non-Hodgkin's lymphoma are varied, and the symptom most frequently associated with the disease is a palpable mass. This is most commonly located towards the superoexternal quadrant of the mammary gland, which may or may not be painful, This manifestation is present in 60.7% of cases, while adenopathies are present in 25% of patients, breast pain is present in 11.9% and local inflammation is present in 10.7%.1,3,6 Other less frequent manifestations are retraction of the nipple or discharge from it, retraction of the skin, erythema and orange skin appearance.3,6
Due to the rarity of non-Hodgkin lymphomas of the mammary gland, signs of the disease may be indistinguishable from those present in patients with breast cancer, so that the diagnosis of the entity usually occurs when a biopsy is taken.3
The imaging evaluation of mammary gland lymphoma may be undertaken by mammography, mammary ultrasound scan, nuclear magnetic resonance or positron emission tomography (PET-CT).
The mammographic changes associated with this entity include the infiltration of lymphomatous deposits in the form of diffuse increased opacities or generalised asymmetries in 16%, and dense mammographic patterns which may occur in 56% of patients. It is possible to identify mammary nodules in 76% of cases; these may be solitary (72%) or multiple (3%) while 72% have indistinct margins, and 56% are not calcified and lobular.7 Up to 28% of them may be bilateral, and ipsilateral axillary metastases may appear in more than 40% of patients; tumour size usually varies from 1 to 5cm.6–8
In study using ultrasound scan, a tumour mass is visible in 90% of cases, while in 10% it is possible to see distortion of breast architecture. The most common finding is the existence of solitary irregular masses. This image is seen in almost half of patients, while margins are indistinct in el 59%, and up to 64% of cases present no subsequent acoustic phenomena. Tumours are predominantly hypoechoic in 59% of cases, mixed hypo- and hyperechoic in 23% of cases and pseudocystic in 18%. When echo-Doppler is used, vascularity is found to be increased in 64% of cases and avascular lesions appear in only 9%, while in 32% of cases there may be ipsilateral axillary adenopathies.6–8
Nuclear magnetic resonance imaging of the mammary gland in the evaluation of primary lymphoma shows large lobular heterogeneously hypointense masses in T1 images and heterogeneously hyperintense ones in T2 with an intense, heterogeneous and swift enhancement and kinetic washing that is typical of malignity.6,8
The recent use of PET-CT has shown intense lesions with increased metabolic activity in from 92% to almost 100% of cases, with a standardised uptake of 16-fluorodeoxyglucose of 10.6. PET-CT is used for diagnosis as well as for patient follow-up, given that this study evaluates the response to treatment, showing residual tumour activity or areas of necrosis or fibrosis.7,8 PET-CT may also be used in women with the suspicion of lymphoma in dense breasts that may darken the mammography image.8 Inflammatory breast cancer and mammary gland metastasis, mainly melanoma, may hinder the differentiation of haematological neoplasias; likewise, lesions smaller than 1cm may give false negatives in PET-CT.8
Histopathological study is essential in primary lymphoma of the mammary gland for diagnosis as well as to establish the treatment and prognosis of the disease.
The most common type of lymphoma that presents in the mammary gland is the diffuse large cell non-Hodgkin's type, which appears in 40% to 70% of cases. Lymphomas associated with mucus membranes usually account for from 0% to 44% of the series.3,9 Young pregnant women usually have Burkitt-type lymphoma. Additionally, cases have been reported of other types of lymphoma, such as follicular, lymphoblastic, lymphoplasmatic, peripheral T-cell lymphoma and true histiocytic lymphoma;3 anaplastic large cell lymphoma is a T-cell lymphoma subtype, which is a rare variety of mammary lymphoma that presents in women with mammary implants.10,11
Mammary gland lymphoma treatment is usually multi-modal, and chemotherapy is the cornerstone here.
Several authors1,3,9,12–15 have defined the role of surgery in this condition. It is usually reserved for diagnostic purposes, solely to obtain a sufficient amount of material to establish the histological diagnosis of the disease. Some authors found surgery to be an adverse prognostic factor, given that it leads to a delay in starting chemotherapy. This reduces disease-free time and increases the relapse rate.3,9,12,15
The optimum treatment for extranodal lymphomas involves the use of chemotherapy. CHOP with or without rituximab is the therapy most widely used for neoplasias of this type.
The only prospective study of mammary gland lymphomas, by Avilés et al.,16 included 96 patients with non-Hodgkin's lymphoma divided into 3 groups according to treatment; the first group was of patients with radiotherapy as the sole treatment. These 30 patients were given 45Gy in 20 fractions with tangential axillary/supraclavicular fields; the second group was composed of 32 patients who were only given chemotherapy, in the form of 6 cycles of CHOP, and the third group composed of 34 patients were treated in multi-modal form. They were given the same dose of radiotherapy and the same cycles of chemotherapy as the previous groups. Complete response was obtained in 66% of the patients in the radiotherapy group, in 59% of the chemotherapy group and in 88% of the patients in the combined therapy group, with a p value of 0.01. The relapse rate was 50% in the radiotherapy group, 43% in the chemotherapy group and only 19% in the combined therapy group. In univariate analysis at 10 years, event-free survival was 50% in the radiotherapy group, 56% in the chemotherapy group and 83% in the combined therapy group. Likewise, overall survival at 10 years was 50% for the radiotherapy and chemotherapy groups and 76% for the combined treatment group. Both results are statistically significant and in favour of the combined treatment group, without benefiting the other two treatment groups. The most common relapse site was the central nervous system, in 9.4% of patients, independently of their treatment group. This finding indicates that the need to use prophylactic radiotherapy for the central nervous system should be evaluated.
Another important retrospective revision by the International Extranodal Lymphoma Study Group9 of 204 eligible patients found in multivariate analysis that the prognostic factors associated with overall survival of diffuse large-cell lymphoma are associated with the international prognostic index (IPI), the use of anthracycline-based chemotherapy and radiotherapy. Progression-free survival is influenced by the IPI and the use of anthracycline-based chemotherapy. For specific cause survival the only statistically significant factor was the IPI. There was a negative association in all of the results measured depending on the extent of the surgery, and the patients treated by radical mastectomy had the worst prognosis for specific cause survival. Factors such as B symptoms, tumour diameter, ganglia invasion, bilaterality and treatment time were not statistically significant. Overall survival for patients who were given anthracyclines was 73% at 5 years and 58% at 10 years; the combination with radiotherapy was the one that gave the best results.
ConclusionsPrimary non-Hodgkin's lymphomas of the mammary gland are a rare entity. The clinical and radiological manifestations of this disease share similarities with breast cancer as well as with other benign diseases that may affect the mammary gland. Confirmation of the disease requires histopathological evaluation as well as immunohistochemical studies. Treatment is similar to that of other extranodal lymphomas and their nodal counterpart. Combined treatment with chemotherapy with or without rituximab and radiotherapy optimise local control, reducing the local and systemic relapse rate of the disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Villalón-López JS, Souto-del Bosque R, Méndez-Sashida PG. Linfoma no Hodgkin primario de la glándula mamaria. Reporte de un caso. Cir Cir. 2017;85:70–75.