The present investigation aims to estimate the feasibility of using eggshell and rice husk ash (RHA) as ingredients to produce calcium silicate board (CSB). The solid-state route was used to prepare the calcium silicate (CS) powder through the mixing of heat treated RHA (∼93% SiO2) and calcined eggshells (∼99% CaO) at 1050°C. CSB specimens were prepared at room temperature by simple curing process followed by mixing of different proportions of CS powder, ordinary portland cement (OPC) and unground rice husk ash (URHA). Several physicals, mechanical and thermal characterizations of the cured specimens were performed. The addition of OPC and URHA with CS were significantly influenced all the properties of CSB. The wastes derived CSB was exhibited low density (<1000kg/m3), comparable bending strength (∼6MPa) and low thermal conductivity (<0.153W/mK). These properties suggest that the waste derived board may be used in the internal lining of building for insulation.
La presente investigación tiene como objetivo estimar la viabilidad del uso de la cáscara de huevo y la ceniza de arroz (RHA) como ingredientes para producir placa de silicato de calcio (CSB). La ruta en estado sólido se usó para preparar el polvo de silicato de calcio (CS) a través de la mezcla de RHA tratada térmicamente (∼93% SiO2) y cáscaras de huevo calcinadas (∼99% CaO) a 1.050°C. Las muestras de CSB se prepararon a temperatura ambiente mediante un proceso de curado simple seguido de la mezcla de diferentes proporciones de polvo de CS, cemento Portland normal (OPC) y ceniza de cáscara de arroz sin moler (URHA). Se realizaron varias caracterizaciones físicas, mecánicas y térmicas de los especímenes curados. La adición de OPC y URHA con CS influyó significativamente en todas las propiedades de CSB. Los desechos derivados de CSB mostraron una baja densidad (<1.000kg/m3), una resistencia a la flexión comparable (∼6MPa) y una baja conductividad térmica (<0,153W/m-K). Estas propiedades sugieren que el tablero derivado de residuos se puede utilizar en el revestimiento interno del edificio para aislamiento.
calcium silicate powder
calcium silicate board
rice husk
rice husk ash
unground rice husk ash
ordinary portland cement
X-ray diffraction
scanning electron microscopy
bulk density
apparent porosity
differential thermal and thermogravimetric analysis
humidity effect
thermal conductivity
Calcium silicate is a group of compounds that can be composed of various ratios of calcium oxide (CaO) and silica (SiO2), e.g., CaO·SiO2 (CaSiO3), 2CaO·SiO2 (Ca2SiO4), 3CaO·SiO2 (Ca3SiO5), and 3CaO·2SiO2 (Ca3Si2O7), which are derived from limestone and siliceous sedimentary rock [1]. CaO–SiO2 system possesses some attractive properties, such as lightweight, fireproof, low thermal conductivity, high-strength, high physical water absorption and excellent bioactivity [2–4]. Due to these unique characteristics of calcium silicate, it is engaged in numerous fields, such as the construction sector, the food processing industry for an anticaking agent, cement, and ceramic, etc. Recently, calcium silicate has been widely used as a new building material to replace the gypsum plasterboard and asbestos cement board. The flame-retardant, strength and moisture resistance of the gypsum board are comparatively poor than calcium silicate board (CSB), and the asbestos board can cause several health issues in the building environs [5]. Lightweight, noise & heat flow reduction and waterproof nature of CSB, make it suitable to be used as wall materials for indoor partition and ceiling in offices, hotels and high rise apartment. Therefore, the global demand for CSB is significantly increased and expected to strong growth in the near future due to infrastructural development and rapid urbanization [6].
Almost all type of CSB is presently manufactured by the combinations of locally available natural ingredients like reactive quartz sand or diatomaceous earth as siliceous material, limestone or ordinary portland cement (OPC) as calcium material and different fiber materials for reinforcing followed by mixing, shaping and curing at a different temperatures or sintering at high temperature [2,7]. The huge use of virgin raw materials for the production of ceramic has caused an enhancement in the deficit level of the natural resources, which is already present in a low amount. In this respect, researchers are tried to find out the new route for the development of ceramic products by consuming numerous waste materials [8–13]. Consequently, the several waste materials have been studied intended for calcium silicate synthesis such as eggshell waste – chamotte (previously fired ceramic) [14], marble scraps – mussel shells [15], bottom ash [16], calcium silicate slag – fly ash [17], waste glass – seashells [7] and zirconium containing silicon slag – caustic liquor [18]. However, as per survey of the literature, no research report has been found to produce CSB using the combination of solid waste as ingredients, i.e., eggshells, rice husk ash (RHA) and unground rice husk ash (URHA); and through the room temperature curing process by mixing of OPC.
Rice is the second most consumed food item globally, and rice paddy production was about ∼758MT in 2017, and this number will increase slowly due to the projected demand of the world population [19]. Rice husk (RH) is the surface cover of the rice grain and its around 20–25wt.% of rice paddy's weight [20]. It is mainly used as animal feed and fuel in the boilers through direct combustion or by gasification because of its significant calorific value (∼800kW/ton) [21]. Subsequently, RH causes new waste through the burning, namely rice husk ash (RHA), which is about 18–22wt.% of the RH [22]. However, RHA contains around ∼92wt.% of active silica (SiO2) [23], and it has great potential to engage in the different sectors such as construction, electronics, biomedical, chemicals, ceramics, and others [24]. Ceramic industries are also used this amorphous silica in place of other silica sources for fabrication of several ceramic products, such as, nano silica for solar cells [25], ceramic membrane [26], refractory [27], cordierite [28], forsterite [29], mullite [30], wollastonite [31], glass-ceramic [32] and silicon carbide [33].
The food processing industry is also generated numerous wastes; eggshells are one of them. It consists of around ∼11wt.% of the egg's weight [34]. It is disposed of in enormous quantities worldwide because of no significant uses. Subsequently, that causes several health issues, environmental pollution and release of bad odor. Therefore, it is an important issue for food industries to the utilization of eggshells [35]. The chicken eggshells contain around 94wt.% of calcium carbonate in the form of calcite (CaCO3), 1wt.% Ca3(PO4)2, 1wt.% MgCO3, and 4wt.% organic substance [36,37]. On the other hand, when eggshell is heat treated at about 900°C, it contains above 99wt.% of CaO [38]. Therefore, it has huge potential to be used as a substitute of natural limestone in the different sectors as a calcium source. At present, many researchers are trying to find the alternative ways, such as replacements of limestone in cement mortar [39], hydroxyapatite [40,41], bio-template [42], cathode ray tube glass foams [43], insulating material [14] and adsorbent [37], to use it without a landfill.
The present work is a new approach to investigate the possibility of utilization of eggshells and rice husk ash as alternative ingredients in the composition of calcium silicate powder that mostly uses virgin raw materials. The board samples are prepared at room temperature by the curing process through the mixing of OPC and URHA. The physical, thermal and mechanical properties of the waste-derived CSB samples are comprehensively investigated and also briefly discussed the effect of humidity on the mechanical strength.
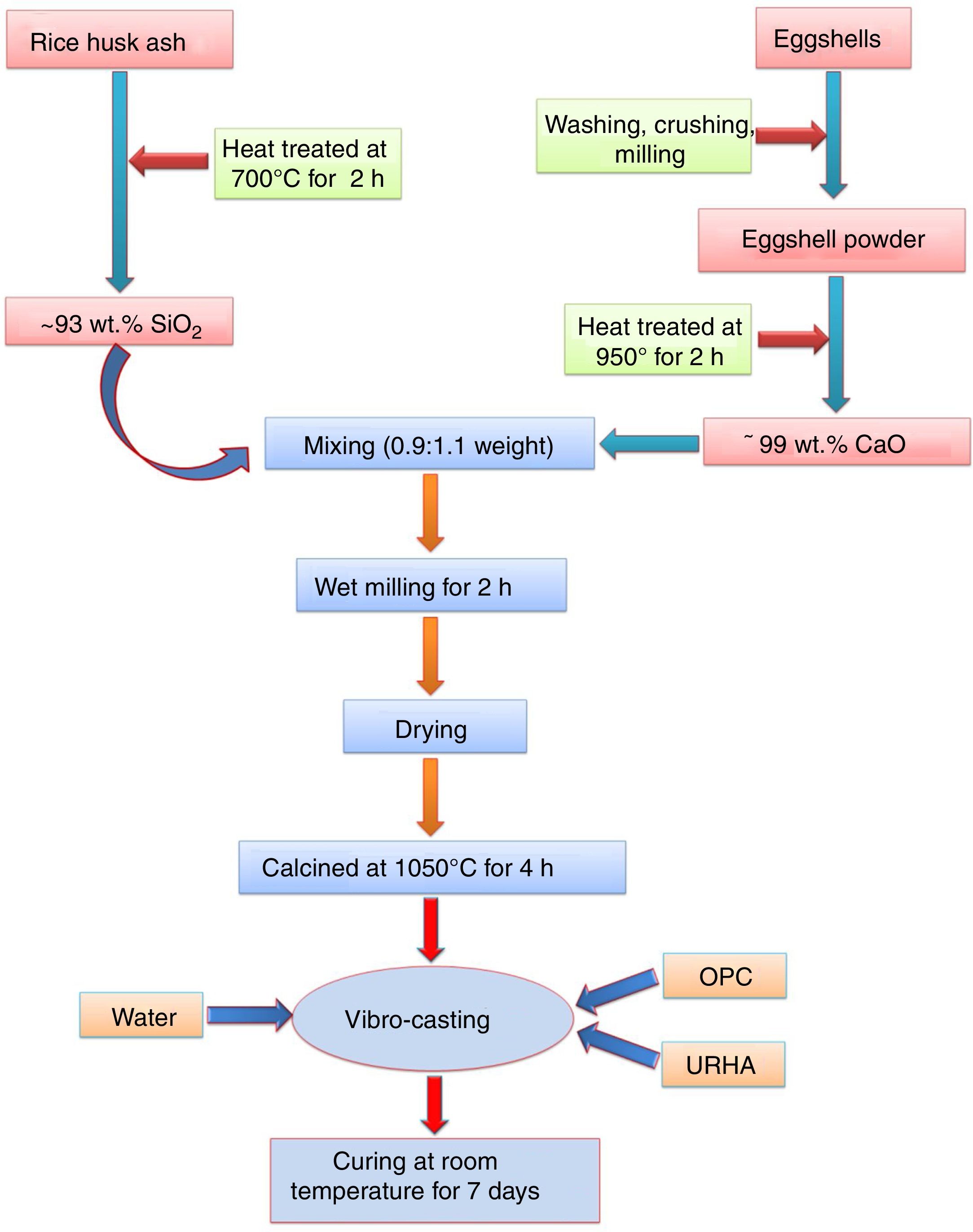
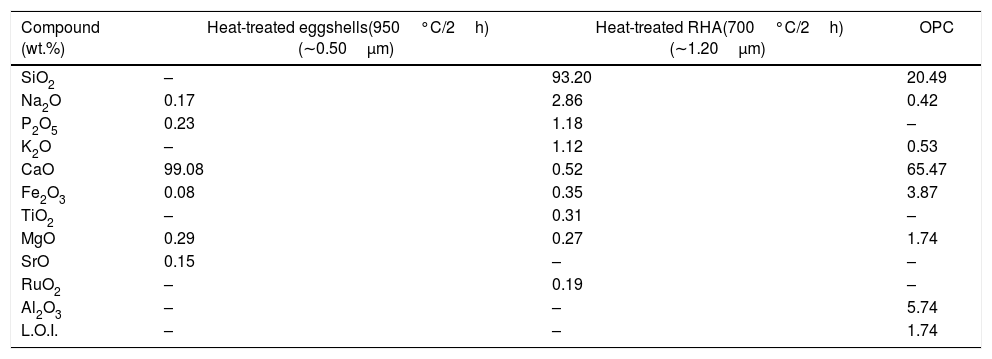
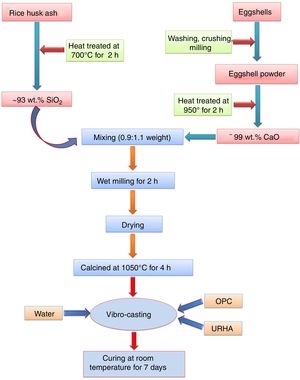
Experimental procedureThe present study was accomplished using RHA, URHA, chicken eggshells and OPC as ingredients; and calcium silicate (CS) powder was synthesized by the conventional solid-state route. The CSB samples were prepared at room temperature by the curing method. The preparation scheme of CSB is presented in Fig. 1. RHA and eggshells were collected from a local rice mill and a nearby restaurant, respectively. The outsize discussion about RHA and eggshells was reported earlier [44]. RHA and eggshells powder were heat-treated at 700 and 950°C respectively, for 2h in the air; and the chemical composition of heat treated powders are tabulated in Table 1. Subsequently, URHA was used as reinforcing media in the CSB system. The OPC was purchased from ACC Limited, India (OPC 43 grade) and chemical composition is presented in Table 1.
Chemical composition of raw materials.
| Compound (wt.%) | Heat-treated eggshells(950°C/2h) (∼0.50μm) | Heat-treated RHA(700°C/2h) (∼1.20μm) | OPC |
|---|---|---|---|
| SiO2 | – | 93.20 | 20.49 |
| Na2O | 0.17 | 2.86 | 0.42 |
| P2O5 | 0.23 | 1.18 | – |
| K2O | – | 1.12 | 0.53 |
| CaO | 99.08 | 0.52 | 65.47 |
| Fe2O3 | 0.08 | 0.35 | 3.87 |
| TiO2 | – | 0.31 | – |
| MgO | 0.29 | 0.27 | 1.74 |
| SrO | 0.15 | – | – |
| RuO2 | – | 0.19 | – |
| Al2O3 | – | – | 5.74 |
| L.O.I. | – | – | 1.74 |
The CS powder formulation was done by the solid-state reaction route through the mixing of 0.9:1.1 weight ratio of heat treated RHA (∼93% SiO2) and calcined eggshells (∼99% CaO). The mixing was done through the wet milling for 1h with 600rpm in water media. Then, the mixture powder was dried at 110°C and calcination was performed at 1050°C for 4h in air. Once again ball milling was done for 30min with 600rpm because agglomeration takes place in the particles after the calcination process.
The excessive air is required to remove the present carbon from the wastes (eggshells and RHA). Therefore, without pre-calcination of raw materials, there is a possibility to present carbon in the calcium silicate. This carbon may be diluted the insulation (thermal conductivity) properties of the board specimens. The pre-calcination step can be skipped and the mixing time can be reduced through the use of different type furnaces, e.g., rotary kiln or cement kiln during calcium silicate formulation. These types of kilns are performed both heating and mixing, simultaneously. Subsequently, less time and low amount of energy are required during milling of eggshells and RHA due to their lower hardness than the conventional source of CaO (limestone) and SiO2 (sandstone or flintstone).
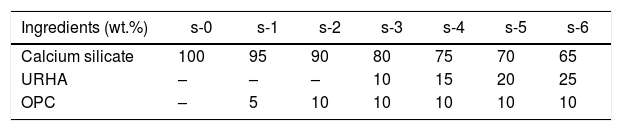
The most facile economic room temperature curing process was used to synthesize the CSB specimens. The batch composition for this process is presented in Table 2. All the raw materials were mixed in a dry condition for 20min followed by addition of 15wt.% water for casting, and mixing was done for 10min. The wet mix was transferred into a properly oiled different shape steel molds, and the flat surface was prepared by vibro-caster for 5min. Then, the specimens were cured at room temperature for about 24h (without autoclave). After that, the samples were released from the molds, and the resulting specimens were taken for curing in a humidity chamber (70–90% relative humidity) for 7 days to yield CSB specimens, as shown in Fig. 2(a). For appropriate results, six specimens of each composition were prepared and all the testing was performed after the drying of samples at 50°C.
Several techniques were used to analyze the physico-mechanical, morphological and humidity effect of CSB specimens. Differential thermal and thermo-gravimetric analysis (DTA–TGA) was done for RHA–eggshells mixed the powder in an oxygen atmosphere with a heating rate of 5°C/min with the help of “KEP-Technologies, Setaram-Scientific & Industrial Equipment, France (Model-Labsys, Serial no-560/51920)”. The crystalline phase identification was performed by the X-ray diffraction (XRD) analyzer with the help of “Rigaku Desktop Miniflex II X-Ray Diffractometer” equipped with Ni filter and Cu Kα radiation (λ) 1.54056Å in the range from 10° to 90° with the step size of 0.02° and scanning rate 2°/min (Model No: HD20972, Rigaku Corporation, Tokyo, Japan). The microstructure of the calcined calcium silicate powder and CSB specimens were observed with a Scanning Electron Microscopy (SEM) (Nova Nano SEM 450, FEI, Netherlands). The bulk density, porosity and water absorption of cured specimens were measured according to the Archimedes principle. Expansion of CSB specimen in water was calculated after dipping in drinking water for 24h at room temperature by the following equation:
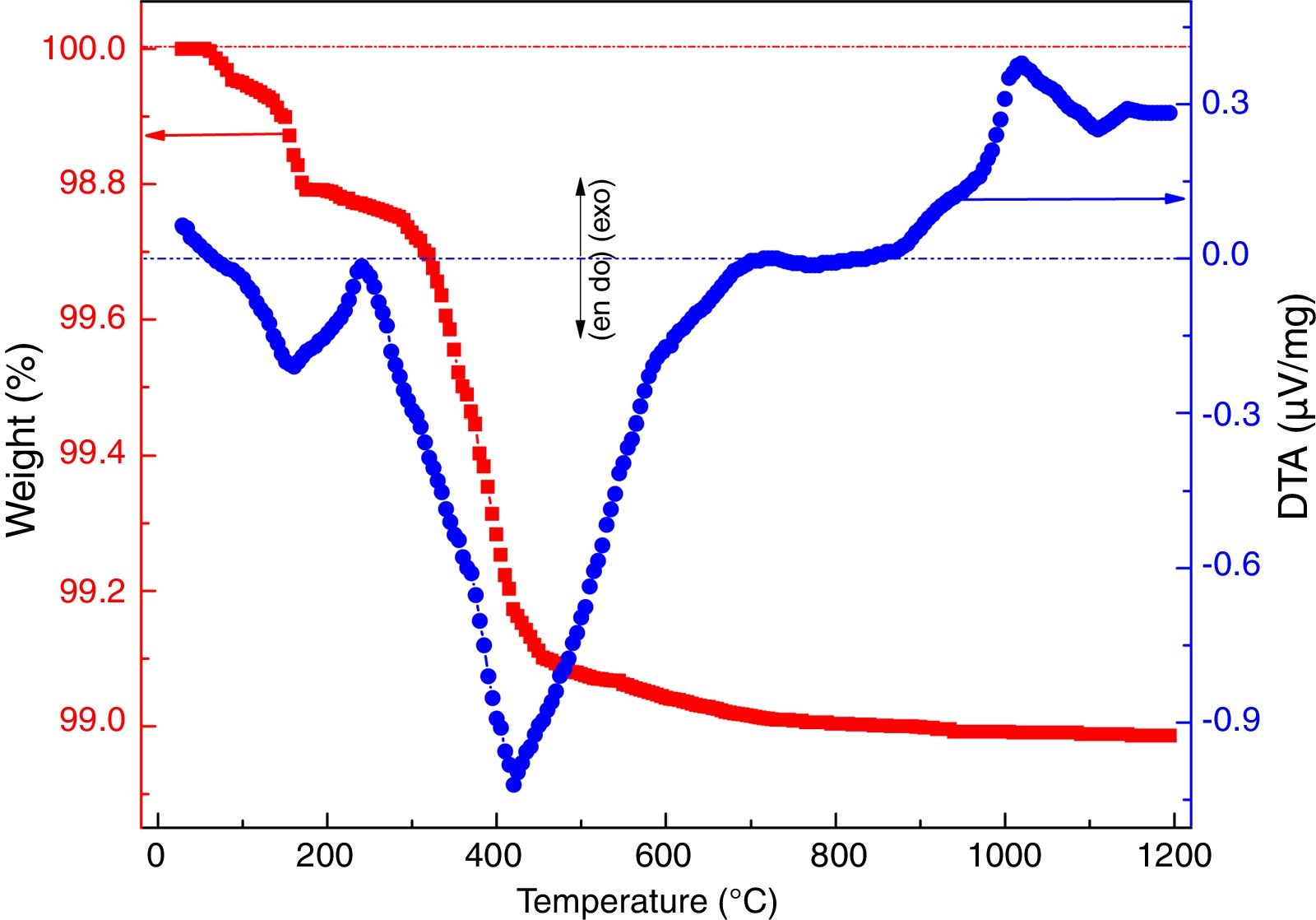
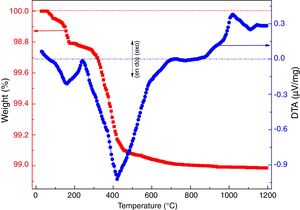
where ve denotes expansion (%); however vb and va denote before and after volume of dipped samples, respectively. Mechanical properties were determined according to the method defined in ASTM C133 by Universal Testing Machine, Tinius Olsen, H10KL-I0129 [45]. The humidity effect (HF) was estimated by the following equation:where cb and ca are associated to before and after the compressive strength of humidified samples, respectively. The thermal conductivity values of the fired samples were measured using a Flashline-4010 instrument (M/s Anter Corp., USA) at room temperature in Argon atmosphere.Results and discussionCharacterization of calcium silicate powderFig. 3 shows the DTA–TGA curve for the CS formulation in a 0.9:1.1 weight ratio heat treated RHA and calcined eggshells. TGA curve demonstrates a little weight loss (∼1wt.%) up to 1200°C because heat treated ingredients are used for the formulation of calcium silicate. The first small weight reduction (0.2wt.%) is detected up to 160°C. It is corresponding to the evaporation of the moisture from the mixture. The next weight loss around ∼0.8wt.% is observed in the range of 200–520°C. It may be due to the conversion of portlandite [Ca(OH)2] to CaO and it attributes an endothermic peak in DTA graph. Most probably, Ca(OH)2 is formed through the moistening of the calcined eggshell (∼99wt.% CaO) from the atmosphere [14]. In addition, the exothermic peaks without weight reduction after 880°C are detected due to the formation of calcium silicates.
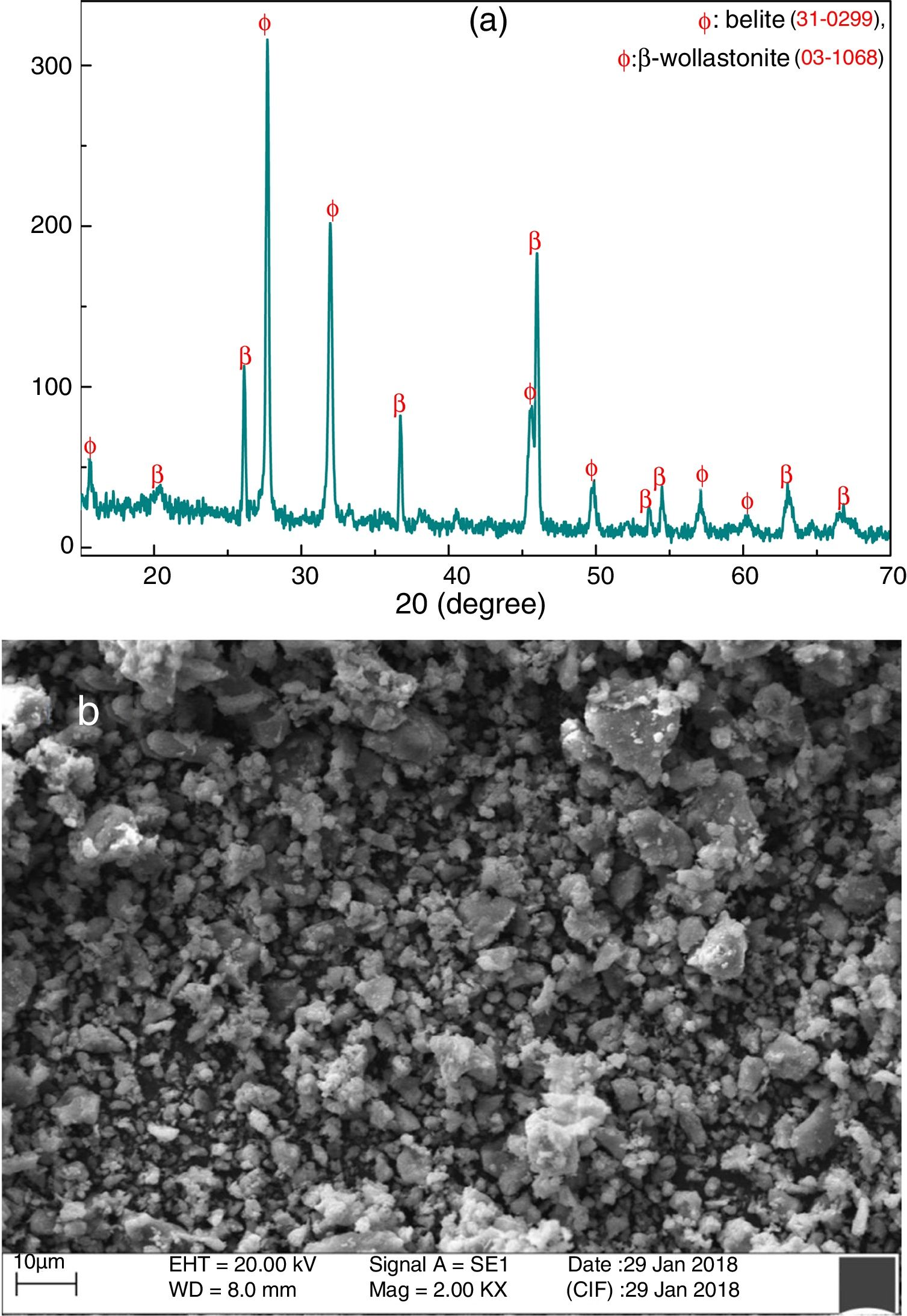
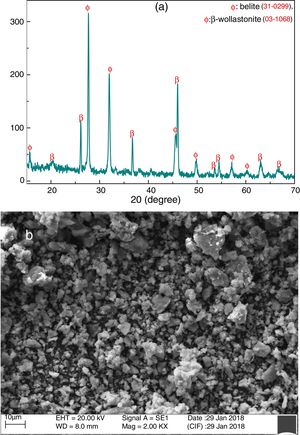
Fig. 4(a) shows the XRD pattern of grounded CS powder at room temperature. It is confirmed that the formation of belite (Ca2SiO4) and β-wollastonite (CaSiO3) and no other phases like unreacted CaO and SiO2 are detected by XRD. It may occur due to the highly reactive of SiO2 and CaO present in RHA and calcined eggshell, respectively. These are potentially reacted with each other through the diffusion mechanism and formed new compounds. Fig. 4(b) shows the SEM micrograph of grounded CS powder. It indicates that the powders are composed mostly of irregular or no preferential shape, and the average particle size is around 2.10μm.
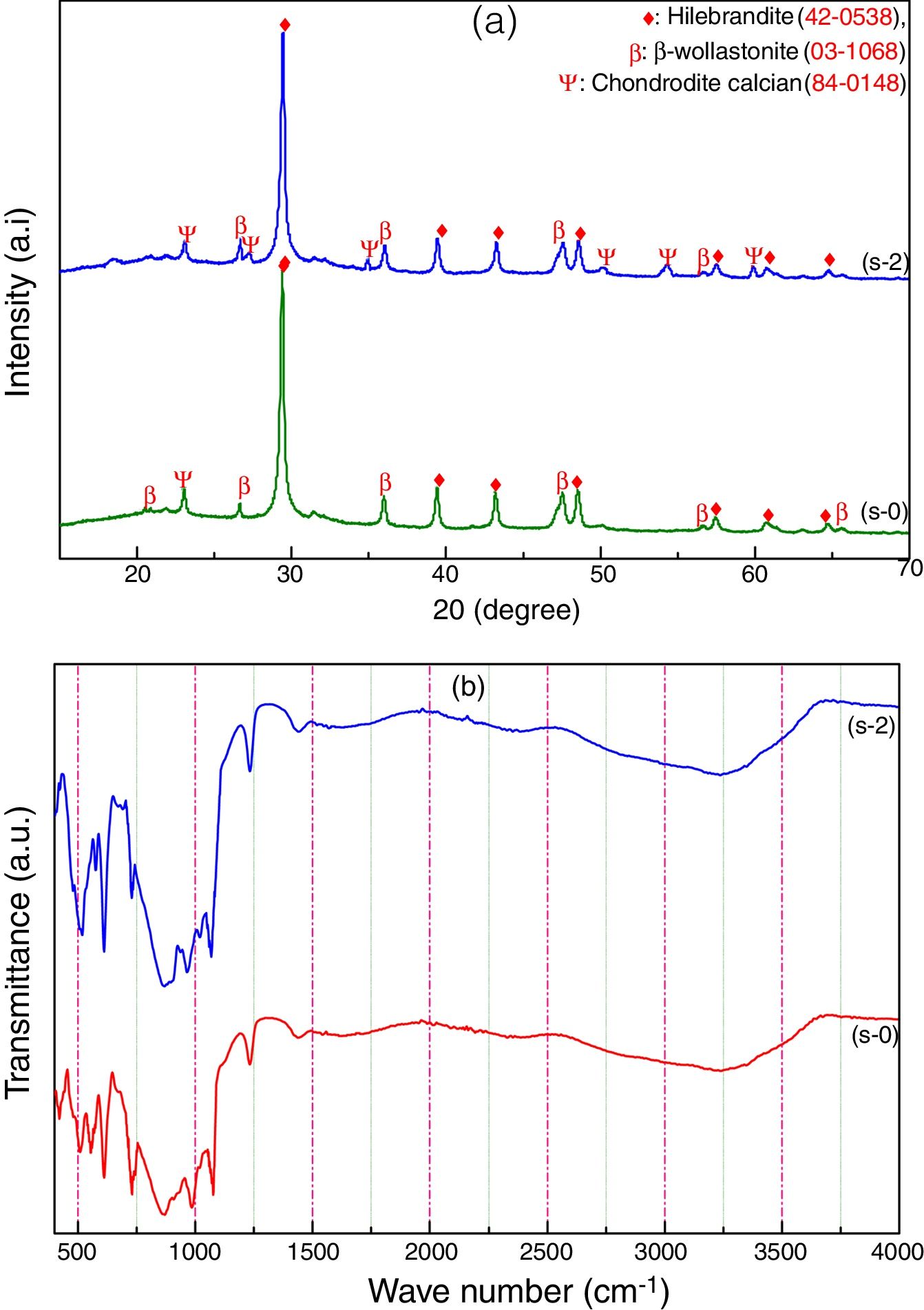
Characterization of calcium silicate boardCSB samples are prepared using CS powder, OPC and URHA as an aggregate or reinforcing material at room temperature by curing method. The main hydration product of CSB is calcium silicate hydrate (C-S-H), which is responsible for achieving a practical strength of CSB [17]. The room temperature XRD analysis of after 7 days curing of s-0 (only CS) and s-2 (90% CS+10% OPC) samples are shown in Fig. 5(a). Hillebrandite (Ca2H2O5Si, orthorhombic, space group number and name: 63, Cc**) and chondrodite calcian (Ca5H2O10Si2, monoclinic, space group number and name: P21/b, 14) are the hydrated product in CSB. It may be formed after the hydration of Ca2SiO4 phase in CS and OPC containing phases like alite (3CaO·SiO2) and belite (2CaO·SiO2). The β-wollastonite (inert) phase is also present in the system as an unreacted fine aggregate. Consequently, the number of C-S-H peaks is increased in the s-2 specimens due to the hydrated phases are increasing with OPC incorporation in CSB. These hydrated phases help to develop practical strength in the CSB through the formation of a 3D network of C-S-H in between the aggregates (β-wollastonite and URHA).
The presence of several chemical bonds in room temperature curing CSB specimens (s-0 and s-2) is detected through FTIR analysis, which is shown in Fig. 5(b). The broad absorption band around 3600–2900cm−1 and 1700–1500cm−1 are attributed to the stretching and bending vibrations of OH groups present in the CSB system, respectively [18,46]. The peaks around 1442, 985 and 423–450cm−1 are ascribed to the presence of Ca–O vibration in the structure [47]. The stretching vibration of Si–O–Si in [SiO4]-tetrahedra is identified through the absorption band at the center around 1230cm−1[48]. A group of the absorption band at 423, 610 and 985cm−1 relates to the presence of Ca–O–Si [49]. The bending vibrations of the bridge and non-bridge of Si–O group are estimated by the 600–450cm−1 spectrums [50].
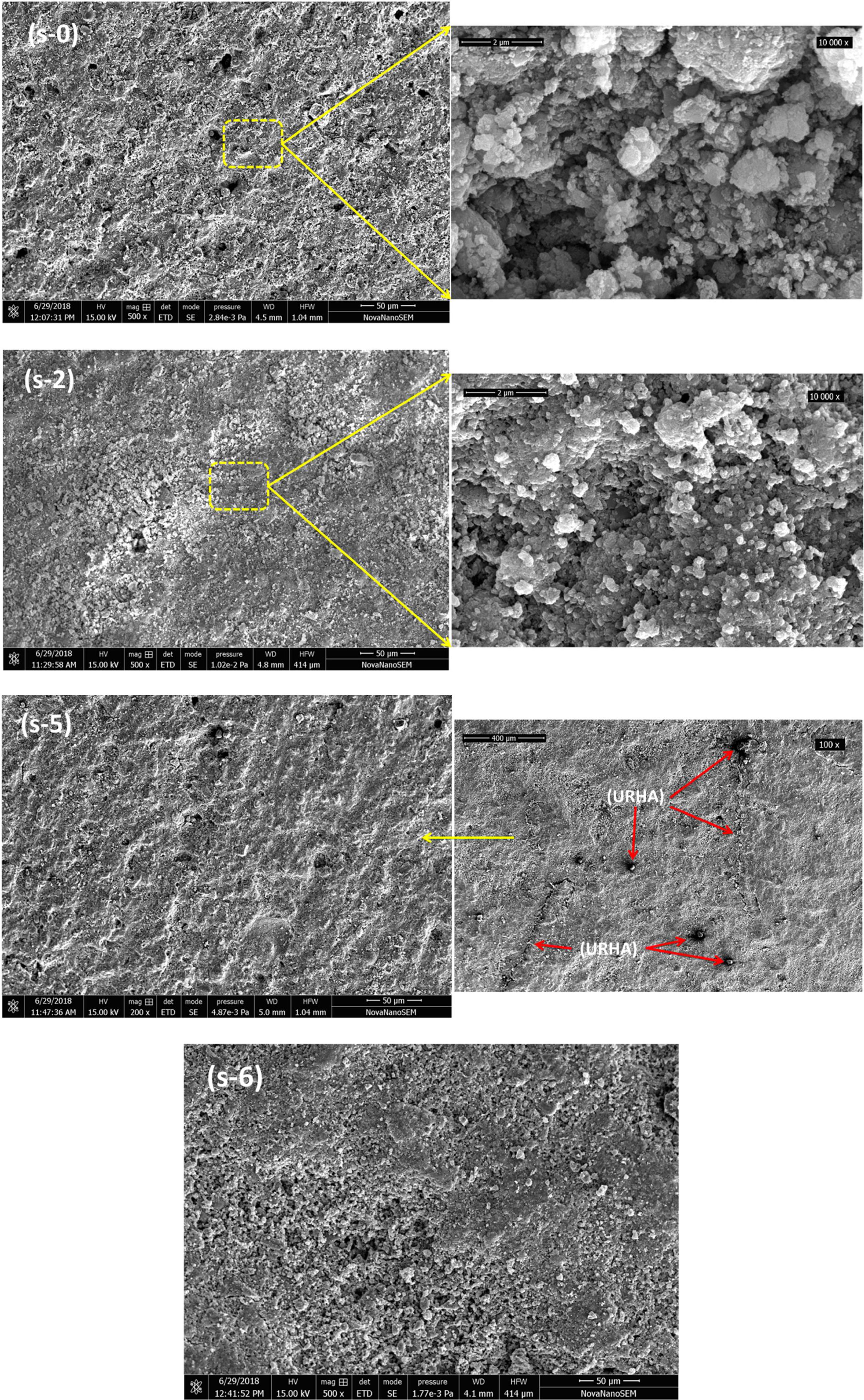
Fig. 6 exhibits the SEM micrographs of the fractured cross-sectional surface of some selectively cured CSB specimens. SEM analyses show a low grade of densification with micropores for s-0 sample. It can be seen that the OPC addition has marked influence on the microstructure of CSB through the reduction in pore size as well as their number. It may take place due to the formation of a higher amount of cementing bonds with OPC incorporation (Fig. 5(a)), and it could help to agglomerate of particles, shows in high magnification (10,000×) image of s-2. However, URHA addition does not create any large alteration on microstructure (≤20wt.%). Low magnification (100×) image of s-5 sample displays the reinforcement of URHA in the hydrated calcium silicate matrix.
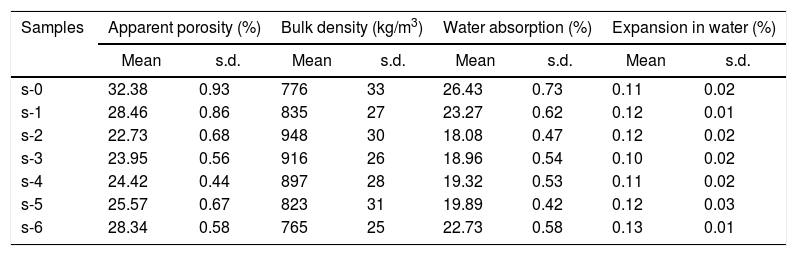
The density and porosity of calcium silicate boards have a lot of importance with regard to the performance and applications of the material. Table 3 shows the mean values of bulk density (BD) and apparent porosity (AP) of cured specimens. It has been observed that the AP significantly reduced with the addition of OPC in the composition while at the same time BD shows an improvement. AP was decreased from 32.38% to 22.73% and BD increases from 776kg/m3 to 948kg/m3 with the addition of 10wt.% OPC in the system. It may ascribe due to the formation of more amounts of hydration products (Fig. 5(a)), which forms a higher number of cementing network. It can help to get closer the particles to each other (Fig. 6). Subsequently, the AP does not expressively influence by the lower amount of URHA (≤20wt.%) addition. On the other hand, a high amount of URHA incorporation in place of CS powder remarkably affects the AP. It may happen due to the reduction of the total amount of hydration products in the CSB system. However, all the CSB samples demonstrated a lower density than water (1000kg/m3), which is virtually represented in Fig. 7. It has been seen that the sample was floating on the water, but after some time it sinks in the water because the sample absorbed water by the open pores.
Apparent porosity, bulk density, water absorption and expansion in water of cured CSB samples.
| Samples | Apparent porosity (%) | Bulk density (kg/m3) | Water absorption (%) | Expansion in water (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| s-0 | 32.38 | 0.93 | 776 | 33 | 26.43 | 0.73 | 0.11 | 0.02 |
| s-1 | 28.46 | 0.86 | 835 | 27 | 23.27 | 0.62 | 0.12 | 0.01 |
| s-2 | 22.73 | 0.68 | 948 | 30 | 18.08 | 0.47 | 0.12 | 0.02 |
| s-3 | 23.95 | 0.56 | 916 | 26 | 18.96 | 0.54 | 0.10 | 0.02 |
| s-4 | 24.42 | 0.44 | 897 | 28 | 19.32 | 0.53 | 0.11 | 0.02 |
| s-5 | 25.57 | 0.67 | 823 | 31 | 19.89 | 0.42 | 0.12 | 0.03 |
| s-6 | 28.34 | 0.58 | 765 | 25 | 22.73 | 0.58 | 0.13 | 0.01 |
The moisture absorption from the atmosphere and expansion in water significantly affects the durability and quality of the board. The moisture absorption is closely related to the open porosity and size of the pores in the board specimens [47]. The mean values of water absorption and expansion in the water of CB samples are illustrated in Table 3. It can be observed, that the value of water absorption decreases from 26.43wt.% to 18.08wt.% with OPC (10wt.%) addition. It may be occurred due to the detraction of open porosity. The URHA addition does not affect the water absorption and expansion as it is a fired ingredient. The overall expansion behavior of CSB in water is very low due to the absence of any expansible phases like free CaO.
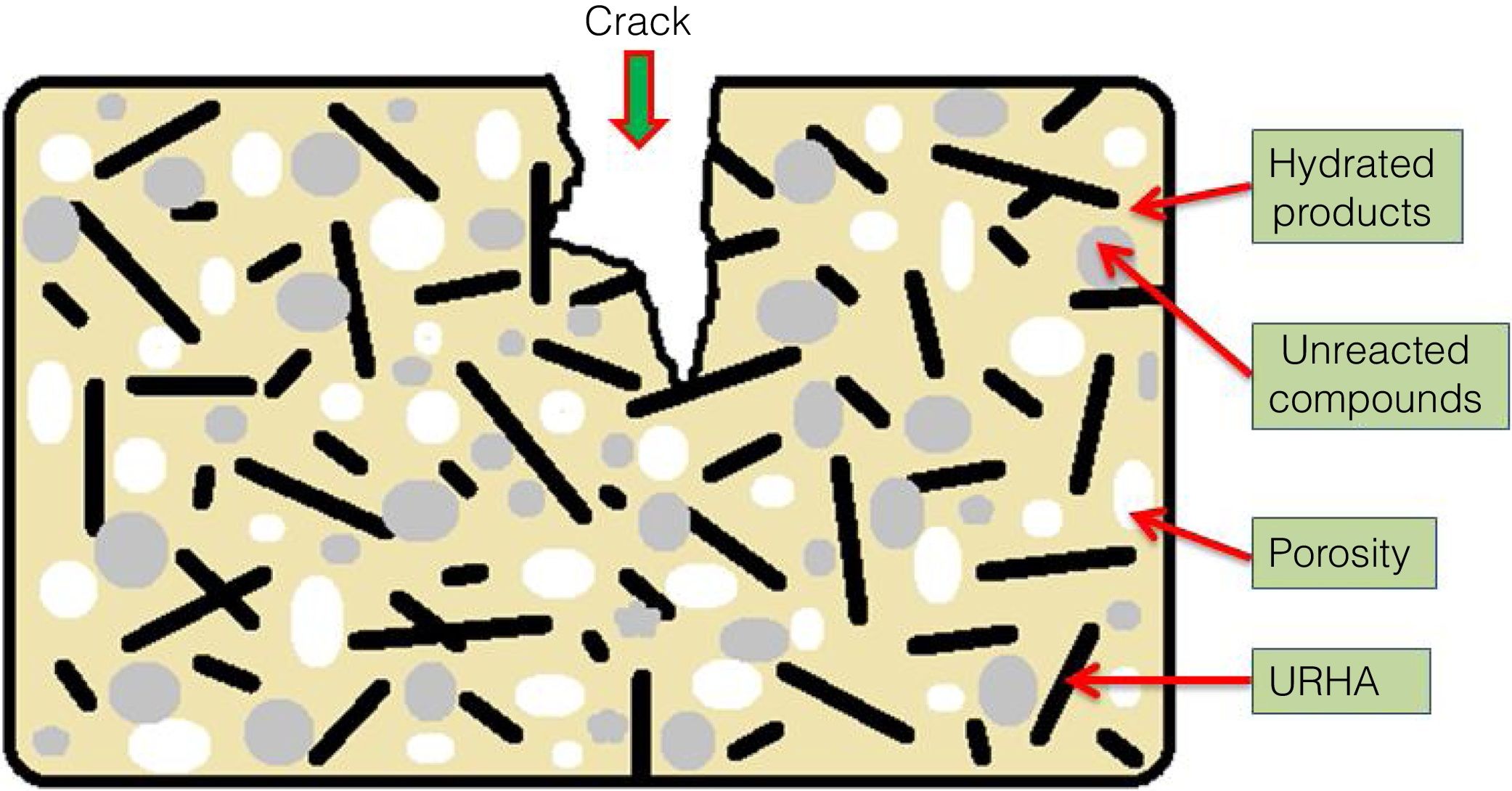
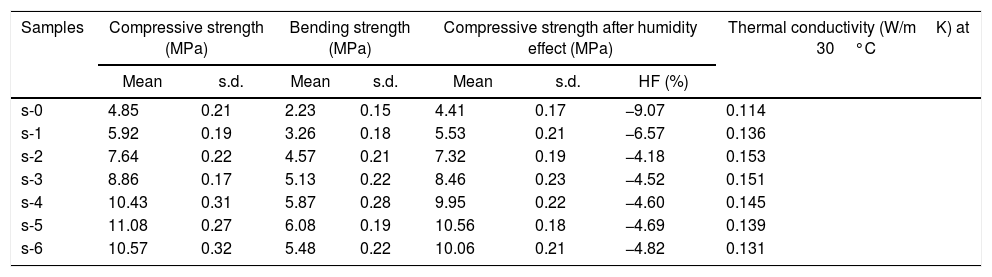
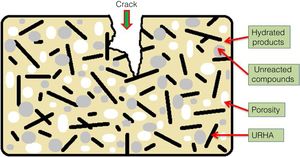
The mean values of bending strength and compressive strength of cured specimens are tabulated in Table 4, measured after 28 days of sample preparation. It can be seen from Table 4 that the mechanical strength values of both bending and compressive strength is boosted through the addition of OPC and URHA (up to 20wt.%) in the composition of CSB. This enhancement with OPC was due to the development of a higher number of cementing bonds, which are attributed to stronger networks in the CSB than only CS containing samples [2]. Furthermore, lower porosity is another reason for improving strength. In the brittle body, porosity induces several promising sites for producing cracks and its spreading [7]. Consequently, URHA in the system performs as reinforcement materials or an aggregate that improves the stress resists ability and prevents the crack propagation in the CSB, its mechanism graphically illustrates in Fig. 8. Conversely, above 20wt.% of URHA is harmful to CSB, i.e., reduced the strength value. It may take place due to the decrease of hydrated phases and an increase of non-ductile an-hydrated material (URHA).
Compressive strength, bending strength, humidity effect and thermal conductivity of cured CB samples.
| Samples | Compressive strength (MPa) | Bending strength (MPa) | Compressive strength after humidity effect (MPa) | Thermal conductivity (W/mK) at 30°C | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | HF (%) | ||
| s-0 | 4.85 | 0.21 | 2.23 | 0.15 | 4.41 | 0.17 | −9.07 | 0.114 |
| s-1 | 5.92 | 0.19 | 3.26 | 0.18 | 5.53 | 0.21 | −6.57 | 0.136 |
| s-2 | 7.64 | 0.22 | 4.57 | 0.21 | 7.32 | 0.19 | −4.18 | 0.153 |
| s-3 | 8.86 | 0.17 | 5.13 | 0.22 | 8.46 | 0.23 | −4.52 | 0.151 |
| s-4 | 10.43 | 0.31 | 5.87 | 0.28 | 9.95 | 0.22 | −4.60 | 0.145 |
| s-5 | 11.08 | 0.27 | 6.08 | 0.19 | 10.56 | 0.18 | −4.69 | 0.139 |
| s-6 | 10.57 | 0.32 | 5.48 | 0.22 | 10.06 | 0.21 | −4.82 | 0.131 |
In Indian weathering condition, the effect of humidity on the mechanical strength (compressive) is a vital characteristic for CSB specimens. In summer, the average temperature and relative humidity of some states in India are above 40°C and 60%, respectively [51]. Thus, the investigation is done at 80% of relative humidity and 50°C atmosphere temperature. Table 4 tabulates the compressive strength values after humidity treatment and % of humidity effect (HF). The result shows a decrement of strength values of CSB for all composition. It may be occurred because of moisture, which is absorbed through the capillary pores in the CSB samples at humidifying environment. The absorbed moisture ascribes to a reduction of cohesion forces between the particles and produces a diagonal bursting effect in the CSB structure. It is enhanced with the applied load [52]. Therefore, the strength value of the CSB samples is reduced. The probability of cracking in CSB structure is increased due to swelling in wall particles by the absorbed moisture. The absorbed moisture pressure with an applied load on the capillary pore wall is another possible reason for the reduction of compressive strength in humidity atmosphere [53].
The thermal properties especially thermal conductivity (κ) measurement is essential for the insulating board to find an application or selected working state and optimized to safe operation processes. Table 4 shows the room temperature κ of CSB specimens. It is observed that the calcium silicate hydrate containing CSB samples show the lower value of κ. It may be happened due to the major phase, calcium silicate hydrate, which exhibits poorer values of κ[14]. However, the OPC addition in the composition is slightly enhancing κ values. It may be due to the porosity which are decreased as the unit weight increased in the sample. On the other hand, the incorporation of URHA in the composition does not have a significant effect on the κ values. It may be happened due to lower κ values of amorphous RHA silica (0.140W/mK), which also acts as a barrier to the flow of heat and attributes to the lower value of κ[54].
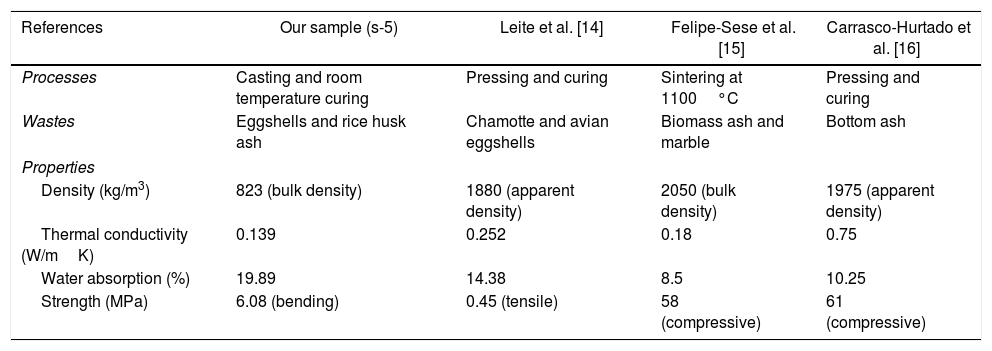
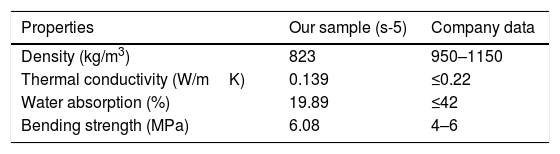
From the above-evaluated characteristic, s-5 specimen exhibits optimum properties. The key properties of s-5 are compared with the obtained results from the literature using other wastes for the fabrication of CSB, as shown in Table 5. However, the acquired synthesis processes are different (not casting and room temperature curing process) like's high-temperature sintering and autoclaved curing method for fabrication of CSB using wastes. Thus, obtained results are huge different from our study. Additionally, the obtained results in s-5 are compared with the technical data of an international supplier company of calcium silicate board [55], as illustrated in Table 6. It can be observed that the characteristics of waste derived CSB are very good compared to the technical data of the company. These promising characteristics and simple preparation method suggest that this work will open a new window to the calcium silicate board production industry for utilization of RHA and eggshell in the commercial production of CSB.
Comparison of properties between s-5 sample and obtained in the literature using other wastes.
| References | Our sample (s-5) | Leite et al. [14] | Felipe-Sese et al. [15] | Carrasco-Hurtado et al. [16] |
|---|---|---|---|---|
| Processes | Casting and room temperature curing | Pressing and curing | Sintering at 1100°C | Pressing and curing |
| Wastes | Eggshells and rice husk ash | Chamotte and avian eggshells | Biomass ash and marble | Bottom ash |
| Properties | ||||
| Density (kg/m3) | 823 (bulk density) | 1880 (apparent density) | 2050 (bulk density) | 1975 (apparent density) |
| Thermal conductivity (W/mK) | 0.139 | 0.252 | 0.18 | 0.75 |
| Water absorption (%) | 19.89 | 14.38 | 8.5 | 10.25 |
| Strength (MPa) | 6.08 (bending) | 0.45 (tensile) | 58 (compressive) | 61 (compressive) |
Comparison of properties between s-5 sample and company data [55].
| Properties | Our sample (s-5) | Company data |
|---|---|---|
| Density (kg/m3) | 823 | 950–1150 |
| Thermal conductivity (W/mK) | 0.139 | ≤0.22 |
| Water absorption (%) | 19.89 | ≤42 |
| Bending strength (MPa) | 6.08 | 4–6 |
Depending on the results obtained in this work, it can be concluded that the eggshells and RHA can be used as ingredients for the production of the sustainable CSB, which can be used for the lining of the inside of rooms as a partition wall or ceiling. The several characteristics of CSB have been examined, and the following results are obtained:
- •
It is detected that the 1050°C calcined CS powder contains belite (Ca2SiO4) and β-wollastonite (CaSiO3) as crystalline phases.
- •
The hydration products of CSB are chondrodite calcian (Ca5H2O10Si2) and hillebrandite (Ca2H2O5Si), which are responsible for providing the strength of the board.
- •
The wastes derived CSB demonstrates a lower density, low thermal conductivity, and good mechanical strength.
- •
OPC incorporation in the composition strongly influences the physical and mechanical properties of CB.
CSB with composition of s-5 (70wt.% CS+10wt.% OPC+20wt.% URHA) exhibits promising characteristic. Therefore, a sample of pilot size based on the composition of s-5 has been prepared as shown in Fig. 2. It leads to economically recycling of wastes in the ceramic industry for CSB manufacturing.
The authors are grateful to the DIC (IIT (BHU) & BHU), India for the financial assistance and also wish to thank CIFC (IIT BHU) for providing facilities.






 CSB specimens.' title='Preparation flowchart of
CSB specimens.' title='Preparation flowchart of  CSB samples and (b) pilot size sample.' title='Image of (a) different size
CSB samples and (b) pilot size sample.' title='Image of (a) different size 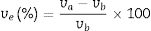
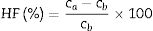
 XRD and (b)
XRD and (b) 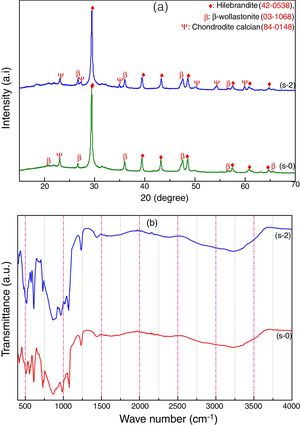 XRD and (b) FTIR analysis of
XRD and (b) FTIR analysis of 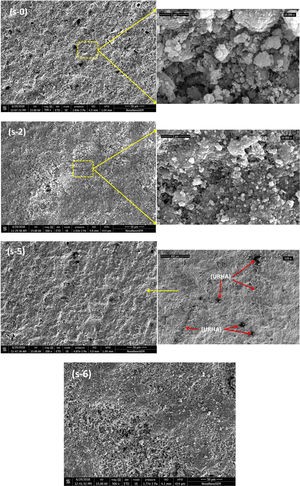 SEM analysis of
SEM analysis of 
 CSB sample.' title='Mechanisms of mechanical strength of s-5
CSB sample.' title='Mechanisms of mechanical strength of s-5 

