Mullite has many excellent properties which can produce refractories with high quality. The fast transformation of andalusite into mullite and silica is around 1653K. In this work, quenching method was employed to study the effect of Y2O3 additive on the transformation of andalusite into mullite. The experiment was carried out in a closed tube furnace at 1553K and 1573K. The transformation of andalusite into mullite was studied thermodynamically. Thermodynamic calculation reveals that the temperature of spontaneous mullitization of andalusite was 1180K. Additional Y2O3 of 3.75wt% was added into different sizes of andalusite powders. Some Y2O3 was found to be reacted with SiO2 and formed as Y2Si2O7 phase. The mullite contents after fired at both 1523K and 1573K for 2h have improved markedly when the andalusite powders were with small sizes (d50=4μm and d50=43μm) but little affection on the mullitization of andalusite with large size (d50=143μm) was found. Moreover, with the addition of Y2O3, the andalusite (d50=4μm) was completely transformed into mullite after fired at 1573K for 2h. The mullite content in the sample of small size after fired at 1573K went up by more than 20%. The viscosity value of generated liquid phase is major determinants of transformation of andalusite into mullite.
La mullita posee excelentes propiedades para producir materiales refractarios de alta calidad. La rápida transformación de andalucita en mullita y sílice es de 1.653K, aproximadamente. En este trabajo se empleó el método de enfriamiento brusco para estudiar el efecto del aditivo Y2O3 en la transformación de andalucita en mullita. El experimento se llevó a cabo en un horno de tubo cerrado a 1.553 y 1.573K. Se estudió termodinámicamente la transformación de andalucita en mullita. El cálculo termodinámico revela que la temperatura de la mullitización espontánea de la andalucita era 1.180K. Se añadió el 3,75% en peso de Y2O3 adicional a diferentes tamaños de polvos de andalucita. Se observó alguna reacción de Y2O3 con SiO2, que se formó como fase Y2Si2O7. El contenido de mullita después de cocer tanto a 1.523K como a 1.573K durante 2h mejoró notablemente cuando los polvos de andalucita eran de pequeño tamaño (d50=4μm y d50=43μm), pero se halló poca atracción en la mullitización de la andalucita de gran tamaño (d50=143μm). Además, con la adición de Y2O3, la andalucita (d50=4μm) se transformó completamente en mullita después de cocerla a 1.573K durante 2h. El contenido de mullita en la muestra de pequeño tamaño después de cocerla a 1.573K aumentó más del 20%. El valor de viscosidad de la fase líquida generada es el principal determinante de la transformación de andalucita en mullita.
As a member of the alumino-silicate minerals, andalusite (Al2O3·SiO2) can be applied in the production of commercial refractories with high content of mullite. Mullite is a solid solution that the Al2O3 to SiO2 mole ratio is in the range of 3:2 to 2:1. The mullite with the composition of 3Al2O3·2SiO2 has many excellent properties: high refractoriness, low thermal expansion, thermal conductivity, good chemical stability, and outstanding mechanical properties at high temperatures. Those properties make mullite meet the requirement of the production of high-quality refractories [1–4]. During heating, mullite and silica are formed by the decomposition of andalusite [5–7]. However, fast transformation of andalusite into mullite only happens at temperature above 1653K [7]. It takes a long time to get enough mullite phases from andalusite when the firing temperature is below 1653K. Therefore, it is essentially important to lower the firing temperature and accelerate the transformation progress.
The transformation of andalusite into mullite has been studied for many years. Most of the researches are based on the kinetic consideration. Two stages were found with different firing temperatures [8]. The mullite phases are restricted to energetically favored lattice sites at temperature below 1653K and the mullite phases grow over the whole body of andalusite phase at temperature above 1653K. Bouchetou discovered that the complete mullitization would be more easily achieved in a fine particle than in a large particle and the impurities in the natural andalusite promotes the ion transport and accelerate the mullitization of andalusite [7]. Some chemicals [5,6,9,10] had attempted to improve the transformation rate. Al2O3, CaO, MgO, TiO2 and AlF3 are the chemicals which can be capable of lowering the mullitization temperature and accelerating the transformation rate. On the other hand, the doping of ZrO2 has adverse effect on the transformation of andalusite into mullite [9]. Although Al2O3 can increase the amount of mullite after firing, Liao suggested that more widespread secondary mullitization and greater expansion would be detected in the experimental sample when the quantity of added alumina reaches to about 15wt% [6].
It is known that the presence of liquid phase can promote the ion transport, especially the liquid with low viscosity value. Doping of most rare earth oxides reduces the melting temperature and lowers the viscosity of soda–lime–silicate glass [11]. As a common rare earth oxide, many researchers study about the effect of Y2O3 on the properties of different glass and the researches revealed that doping appropriate amount of Y2O3 is an effective way of lowering the viscosity of silica-rich melts.
This study focuses on the transformation of andalusite into mullite by addition of rare earth oxide Y2O3. By freezing the whole system (the sample along with crucile could be quenched), the effect of additive Y2O3 on the transformation rate of andalusite into mullite and the transformation mechanism could be revealed successfully. The transformation mechanism is discussed thermodynamically and kinetically. The Gibbs energy of the reaction was calculated in this paper.
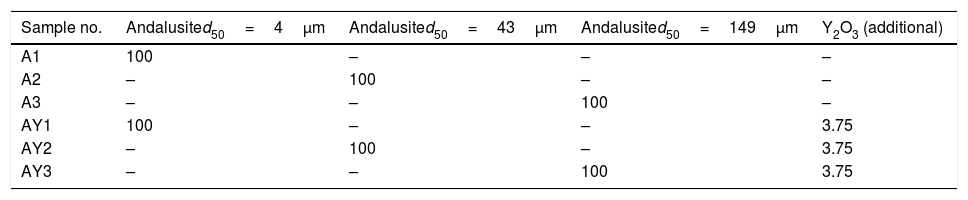
ExperimentalMaterials preparation and quenching methodAndalusite powders of three different sizes, named as A1–A3, were supplied by a commercial supplier. The minerals and Y2O3 powders (Sinopharm Chemical Reagent Co., chemically pure analytical reagent) were dried at 373K for 24h before experiment in order to remove the moisture.
Firstly, different amount of Y2O3 was added to sample to determine best content of Y2O3 in the mixture. The results showed that the addition of Y2O3 should not be more than 3.75wt%. And then, to study the effect of Y2O3 on the mullitization of andalusite, the powders of andalusite and Y2O3 were mixed in an agate mortar in predetermined ratio for the sample preparation.
Finally, three samples were prepared, namely AY1–AY3. Approximately 2g of the mixture was packed into an alumina crucible (inner diameter 9mm and inner depth 18mm). On the other hand, the pure andalusite powders, namely A1–A3, weighting about 2g, were prepared for parallel comparison study.
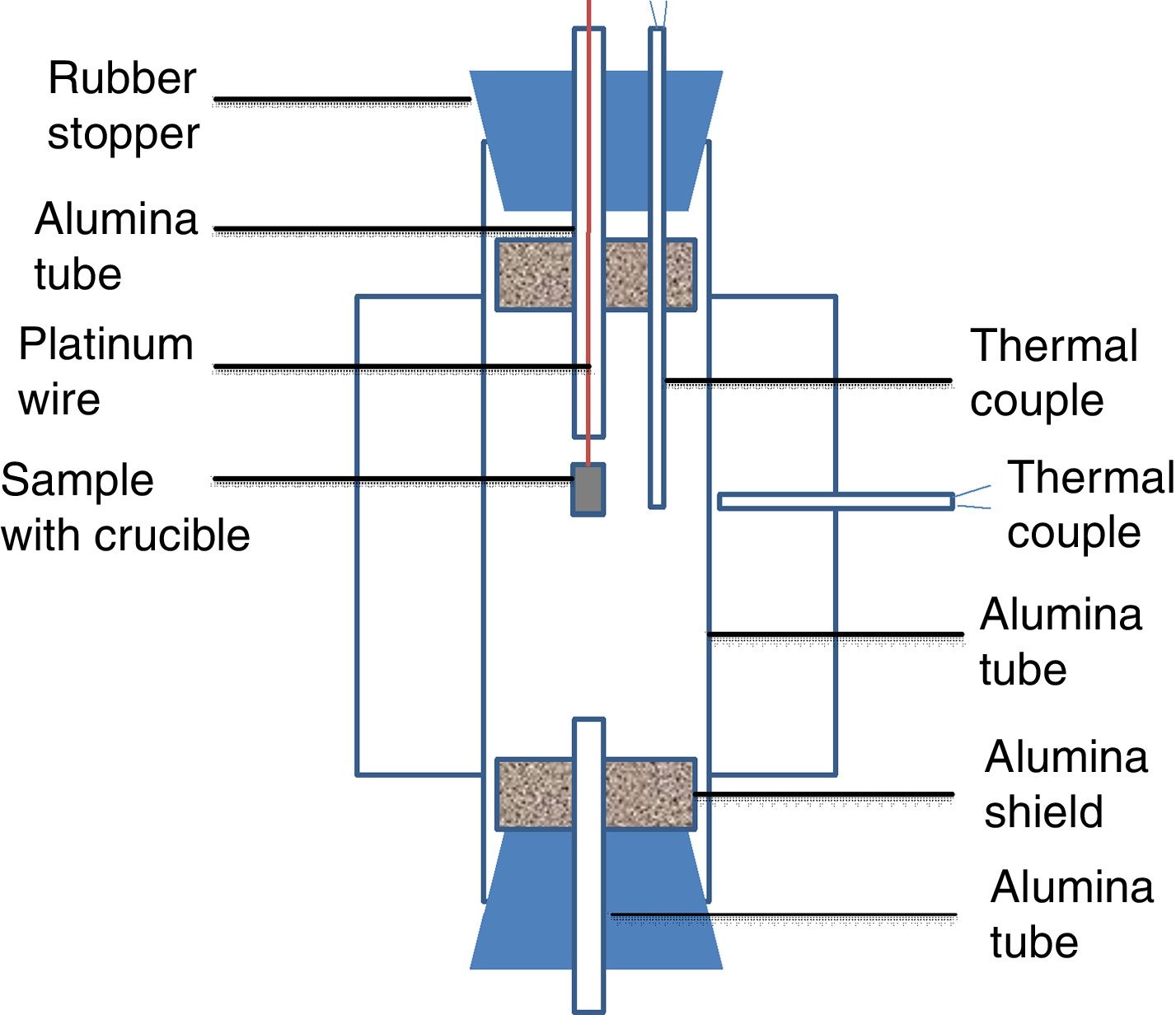
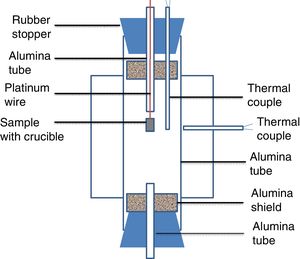
Experimental setup in this study was shown in Fig. 1. A vertical tube furnace with MoSi2 heating elements was employed. The alumina tube was introduced as the reaction chamber. The alumina tube in the furnace was closed by two rubber stoppers. Two radiation shields were placed at both ends to protect the rubber stoppers from the radiation. Two small holes were drilled in the rubber stopper of the upper part. The small alumina tube was gone through a hole and the thermal couple was placed in another hole. The sample along with alumina crucible was hung on a platinum wire in a small alumina tube. The small alumina tube could keep the wire straight and allowed the crucible drop smoothly under the gravity. The wire was clamped by a clamp. A B-type thermocouple was placed just beside the crucible to read the real temperature of the sample. A small alumina tube was placed in the rubber stopper at the lower part of the reaction chamber to keep the pressure in the tube constant. To protect alumina tube in the furnace from thermal shock, the heating rate of 3K/min was set. The sample was hold at their desired temperature for 2h. Thereafter, the sample with crucible was dropped into cold water for quenching.
Sample analysisTo determine the chemical compositions and size distribution of andalusite powders, three different andalusite powders were analyzed by X-ray fluorescence (XRF) analyzer (Axios-Advanced, PANalytical B.V., Netherlands) and laser particle size analyzer (Mastersizer 2000, Britain) separately before experiment. The results of chemical compositions are listed in Table 1 and batch formula along with particle size of different samples is given in Table 2. In general, many impurities such as Na2O, K2O, CaO, MgO and Fe2O3 are associated with andalusite according to the geographical reason. Iron oxide is usually in solid solution in andalusite. The samples were crushed into powders after quenching and divided into two parts. A portion was subjected to X-ray diffraction (XRD) (Rigaku Rint D/max-III, Japan) to identify the phases of those samples and then the rest powders were analyzed by scanning electron microscopy (SEM) (JSM-5610LV, Japan) after Au–Pd coating. The chemical compositions of different phases were semi-quantified by EDS which is attached to SEM.
The content of mullite phase in all samples was analyzed by XRD. Chrome trioxide (Cr2O3) as internal standard was used to quantify mullite phase (3Al2O3·2SiO2, known 3:2 mullite). The phase compositions of sintered samples were measured by an X-ray diffractometer (XRD) (Model D/Max-Ra, Rigaku., Japan) with the following conditions: Cu Kα radiation, and operating voltage of 40kV, operating current of 30mA. The mass ratio of mullite in the sample is calculated through quantitative phase analysis by using the Rietveld method which is conducted by the Whole Pattern Fitting function of Jade. Two main effects are considered here to reduce the residual error.
- 1.
The sample should be grinded into fine powders, because the massive pieces in the sample reduce the preferred orientation.
- 2.
To ensure the accuracy and precision of Rietveld analysis, the scanning parameters of the XRD measurement should be set adequately, since the range of 2θ values, step size and counting time have an obvious effect on the quality of the obtained data.
In this case, the samples were measured from 5° to 80° (2θ) by XRD analysis. A step size is controlled as 0.02° and a counting time is 2.5s per step.
It must be noticed that the andalusite content in the raw material is 80.53wt% according to the calculation of the chemical composition listed in Table 1. On the other hand, only solid phases were revealed by the XRD patterns. The XRD patterns did not consider the presence of amorphous phase. The content of mullite in the sample after completely transformation must be less than 100wt% consequently. The content of mullite in the sample after fully transformation was calculated as 70.58wt% according to the decomposition reaction.
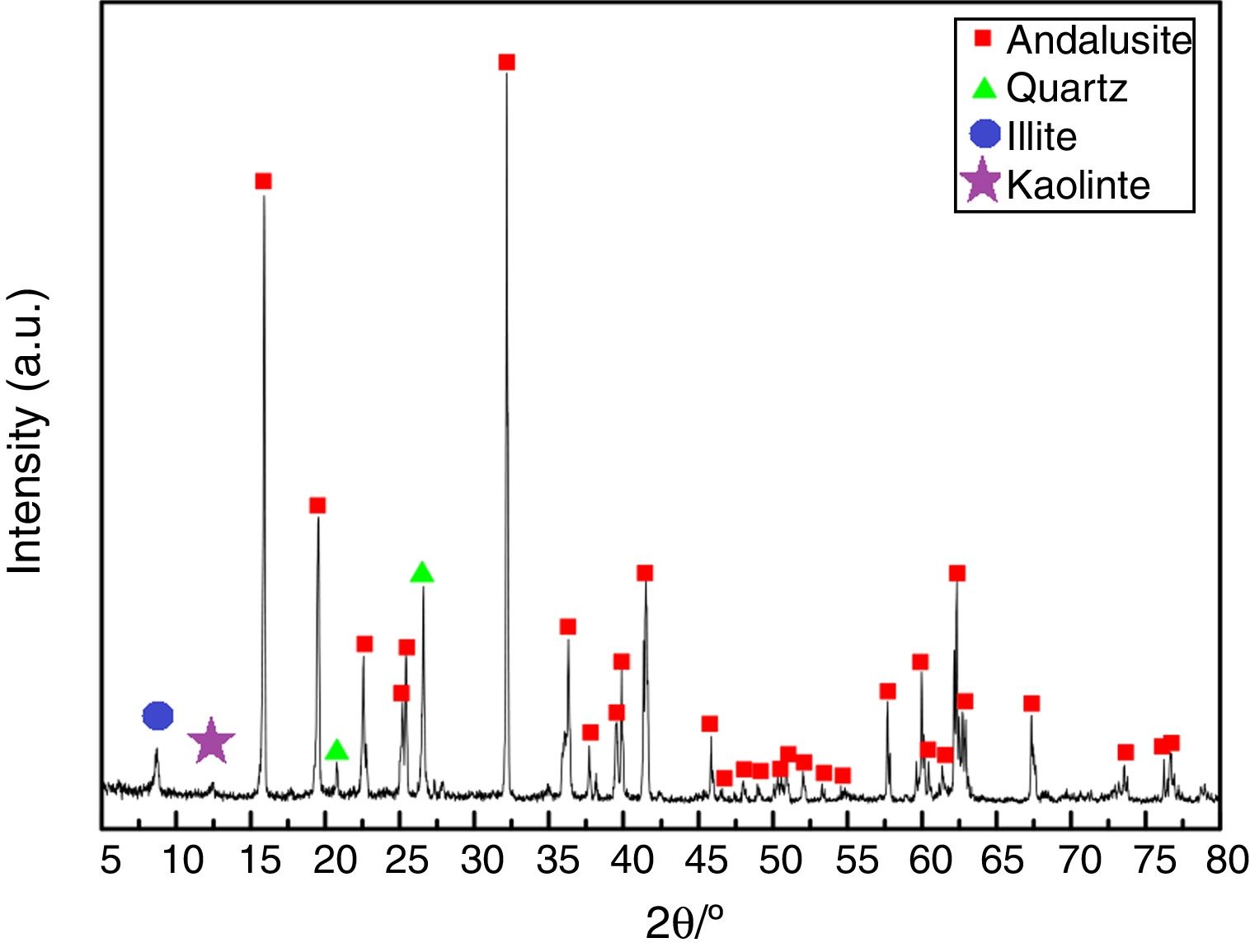
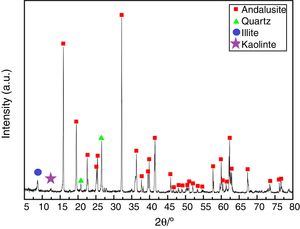
ResultsAll three andalusite samples are identified by X-ray diffraction and they are found to be with the same phases. Fig. 2 presents the X-ray diffraction patterns obtained from the raw materials. Andalusite sample in this study contains andalusite, quartz, kaolinite and illite as crystalline phases. Quartz and andalusite are clearly identified by the X-ray patterns. Illite and kaolinite are very hard to identify because the intensity of those peaks are really weak. However, kaolinite and illite are the well-known minerals associated to andalusite as minor impurities according to the geographical reason, thus, peaks identified at 2θ=12.5° and 2θ=8.8° are the main diffractions of kaolinite and illite.
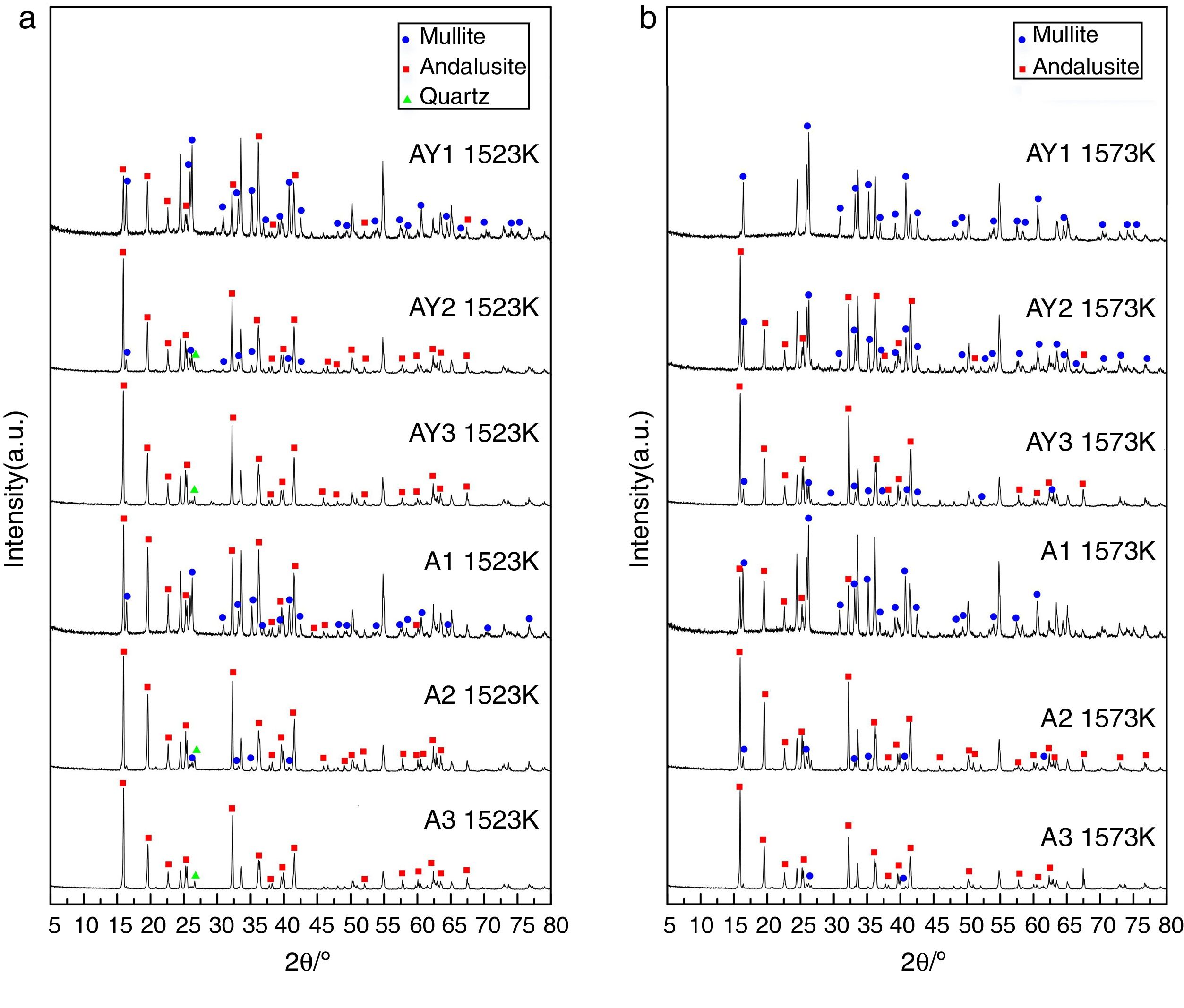
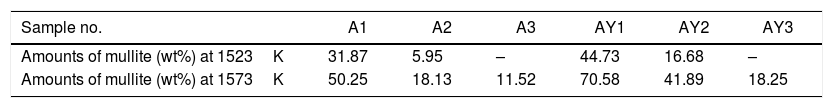
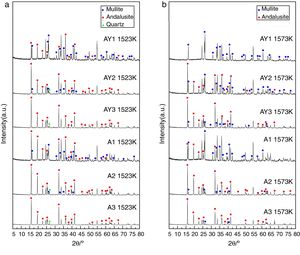
The transformation of andalusite into mullite is closely related to the size of andalusite powders and the soaking temperature. Fig. 3 presents the X-ray diffraction patterns obtained from different samples after fired at 1523K and 1573K separately. As shown in Fig. 3(a) and (b), the mullitization of andalusite is found to be activated with temperature increasing but retarded with growth of the powder size of andalusite. Table 3 is the content of mullite in different samples after experiment. With the addition of Y2O3, the mullite content of sample AY1 and sample AY2 after fired at 1523K and 1573K goes up by more than 25wt%, compared with sample A1 and A2. The results reveal that the addition of Y2O3 into sample with small size (d50=4μm and d50=43μm) strongly improves the transformation efficiency of andalusite into mullite. Even sample fired at lower temperature, the transformation of andalusite into mullite is found to be efficient. However, the content of mullite in the sample with large size does not change significantly.
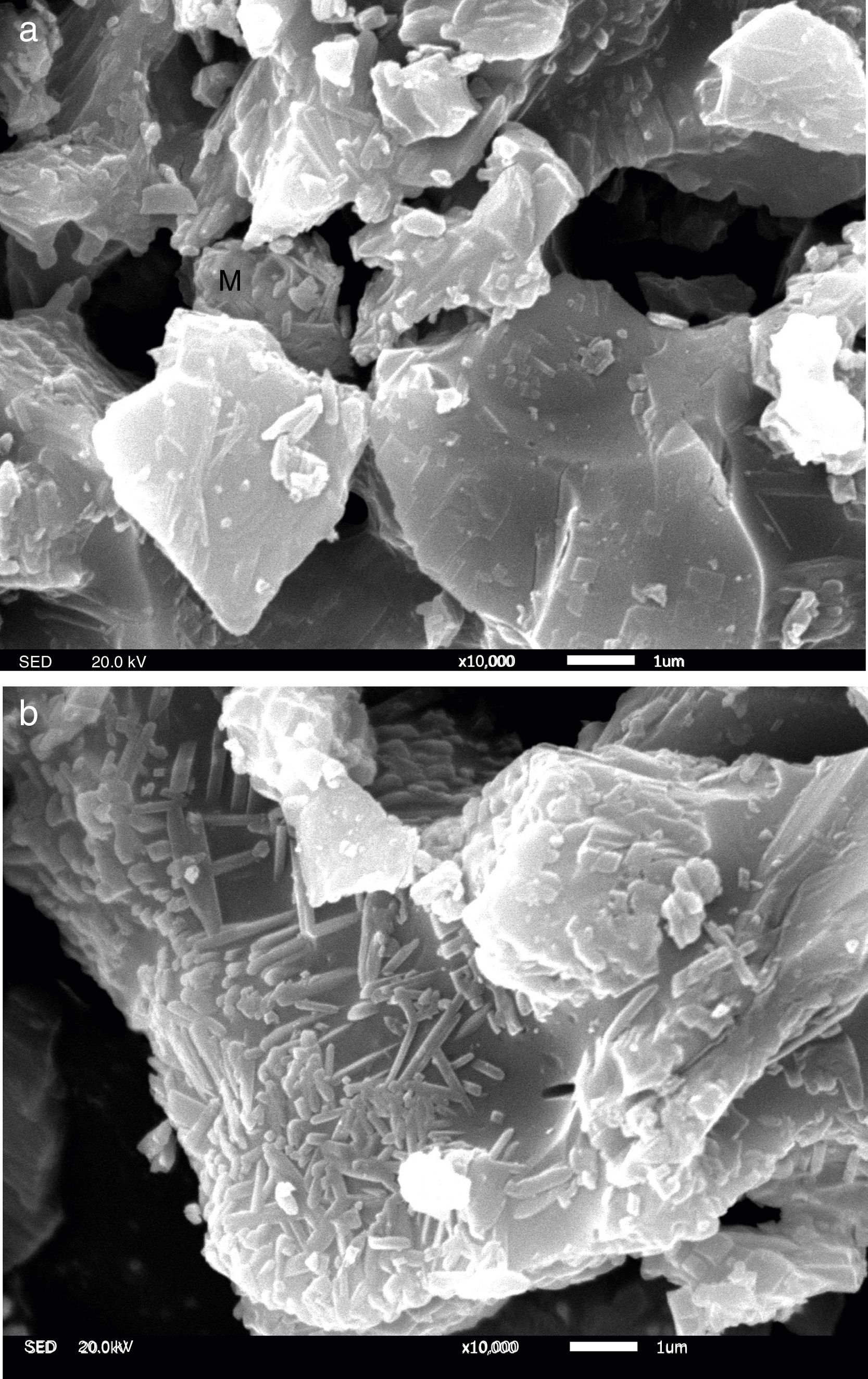
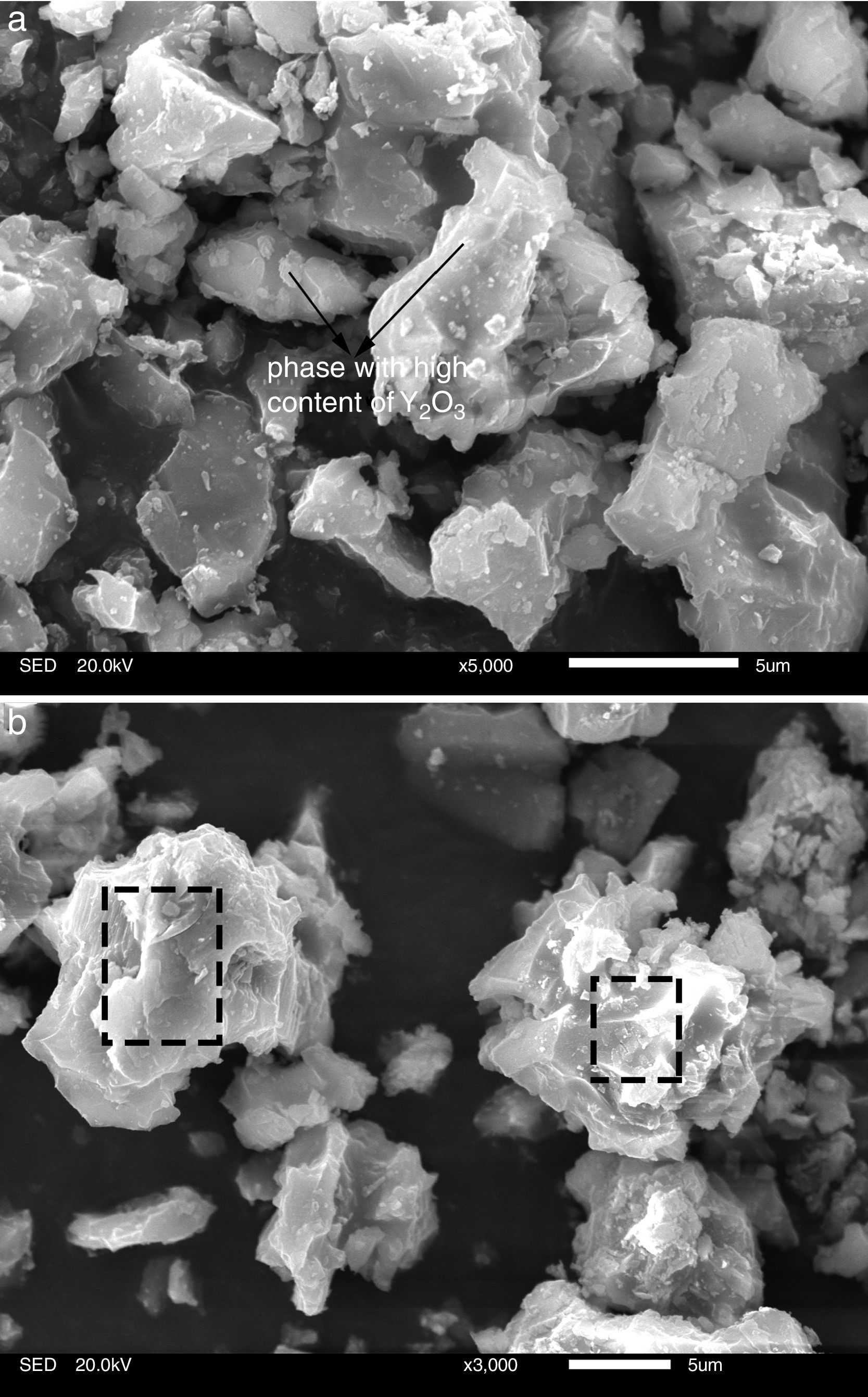
To gain an insight into the effect of Y2O3 on the mullitization of andalusite, the SEM is employed to reveal transformation mechanism. A part of sample A1 and AY1 after fired at 1573K were etched in 5wt% HF solution for 60s to characterize the microstructure of those samples and the other part of those two samples were subjected for EDS analysis without etching.
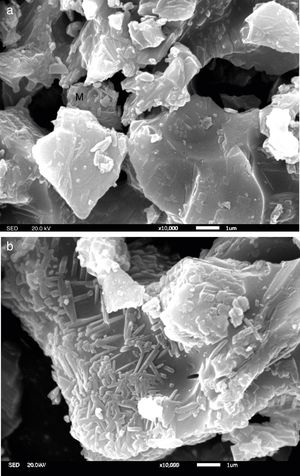
Fig. 4 presents the microstructures of sample A1 and AY1 respectively. As shown in Fig. 4(a), without the addition of Y2O3, a few mullite phases can be found but not many. On the other hand, Fig. 4(b) presents that mullite is clearly found on the granule surface and the growth of mullite is in the same direction.
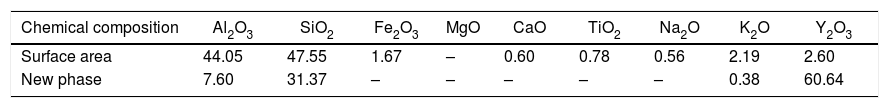
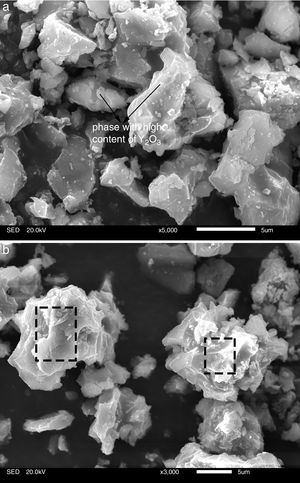
Fig. 5 presents SEM microphotographs of different regions of sample AY1 after fired at 1573K without etching. Fig. 5(a) and (b) focuses on the new phase and the chemical composition of the surface area respectively. Because XRD analysis is very hard to find out the element with low content (less than 5wt%) in the sample, EDS analysis is used to figure out the trace of element Y. The white phase in Fig. 5(a) is found to be with high content of Y2O3 and the area analysis of chemical composition of Fig. 5(b) shows that small amount of Y2O3 also exists in the surface area. The results of EDS analysis of Fig. 5(a) and (b) are listed in Table 4. EDS analysis indicates that Y2O3 and other oxides formed as a glassy phase. At the same time, the following reaction (1) happens:
The results of EDS analysis of Fig.5(a) and (b).
| Chemical composition | Al2O3 | SiO2 | Fe2O3 | MgO | CaO | TiO2 | Na2O | K2O | Y2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Surface area | 44.05 | 47.55 | 1.67 | – | 0.60 | 0.78 | 0.56 | 2.19 | 2.60 |
| New phase | 7.60 | 31.37 | – | – | – | – | – | 0.38 | 60.64 |
The transformation of andalusite into mullite is studied thermodynamically. The definite integration form (2) is employed to calculate the Gibbs energy of reaction (3):
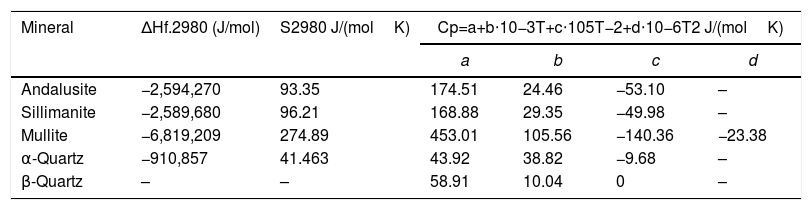
where ΔrGmθ is the standard reaction Gibbs free energy (J/mol), ΔrHmθ is the standard reaction enthalpy (J/mol), ΔrSmθ is the standard reaction entropy (J/(molK)) and T is the temperature in Kelvins (K).The standard Gibbs energy of formation, standard formation enthalpy and entropy of different minerals are listed in Table 5[12–15]. The approximation used for standard heat capacity is of the term (4):
Thermodynamic data of different minerals.
| Mineral | ΔHf.2980 (J/mol) | S2980 J/(molK) | Cp=a+b⋅10−3T+c⋅105T−2+d⋅10−6T2 J/(molK) | |||
|---|---|---|---|---|---|---|
| a | b | c | d | |||
| Andalusite | −2,594,270 | 93.35 | 174.51 | 24.46 | −53.10 | – |
| Sillimanite | −2,589,680 | 96.21 | 168.88 | 29.35 | −49.98 | – |
| Mullite | −6,819,209 | 274.89 | 453.01 | 105.56 | −140.36 | −23.38 |
| α-Quartz | −910,857 | 41.463 | 43.92 | 38.82 | −9.68 | – |
| β-Quartz | – | – | 58.91 | 10.04 | 0 | – |
The values of a, b, c and d of different mineral in Eq. (4) are given in Table 5.
In the calculation, the reaction enthalpy, entropy and heat capacity are obtained according to Eq. (5).
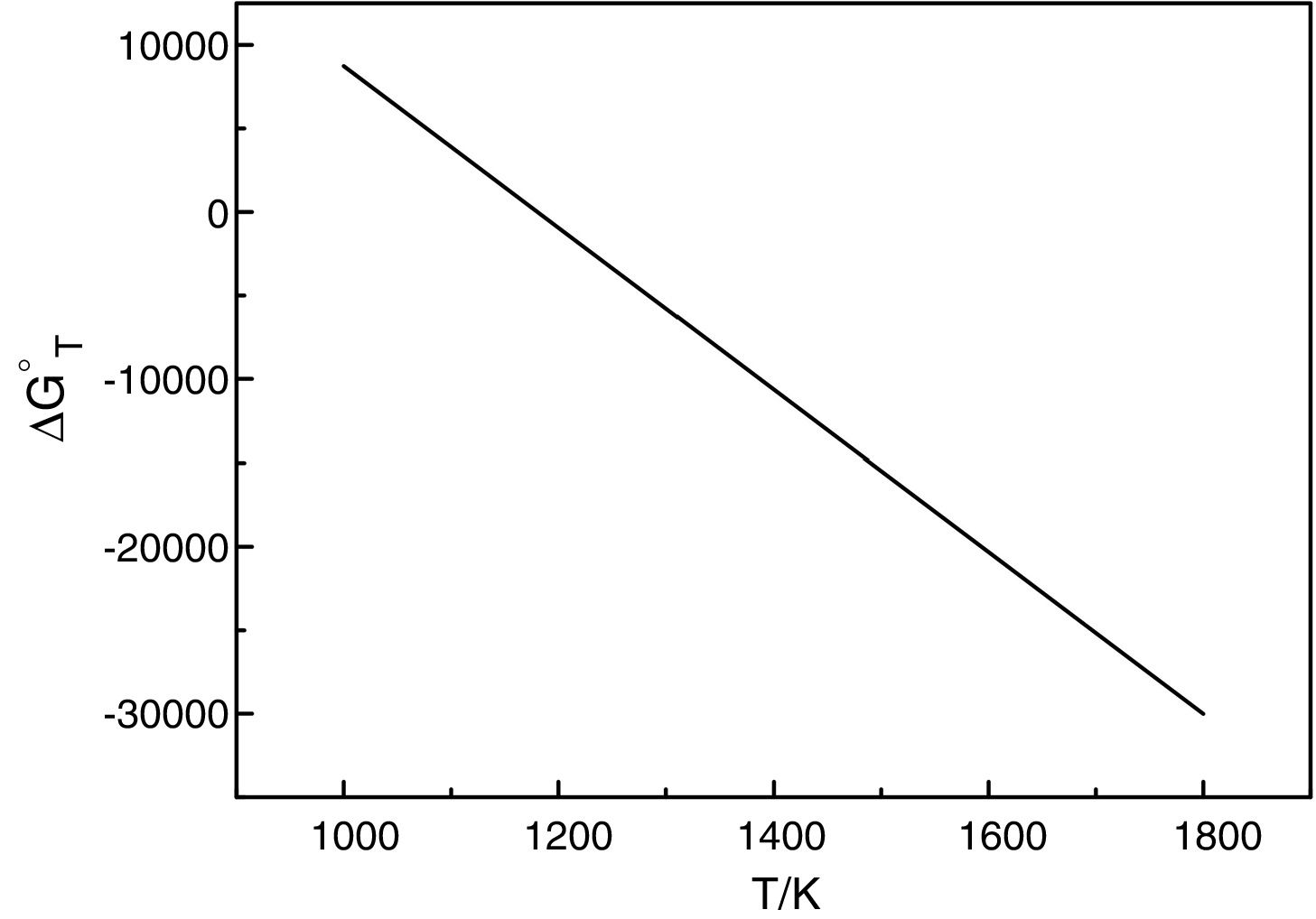
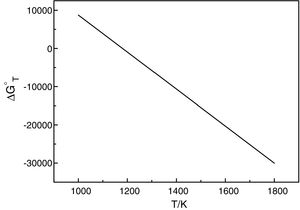
It should be pointed out here that, the phase transformations of quartz and andalusite must be considered. The phase transformation happens at 847K and 1049K respectively [12]. Consequently, the definite integration form equation (2) is divided into three sections. For the conversion of andalusite to mullite and quartz, the relationship between the reaction Gibbs energy of reaction (3) and temperature is shown in Fig. 6. The figure presents the Gibbs energy as the function of the temperature range between 1049K and 2000K. The linearized expression (6) has been achieved:
The relationship between the reaction Gibbs energy of reaction (3) and temperature.
According to Eq. (6), the reaction (3) can happen at above 1180K. A small amount of mullite was tracked at 1373K [7] but time required at 1180K of reaction (3) is too long. The calculated results are not so far different from experimental results. So, this calculation can provide the useful references for the industry. However, thermodynamic calculation only tells whether the reaction happens, the kinetics of this reaction is more important for the industrial production.
Kinetic considerationEffect of andalusite particle sizeThe size of the particle is one of the kernels to accelerate the transformation speed of andalusite [7]. The smaller size of andalusite particles signifies that specific surface area of the particles is larger. The number of nuclei increases when a particle is split. In industry, small particles of minerals are commonly prepared by physical grinding. The ground minerals with small size always have large specific surface area and many defects. The large specific surface area and defects make those small particles contain large number of grain boundaries and cleavage planes.
The transformation of andalusite into mullite becomes activated at above 1653K [7,16]. Below the critical temperature, the mullitization of andalusite is limited to some extent and simply taking far too long, even the andalusite particles are with small size. The suggestion is given as follows: the nuclei of the production mullite are always formed first at energetically favored sites of andalusite lattice. On the other hand, those nuclei are recognized as “germ nuclei” and they can be served as “growth nuclei” [8,17], if the temperature meets the requirement. But the formation of growth nuclei from germ nuclei is time consuming. Thus, the mullitization of andalusite takes long time before the temperature goes up to 1653K. As mention above, the smaller particles are always with more grain boundaries and cleavage planes, so the particles with smaller size will have larger number of germ nuclei. Consequently, more mullite phases are obtained. For purpose of industrial manufacture, the mullitization of andalusite should be efficient in a short time at low temperature.
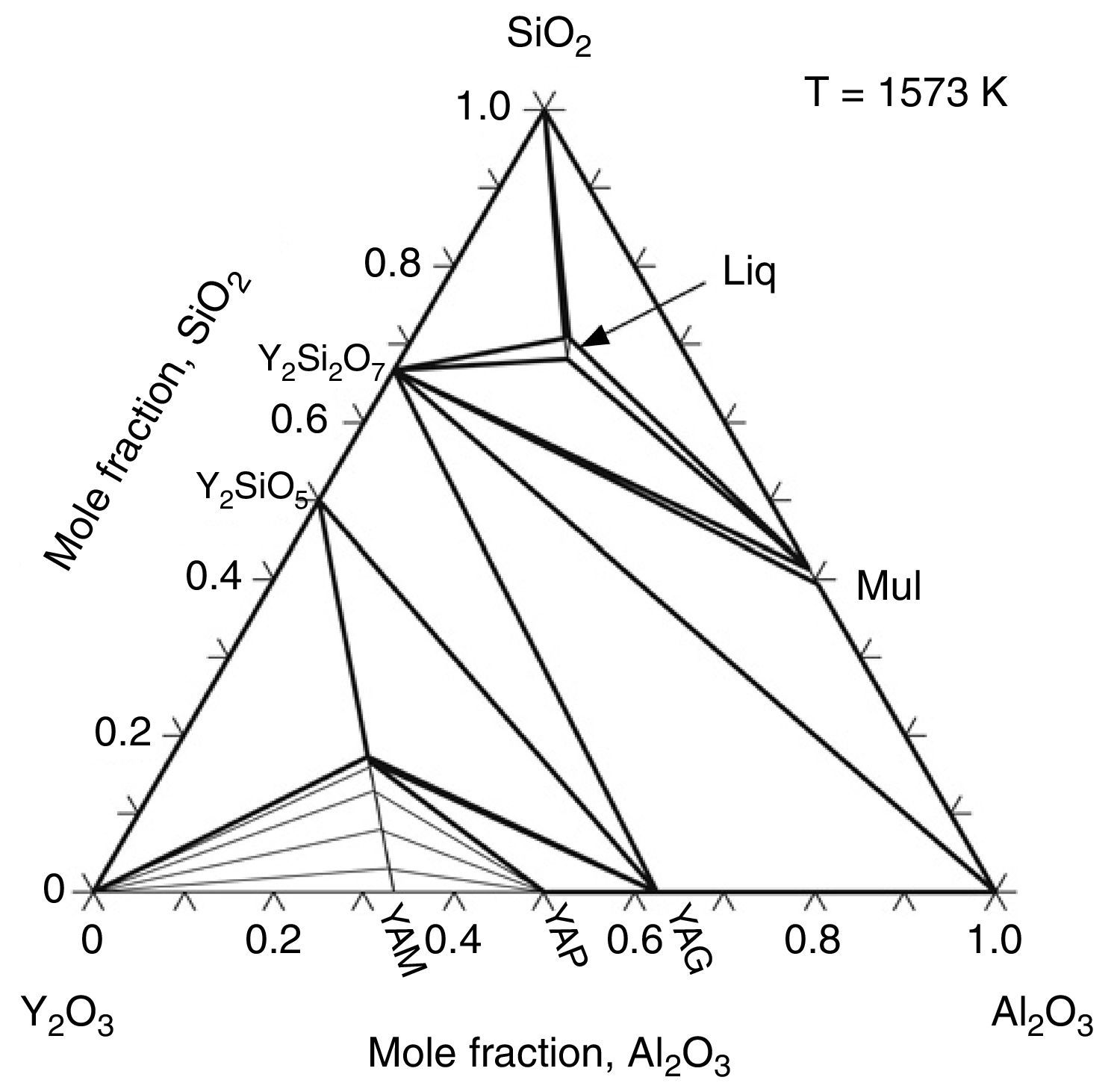
Effect of SiO2The productions of reaction (3) are silica and mullite. The existence of quartz is ineffectual to the transformation of andalusite into mullite at high temperature. It should be considered from two aspects. Firstly, silica-rich liquid phase is generated during the firing process [7,8] and the viscosity of such liquid phase during the sintering is very high according to the high content of silica in the liquid phase. Previous study [18] showed that the transformation of andalusite into mullite had been retarded at 1573K even the andalusite was enveloped by liquid phase and the liquid phase contained more than 10wt% of alkaline oxides (K2O and Na2O). Due to the different chemical composition of mullite and andalusite, the transformation of andalusite into mullite is accompanied by the ions diffusion into and out of andalusite structure. The high viscosity of liquid phase has negative effect on the ions diffusion and therefore slower the transformation rate of reaction (3). Secondly, to obtain more mullite germs, the consumption of silica is necessary. Andalusite is formed in the contact metamorphic rocks. Generally, the content of andalusite in the ore deposit is limited, so it needs to be used after beneficiation. Therefore, impurities in mineral materials cannot be completely removed. Metamorphic rock is another type of rock which is naturally metamorphosed by different rocks under the high temperature and high pressure. SiO2 is easily recrystallized to generate quartzite under high temperature and high pressure, and its main phase is quartz. Many andalusite minerals are accompanied with quartz, as shown in this study. In this study, according to the XRD analysis, quartz phase is found with andalusite and this impurity gives negative impact on reaction (3), since the products of reaction (3) contain SiO2. However, Y2O3 can get rid of the excess quartz and formed as phase Y2Si2O7. The EDS analysis of a particle indicates that the Y2Si2O7 is formed, as shown in Fig. 5 and listed in Table 4. On the other hand, liquid phase is found at 1573K according to the phase diagram as shown in Fig. 7[19].
The isothermal section of Y2O3–Al2O3–SiO2 system at 1573K [19].
The eutectic point of Al2O3–SiO2 is very high [20] which mean that the liquid is very hard to be generated if andalusite is pure. However, the generation of liquid phase during heating has positive effect on the promotion of chemical transportation. The presence of liquid phase promotes the transportation rate of different ions and thus accelerates the diffusion process. As shown in Fig. 4(b), the mullite phases cover the surface of the andalusite. On the other hand, the natural mineral andalusite always contains many impurities. It should be mentioned here that Y2O3 addition increases liquid content at temperatures higher than 1573K [19]. The generation of liquid phase at low temperature is mainly due to the impurities of the andalusite. Specially, the iron oxide and alkali oxide are detectable by XRF and those impurities help the generation of liquid phase at low temperature during the heating. The phases such as alkaline oxides, kaolin and illite are playing an important role in the evolution of liquid phase and they are the beginning point of the liquid formation in the sample. As an example 1.5wt% of K2O/Na2O in andalusite produces 13–15wt% of liquid phase at 1573K [20]. Those impurities have three important effects on the transformation process, according to the previous research [7]:
- (1)
The eutectic point of Al2O3–SiO2 is above 1723K. With the presence of alkaline oxides, the liquid phase generates at lower temperature.
- (2)
The content of liquid phase in the sample increases.
- (3)
Low viscosity value is obtained by the presence of impurities, especially alkaline oxide. The liquid phase is with high viscosity value if impurities are not presented but small amount of alkaline oxides halves the viscosity value of the liquid phase at the temperature below 1723 [7]. But the generated liquid phase at surface area is still with extremely high viscosity.
The generation of liquid phase is also important for the consumption of excess silica. The formation Gibbs energy of reaction (1) is expressed as Eq. (7)[19]:
Eq. (7) shows that the Y2Si2O7 can be obtained at really low temperature before the generation of liquid phase thermodynamically. However, without driving force, solid–solid reaction always takes long time. With temperature arising, more energy is used to provide the driving force which is helpful for reaction (1) happening. On the other hand, the presence of liquid phase promotes the reaction and makes the Y2Si2O7 nuclei grow.
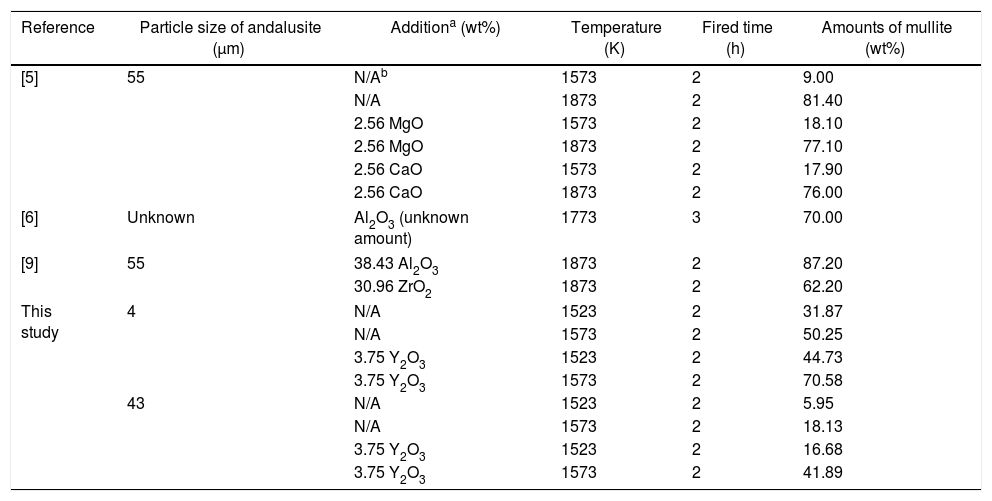
Effect of Y2O3Yttria has two functions: to consume the silica and lower the viscosity value of liquid phase. The most considerable effect of diffusion rate is to lower the viscosity value of the liquid phase. Consequently, the viscosity of the liquid phase strongly related to the reaction rate. On the other hand, EDS analysis reveals that some Y2O3 indeed dissolve into the siliceous liquid. It was reported [11] that Y2O3 decreases the melting temperature and the viscosity of soda–lime–silicate glass. Table 6 is the results of mullitization of andalusite by addition of different chemicals according to different researchers. It is indicated that MgO and CaO [5] are also two chemicals which can encourage the fast transformation of andalusite into mullite and the results are coincident with the present study, because the addition of those two chemicals can also decrease the viscosity of generated liquid phase. However, as shown in Table 6, the Y2O3 has more remarkable effect on the mullitization of andalusite. A reasonable explanation is that compared with MgO and CaO, Y2O3 strongly lower the viscosity value of liquid phase. It has been reported that with addition of small amount of Y2O3, dramatic result of lowing the viscosity value of soda–lime–silicate liquid phase can be achieved. The viscosity value is even lower than that of liquid with addition of alkaline oxides [11]. Y3+ has larger ionic radius which creates more non-bridging oxygen (NBO) atoms in the glass system and broadening the distribution of Qn (Qn is tetrahedron structural units with n=(1–4) non-bridge oxygen) structural units.
The results of mullitization of andalusite by addition of different chemicals according to different researchers.
| Reference | Particle size of andalusite (μm) | Additiona (wt%) | Temperature (K) | Fired time (h) | Amounts of mullite (wt%) |
|---|---|---|---|---|---|
| [5] | 55 | N/Ab | 1573 | 2 | 9.00 |
| N/A | 1873 | 2 | 81.40 | ||
| 2.56 MgO | 1573 | 2 | 18.10 | ||
| 2.56 MgO | 1873 | 2 | 77.10 | ||
| 2.56 CaO | 1573 | 2 | 17.90 | ||
| 2.56 CaO | 1873 | 2 | 76.00 | ||
| [6] | Unknown | Al2O3 (unknown amount) | 1773 | 3 | 70.00 |
| [9] | 55 | 38.43 Al2O3 | 1873 | 2 | 87.20 |
| 30.96 ZrO2 | 1873 | 2 | 62.20 | ||
| This study | 4 | N/A | 1523 | 2 | 31.87 |
| N/A | 1573 | 2 | 50.25 | ||
| 3.75 Y2O3 | 1523 | 2 | 44.73 | ||
| 3.75 Y2O3 | 1573 | 2 | 70.58 | ||
| 43 | N/A | 1523 | 2 | 5.95 | |
| N/A | 1573 | 2 | 18.13 | ||
| 3.75 Y2O3 | 1523 | 2 | 16.68 | ||
| 3.75 Y2O3 | 1573 | 2 | 41.89 | ||
It should be mentioned here that the number of “germ nuclei” is the source of mullite phases, which is strongly related to the mullite content in the sample after fired. As the results listed in Table 3, mullite phases in sample A3 and AY3 after fired at 1523K are not detectable according to the XRD analysis. Sample A3 is with the largest size among those three samples which contains less grain boundaries and cleavage planes. Because not all the “germ nuclei” can be served as “growth nuclei” and some of them are swallowed by the growing grains [17], thus, if the size of andalusite is too large to get enough energetically favored sites, only a few “germ nuclei” develop into “growth nuclei” which means the content of mullite in the sample is extremely low. Regardless of whether Y2O3 powders are added into andalusite or not, mullite phases in the sample with the large size are not detectable consequently.
ConclusionsThe transformation of andalusite into mullite by addition of Y2O3 powders was studied experimentally by quenching method. The spontaneous transformation occurred at above 1180K according to the thermodynamic calculation. The kinetic consideration was more important for the practical applications. Transformation of andalusite into mullite is enhanced if grain size of andalusite is reduced. The addition of Y2O3 reduces SiO2 content and increases liquid content at temperature higher than 1573K. On the other hand, the addition of Y2O3 remarkably lowered the viscosity value of generated liquid phase. The low viscosity value of liquid phase accelerated the ion transportation and produced fast transformation of andalusite into mullite.
The financial supports on the Project 51502230 from National Natural Science Foundation of China are gratefully acknowledged.






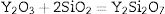
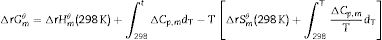
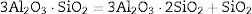
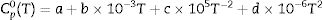
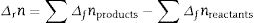
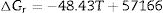
![The isothermal section of Y2O3–Al2O3–SiO2 system at 1573K [19]. The isothermal section of Y2O3–Al2O3–SiO2 system at 1573K [19].](https://static.elsevier.es/multimedia/03663175/0000005800000004/v1_201907260835/S0366317518300797/v1_201907260835/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


