
Edited by: Marco Arrese - Pontifical Catholic University of Chile, Santiago, Chile
Last update: November 2023
More infoThe most common primary liver tumors are hepatocellular carcinoma and cholangiocarcinoma. They constitute the sixth most common neoplasia and the third cause of cancer-related deaths worldwide. Although both tumors may share etiologic factors, diagnosis, prognostic factors, and treatments, they differ substantially in determining distinctive clinical management. In recent years, significant advances have been made in the management of these neoplasms, particularly in advanced stages. In this review, we focus on the most relevant diagnostic, prognostic, and treatment aspects of both, hepatocellular carcinoma and cholangiocarcinoma, underlying their applicability in Latin America.
In recent years, there has been increasing evidence-based data regarding diagnosis, prognosis assessment, and treatment approaches for primary liver tumors. Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer [1] and its occurrence is closely associated with the presence of chronic liver disease. Cholangiocarcinoma (CCA) constitutes 10–20 % of primary liver tumors, and its incidence is steadily increasing worldwide. CCAs are heterogeneous and are commonly classified according to their anatomical localization as intrahepatic (iCCA), perihiliar (pCCA), or distal (dCCA) [2,3]. Each anatomical subtype is characterized by unique genetic aberrations, clinical presentations, and treatment options [3]. Although iCCA and HCC share associated risk factors, a relevant proportion of CCAs do not have an identifiable risk factor. In this review, we will focus on the most recent advancements and the diagnostic and treatment challenges in the management of patients with primary liver cancer, underlying the Latin American social, economic, and epidemiological perspectives.
2Epidemiological perspective for primary liver tumorsHCC comprises more than 80 % of primary liver tumors and is ranked as the sixth most frequent malignancy and third most common cause of cancer-related deaths worldwide [4]. Sex and race-ethnicity disparities in HCC incidence have been described; men are disproportionately affected compared to women [1]. On the other hand, Hispanics have experienced the largest increase in HCC incidence among all racial/ethnic groups, with an estimated 4.7 % increase in incidence each year since 2000 (vs. 4.3 % in blacks and whites and 0.6 % in Asians) [5]. The reported HCC incidence rate in Latin America ranges between 5 and 7 cases per 100.000 persons/year, and 4.4 % of HCC cases worldwide are diagnosed in this region (around 39,450 cases per year) [1,4,6].
Liver cirrhosis is the main risk factor associated with HCC development and chronic hepatitis B (HBV) and C (HCV) viral infections are the most frequent etiologies [7]. Universal HBV vaccination has dramatically reduced the incidence and mortality of HBV-related HCC [8], and on the other hand, effective antiviral therapies had led to a progressive decrease in HCV-related HCC [9]. A prospective multicenter Latin American cohort study including 1400 patients reported a relative HCC risk reduction of 73 % following HCV eradication, but this risk is not completely eliminated, particularly in patients with clinically significant portal hypertension [10]. The increasing prevalence of obesity, diabetes mellitus, and sedentary lifestyles, has led to a new pandemic of metabolic dysfunction associated steatotic liver disease (MASLD). This chronic liver disease has become one of the leading causes of HCC in developed countries [11]. Different definitions of this entity have raised caution when interpreting population studies analyzing large secondary databases [11].
The etiology of HCC in Latin America has changed over the past few decades. Before 2017, the main causes of HCC were HCV, HBV, and alcohol abuse [12,13]. Since then, MASLD-related HCC has quadrupled from 9 % to 37 % and has become the leading cause of HCC by 2021 [14]. A matter of debate is still open whether HCC prevention is effective with the use of metformin, statins [15], or aspirin [16,17] in this population.
The incidence of CCA is increasing worldwide, mainly at the expense of iCCA. Controversy remains regarding whether it is a true increasing incidence figure or rather a misclassification bias of cancer registries, improved diagnostic methods, greater awareness of the disease, and increased utilization of biopsies in indeterminate liver lesions [18]. Several risk factors are associated with CCA, most of which are linked to the chronic inflammation of the biliary epithelium and biliary stasis. Specific factors, such as the presence of cirrhosis, viral hepatitis, or fluke infections in endemic countries, have been clearly associated with iCCA [19] Additionally, recognized risk factors, such as obesity, metabolic syndrome, and excessive alcohol consumption have potentially contributed to the rising incidence of CCA [2,3,18,19]. However, most CCA cases do not present identifiable risk factors, which makes it difficult to identify at-risk patients for whom early diagnosis by surveillance may be advisable.
3Surveillance and diagnostic challengesHCC is a clear example of a potentially “screenable” cancer since it accomplishes most of the principles applied for surveillance programs [20]. Although robust evidence of decreased cancer-related mortality is lacking, the overall amount of evidence supports surveillance in high-risk groups of patients [21,22]. Optimizing HCC surveillance is a major challenge to improve the prognosis of this malignancy. Therefore, efforts should be made to develop models aimed at individualized HCC risk assessment (personalized surveillance) [23,24]. Although abdominal ultrasound has shown acceptable screening accuracy under expert hands, its real-life performance is suboptimal. On the other hand, current biomarkers (particularly AFP) do not significantly improve screening accuracy, increasing costs and potential harms [23]. Finally, improving patient education to increase surveillance adherence (longitudinal repetition of the diagnostic tool over time), such as mailed outreach strategies, patient navigation, clinical reminder systems, and partnership reinforcement with primary care providers, may improve HCC surveillance applications in real life [23]. In contrast, surveillance for CCA is unreliable, with scarce evidence supporting its efficacy [25]. The only patients who may benefit are those with primary sclerosing cholangitis, for whom most guidelines recommend surveillance by annual contrast-enhanced magnetic resonance cholangiopancreatography (MRCP). Surveillance for iCCA is even more questionable in patients with cirrhosis (for whom HCC surveillance by biannual ultrasound is already recommended). [26]
Non-invasive imaging is accepted for HCC diagnosis relying on specific radiological signs including the presence of tumor arterial contrast enhancement followed by contrast wash-out in the portal venous and/or delayed phases in patients at increased risk of HCC (cirrhosis irrespective of etiological factor, chronic HBV even without advanced fibrosis, and advanced fibrosis stages in chronic HCV) [21,22]. Despite these specific imaging criteria, non-invasive diagnosis is not feasible in a proportion of patients and pathological confirmation is required. Consequently, major efforts have been made to further improve the diagnostic accuracy of non-invasive criteria. Organ-specific contrast agents, such as gadoxetic acid, have shown promising results [26]. Regrettably, gadoxetic acid was not superior to extracellular contrast media in prospective studies conducted in Europe, and the evaluation of the hepatobiliary phase increased the sensitivity, but at the cost of decreasing specificity [27,28]. The most accurate pattern was arterial phase enhancement, followed by contrast washout, which was evaluated exclusively during the portal phase [27,28]. Another controversy is the use of the LI-RADS system proposed by the American College of Radiology, and updated several times until its latest version in 2018 [29]. It classifies the full spectrum of liver lesions in categories, ranging from benign (LR-1) to HCC (LR-5) or other malignancies (LR-M), each one with expected probabilities of HCC [30]. However, the conjunction of confidence intervals between LR-4 and LR-5 probabilities, and interobserver agreement are all concerning factors [31,32]. The high proportion of HCC lesions in LR-3 nodules previously detected by ultrasound [33], combined with the uncertainty regarding the need to distinguish between LR-4 and LR-5, as both categories have a very high probability of HCC diagnosis [33,34], limits the applicability of LI-RADS to clinical-decision making processes.
4Therapeutic approach for primary liver tumorsThe treatment management of primary liver tumors is complex, and a multidisciplinary approach is key to diagnosis, staging, and initial and sequential treatment approaches. Expert hepatologists, surgeons, radiologists, interventional radiologists, oncologists, pathologists, nurses, palliative care givers, and social workers collaborate to enhance access to and the quality of care for new cancer therapies. Clinical practice guidelines summarize the current state of knowledge and evaluate the quality and degree of scientific evidence available for each intervention, aimed at stating different recommendations. However, the final decision must be conducted by the patient's care responsible, either physician or even better, under a multidisciplinary tumor board, which needs to integrate all patients’ characteristics, preferences, and social context, and recommend a given path of individualized care.
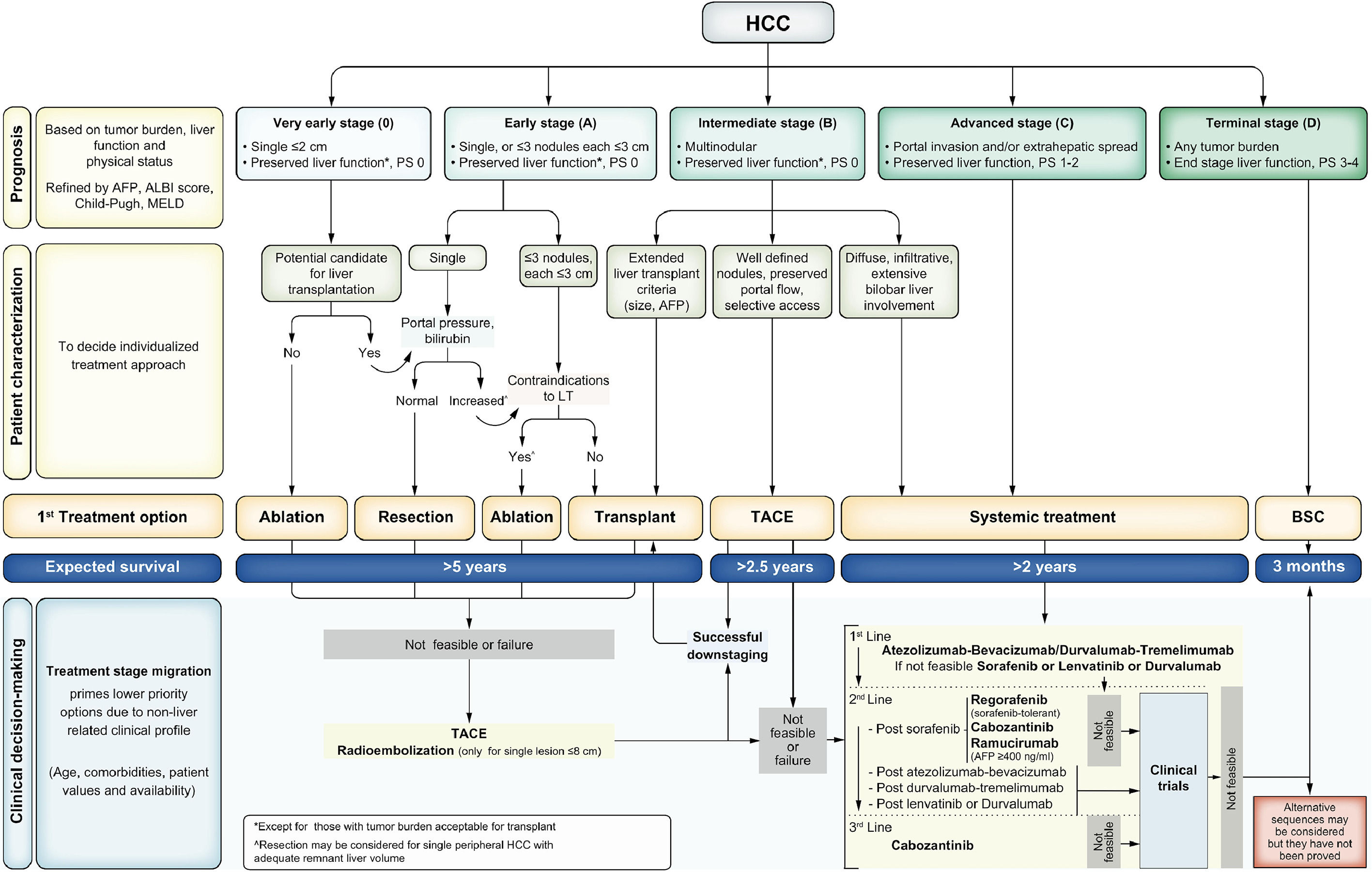
The prognosis assessment and management approach for HCC is framed by the Barcelona Clinic Liver Cancer (BCLC) staging system (Fig. 1) [35]. The BCLC staging system considers the tumor burden, degree of liver function impairment, and ECOG performance status, recognizes five tumor stages (very early, early, intermediate, advanced, and terminal stages), and links each stage with the recommended treatment approach based on the best available scientific evidence. Refinements such as the assessment of liver function using the ALBI score [36] and the incorporation of alpha-fetoprotein (AFP) values have been implemented in the latest BCLC version to further improve prognosis prediction and guide treatment approaches. Finally, it is worth stressing that these recommendations should be individualized and not considered dogmatic statements. In fact, the specific profile of an individual patient may induce a shift in the recommendation to an option that would be considered a priority for a more advanced stage (treatment stage migration). In addition, when patients present treatment failure or progression but still fit into their initial BCLC stage, a therapy corresponding to a more advanced stage should be considered (untreatable progression) [37,38].
The latest Barcelona Clinic Liver Cancer staging system [35]. AFP: Alpha-fetoprotein; ALBI: Albumin to Bilirubin ratio; BCLC: Barcelona Clinic Liver Cancer; LT: Liver Transplantation; PS: performance status; SIRT: Selective Internal Radiotherapy; TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization.
Compared to HCC, CCA is an orphan cancer, with scarce information regarding its natural history and prognostic factors. The relatively low incidence, high heterogeneity according to anatomical localization, and limited information from prospective studies with an adequate tumor stage and follow-up hamper the prognosis assessment of CCA, and the treatment approach is simplified according to resectability [39]. The most accepted staging system is the TNM proposed by the American Joint Committee on Cancer (AJCC) [40]
5Challenges of liver resectionSurgical resection remains the cornerstone therapy for primary liver tumors. The feasibility of liver resection depends on the quality of the parenchyma and the extent of resection required. As previously highlighted, HCC arises in most cases in the setting of advanced chronic liver disease, and the best results are achieved in the absence of clinically significant portal hypertension (CSPH) [41,42]. In the presence of CSPH, resection should be considered a significant risk factor for decreased mortality and postoperative liver decompensation. In this setting, liver transplantation (LT), which offers longer survival, should be considered as the treatment of choice. If not feasible due to comorbid conditions contraindicating LT or surgery, ablation offers similar outcomes with less risk for single tumors. Advances in minimally invasive surgery, particularly the laparoscopic approach, have allowed the expansion of resection indications when the tumor is in the appropriate location, with minor degree of CSPH and preserved liver function (MELD score <10 points) [43–45]. This individualized approach is not recommended for large liver resections; therefore, it should only be conducted through minor liver resections (segmentectomies). Although cohort studies of resection in multifocal tumors within the Milan criteria report encouraging survival results [46], LT remains the best option, and prospective data are needed to establish the effectiveness of resection compared to locoregional therapy. In this regard, there may be a small proportion of ideal HCC candidates for liver resection.
On the contrary, liver resection remains the cornerstone and primary treatment for CCA. The main challenge in determining resection eligibility revolves around assessing tumor extension. The appropriateness of surgery varies depending on the location of CCA, requiring distinct approaches for dCCA, pCCA, and iCCA. Regarding the evaluation of tumor extension, the use of PET scans remains a subject of debate, although it is recommended by major clinical practice guidelines [47,48]. In cases of iCCA and cirrhosis, liver resection should adhere to principles similar to those used for HCC, including the assessment of liver function reserve, consideration of the extent of resection, and evaluation for the presence of CSPH
6Liver transplantation for primary liver tumors: where are the limits?LT is an excellent treatment option for HCC because of its capacity for complete tumor removal, including any undetected liver micrometastases, and complete resolution of the underlying chronic liver disease. Regrettably, donor shortage is a significant barrier to its widespread application, demanding optimize selection of patients in whom post-LT expected 5-year survival is greater than 50–60 % [49]. To ensure equitable access to LT and reduce dropouts from the waiting list due to tumor progression, transplant allocation policies should adjust a fine balance between offer and demand of organs to provide timely access to LT, but at the same time allow an observation period to identify biologically aggressive tumors associated with a higher risk of post-LT recurrence.
Initially, LT for HCC resulted in poor post-transplant survival outcomes, owing to the high incidence rate of HCC recurrence. Following the 1980s, during the next decades, incidental pathology analysis promoted the proposal of the Milan criteria [50]. This morphological proposal of 1 lesion not larger than 5 cm in diameter or up-to 3 lesions, none of them above 3 cm in diameter, showed promising 5-years post-LT survival and recurrence rates. This huge change in the history of LT established the Milan criteria as the LT gold standard selection model. However, following this morphological criteria, other authors proposed “expanding” such criteria, modifying the number of nodules and/or diameters [51,52]. These criteria were so called the “expanded criteria” because there was an increase in the number of potential HCC candidates for LT, but they did not stratify the risk of recurrence in patients within Milan criteria (Table 1) [51–60]. Over the last years, several LT selection models have been proposed, such as the AFP model [61] or Metroticket 2.0 [54,62], “composite models” including not only morphological variables (size and number) but also AFP values before LT, which have shown superior discrimination power of HCC recurrence when compared to the Milan criteria. In contrast to the expanded criteria, these composite models optimize the transplant selection process either in patients within or beyond the Milan criteria, without a significant increase in the number of listed patients for LT [63].
Liver Transplant criteria including morphometric and biomarker data.
These mathematical models were developed using regression models, predicting the event of interest (either death or HCC recurrence after transplantation) on “average” (meaning population effects). Thus, applying these models to individual patients might not be perfectly accurate since most of these selection models did not evaluate/incorporate intra individual variability [64] nor dynamic changes of AFP values [65]. Furthermore, limited tumor progression beyond the Milan criteria over the waitlist period may not be associated with an increased recurrence risk [66]. The performance of these mathematical models is often evaluated through rigorous techniques by analyzing their ability to discrimination and calibration [67–69]. In the context of LT, time-to event discrimination and calibration should be estimated. Moreover, there might be deaths without recurrence after LT that preclude to observe the development of recurrence even in high-risk patients. These events compete for observing the main event of interest, HCC recurrence, and thus time-to-recurrence models after LT should be modelled through competing risk regression [70]. Most importanly, discrimination indexes of time-to-event analysis are difficult to interpret for the clinician, and a numerical or even statistically significant difference between models might not show a clinically significant value. Moreover, the discrimination power of a mathematical model that includes continuous data may increase discrimination, but this may not result in clinically meaningful reclassification of risks. In Latin America, the AFP model has been previously validated, and the application of composite models may not substantially increase the number of HCC patients included in the list or the waiting list mortality in patients without HCC following other organ allocation policies [63]. Furthermore, patients within or beyond the Milan criteria presenting with an AFP-score ≤ 2 points presented similar 5-year survival and recurrence rates as those within the Milan criteria [63]. Finally, there is not a significant difference in the net reclassification of risk stratum between the AFP model and the Metroticket 2.0 underlying that both models could be use in the clinical setting [71].
Finally, the benefit of locoregional treatments aimed at reducing tumor burden and allowing patients to be eligible for LT (“downstaging”) was recently confirmed in a randomized phase IIb/III trial [72]. However, there are still several uncertainties, such as the initial tumor burden limit for patient selection to attempt downstaging, the acceptable treatment approaches, or criteria for considering downstaging success, the observation time, and to what limit should the tumor burden be reduced [73]. Although most data come from retrospective cohorts at high risk of bias, there is agreement that a tumor burden limit regarding size and number should be established since the baseline tumor stage determines the success of the strategy and the risk of recurrence despite successful downstaging [74–78]. In addition, biomarkers such as AFP optimize the selection of patients for downstaging [75,77,78]. Finally, recent advances in systemic treatments, including immune checkpoint inhibitors and their potential combination with locoregional treatments, have prompted a discussion regarding their role in downstaging, but data to support their use in this setting are still scarce and immature [79].
LT for CCA is contraindicated in dCCA; however, it may be an option in selected cases of pCCA and iCCA [80]. More recent retrospective studies have shown encouraging results in terms of OS when extensive patient selection was performed. The protocols for selecting patients were different for pCCA and iCCA. Patient selection and neoadjuvant chemoradiation protocols are crucial in the setting of LT for pCCA. The Mayo Clinic proposal is a frequently evaluated protocol that includes specific criteria such as lesion size, absence of extension below the cystic duct, and exclusion of lymph node metastases. Although vascular encapsulation and conduit extension are not contraindications for neoadjuvant treatment, they are associated with a worse prognosis. Neoadjuvant therapy for pCCA involves external beam radiation, concurrent 5-fluorouracil and brachytherapy, followed by capecitabine maintenance until LT. Staging laparoscopy is recommended before transplantation, including complete abdominal examination, routine lymph node biopsy, and biopsy of suspicious lesions. The timing of surgical staging is debated, especially in patients with primary sclerosing cholangitis (PSC) and advanced liver disease. However, PSC patients with pCCA typically have a lower probability of dropout than those with de novo pCCA.
In iCCA, selection based on tumor burden is of great relevance, as demonstrated in an international multicenter study that included 48 patients with incidental iCCA who underwent LT. Among them, 15 had "very early" iCCA (single tumor ≤ 2 cm), and 33 had advanced iCCA (single >2 cm or multifocal). In the very early cohort, the 5-year cumulative risk of recurrence and survival was significantly higher (18% vs. 61 % and 65% vs. 45 %, respectively) [81]. Microvascular invasion and poor tumor differentiation have been identified as predictors of tumor recurrence. The remaining 33 patients (advanced iCCA group) were further divided into an intermediate stage (single tumors 2.1–3 cm, not poorly differentiated) and an advanced stage (all other tumors), with the intermediate stage showing better overall survival rates [81]. More recently, a meta-analysis of 18 studies reported a 5-year OS for very early iCCA of 71% vs. 48 % for advanced iCCA [82]. Remarkably, all of these data came from retrospective studies, including mostly LT patients in whom iCCA was incidental finding. Thus, prospective studies with well-defined inclusion criteria and predefined post-LT imaging follow-up are urgently needed. The evidence supporting LT for locally advanced, unresectable iCCA is even scarcer because most studies are single-center, retrospective, and include a low number of patients and heterogeneous populations in terms of tumor stage and neoadjuvant approach [83,84]. The response to neoadjuvant chemotherapy, especially in the context of new personalized target therapies, may help identify patients with biologically less aggressive tumors in whom LT may offer adequate outcomes [39].
7Locoregional treatmentsTransarterial chemoembolization (TACE) is the treatment of choice in patients with intermediate HCC or BCLC-B, based on two RCT [85,86]. Remarkably, the intermediate stage comprises a heterogeneous population, and the 2022 BCLC version stratifies the BCLC-B stage into 3 groups of patients according to tumor burden, liver function, and applicability of being able to selectively perform TACE [35] The median OS of intermediate HCC patients treated with TACE is 30–40 months when adequate patient selection and the use of state-of-the-art and super-selective techniques are in place [87,88]. The use of drug-eluting beads has enabled standardization of this procedure, resulting in higher reproducibility and tolerability of the treatment, but without superior survival benefit or higher response rates than conventional TACE [87,89]. Available studies comparing bland embolization with TACE are not informative, as the included population does not match the profile of patients for whom TACE would be recommended [85,90,91]. A pivotal consideration arises when determining the point at which a tumor becomes refractory to TACE. While TACE failure has historically been defined as the transition from BCLC B to stage C, TACE refractoriness extends treatment failure to encompass patients who exhibit no objective response after undergoing two consecutive sessions of TACE. In such cases, these patients should be considered for subsequent therapy lines with the objective of preserving liver function and affording patients the benefit of effective systemic treatment options. Various retrospective clinical studies have supported this approach, noting improved survival among patients who expeditiously transitioned to sorafenib once they met the criteria for TACE refractoriness in contrast to those who persisted with repeated TACE sessions [92,93]. Similar to TACE refractoriness, the exclusion of TACE therapy in cases classified as BCLC stage B hinges upon the potential risk of treatment-associated hepatic dysfunction. This subgroup of patients frequently presents with extensive, infiltrative, and diffuse bilobar involvement, and selectivity in angiographic treatment cannot be assured. In recent years, several studies have assessed the benefits of combining different anti-angiogenic agents with TACE (Table 2), but none showed survival benefits, and their use is not recommended [94–100]. Ongoing trials are evaluating the benefits of adding immune checkpoint inhibitors (ICI) to TACE in the intermediate stage.
Clinical trials evaluating combined chemoembolization and systemic therapies.
| Study | Population | Design/Intervention | Results |
|---|---|---|---|
| BRISK-TA trialHepatology 2014[94] | n = 502BCLC B, ECOG 0–1Child Pugh A/B | Randomized 1:1. Phase III• Brivanib + TACE DEB o cTACE• Placebo + TACE DEB o cTACE | OS and TTP similar.(mRECIST)*Study was discontinued |
| SPACE trialJ Hepatology 2016[95] | n = 307BCLC B,ECOG 0–1Child Pugh A (99 %) | Randomized 1:1. Phase II• Sorafenib + TACE DEB• Placebo + TACE DEB | Similar TTP [HR 0.79 (95 % CI 0.59;1.08) and PFS (mRECIST)ORR 35.7% vs 28.1 %.Median survival NR |
| ORIENTAL trialLancet 2017[158] | n = 889BCLC B/C (Vp1–2), ECOG 0–1Child Pugh A | Randomized 1:1. Phase III• Orantinib + TACEc• Placebo + TACEc | OS no benefitTTP better orantinibTime-to-TACE failure and EH/MVI progression without differences. |
| TACE-2 trialLancet 2017[96] | n = 313BCLC B, ECOG 0–1Child Pugh A | Randomized 1:1.Phase III• Sorafenib + TACE DEB• Placebo + TACE DEB | PFS, OS no significant diferencesORR 36% vs 31 %.(RECIST 1.1) |
| TACTICS trialGut 2019[97] | n = 156BCLC B-C, ECOG 0–1Child Pugh A/B | Randomized 1:1,open-label. phase III• Sorafenib + TACEc• TACEc | Time-to-unTACE progression benefit.ORR 71% vs 62 %.(RECICL) |
| STAH trialJ of Hepatology 2019[159] | n = 300BCLC B-C, ECOG 0–1Child Pugh A/B | Randomized 1:1.Phase III• Sorafenib + TACEc• Placebo + TACEc | No significant benefit in OS and PFS |
| LAUNCH trialJ Clinical Oncol 2022[160] | n = 338BCLC C, ECOG 0–1Child Pugh A | Randomized 1:1.Phase III• Lenvatinib + TACE• Lenvatinib | OS HR 0.45(95 % CI 0.33;0.61)PFS HR 0.43(95 % CI 0.34;0.55)ORR 54% vs 25 %.(mRECIST) |
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; TACE, transarterial chemoembolization; DEB, drug eluting beads; RECIST, response evaluation criteria for solid tumors; OS, overall survival; PFS, progression free survival; RECICL, response evaluation criteria in cancer of the liver. ORR, objective response rate; EH, extrahepatic metastasis; MVI, macrovascular invasion; Vp1–2, portal vein tumor thrombosis. NR, not reached; TTP, time-to-progression; HR, hazard ratio.
Transarterial radioembolization (TARE), also known as Selective Internal Radiotherapy (SIRT), has been proposed as an alternative treatment option for primary liver tumors. In the setting of HCC, several retrospective studies including heterogeneous populations have shown an adequate safety profile and efficacy in terms of radiological tumor response [101–103], establishing the rationale to carry out clinical trials comparing TARE vs. sorafenib [104,105] or the addition of TARE to sorafenib vs. sorafenib alone [106]. Regrettably, all three studies failed to demonstrate the benefit of TARE in terms of survival despite showing a higher rate of local tumor response and an adequate safety profile. The importance of personalized dosimetry was suggested by a sub-analysis of the SARAH trial [107] and replicated in the DOSISPHERE-01 trial, an open-labeled, phase 2 trial, in which personalized dosimetry significantly improved survival compared to the standard radiation dose (median OS of 26.6 vs. 10.7 months) [108]. Thus, TARE may serve as an alternative intra-arterial therapy for HCC in the early and intermediate stages. The best results were obtained in non-resectable single lesions <8 cm in diameter, delivering a super-selectively high radiation dose (median absorbed dose of 410 Gy), as shown in the LEGACY study [109]. This was a multicenter, retrospective study that included 162 patients with single lesions of up to 8 cm (median size 2.7 cm) treated with TARE, achieving an objective response rate (ORR) of 88 %, with a median duration of response of 6.5 months [109]. These results in the initial stages were confirmed in the recent RASER study [110]. However, controversy remains regarding the intermediate stages. Retrospective studies have suggested that TARE offers a longer time to progression (TTP) and a more durable tumor response than TACE, although with no clear benefit on survival, and comparison of TTP between the two modalities can be difficult given post-radiation changes, complicating the interpretation of response [111]. More recently, a phase 2, randomized controlled trial (TRACE trial), comparing TARE vs. TACE-DEB in BCLC A or B patients has shown a significant benefit PFS in the TARE arm [17.1 vs 9.5 months; HR 0.36 (95 % CI 0.18;0.70)] [112]. Concerns regarding the adequacy of the control arm and follow-up consistency limit the data reliability.
TARE was also evaluated in iCCA. Although suggested as a promising treatment approach for iCCA and included as a treatment option in the most updated clinical practice guidelines [47,48], most studies were conducted in single centers, including a small number of patients with heterogeneous inclusion criteria [113–120]. Recently, Edeline et al. conducted an emulated target trial using individual patient data from several prospective trials aimed to compare chemotherapy alone or in combination with TARE as the first-line therapy in locally advanced iCCA. Causal inference models of inverse probability of treatment weighting (IPTW) methods were used after a propensity score matching analysis to minimize confounding and intervention bias. Patients treated with chemotherapy and TARE had significant OS [median OS 21.7 months (95 %CI: 14.1; not reached) vs 15.9 months (95 %CI: 9.8; 18.9), HR=0.59 (95 %CI: 0.34; 0.99), p = 0.049] and PFS compared to those treated with chemotherapy alone [median 14.3 months (95 %CI: 7.8; not reached) vs 8.4 months (95 %CI: 5.9; 12.1), HR=0.52 (95 %CI: 0.31; 0.89), p < 0.001] [121] These promising results, which should be confirmed by factual and counterfactual evidence, represent a promising treatment approach in the management of patients with locally advanced iCCA.
8Systemic treatment in liver cancer8.1Hepatocellular carcinomaThe irruption of immunotherapy as effective therapy for HCC has completely revolutionized the treatment armamentarium and improved the expected survival to unexpected levels decades before. The initial demonstration of the survival benefit of sorafenib compared to placebo was a breakthrough in the management of HCC and opened a window of opportunity for testing targeted therapies for this devastating disease [122,123]. After a decade of failures, in the last five years, we have witnessed a plethora of new agents/combinations that have completely changed the landscape of systemic therapy for HCC (Table 3) [122–132]. Systemic treatment is the recommended option for BCLC-B patients who are not candidates or have progressed to TACE, and for BCLCC patients [133]. First-line management of advanced HCC has evolved substantially in the past few years, and immunotherapy–based combinations are the preferred first-line option, keeping tyrosine kinase inhibitors (TKIs) as an alternative in cases of contraindications to immunotherapies (Fig. 3). Both atezolizumab (anti-PD-L1) combined with bevacizumab (anti-VEGF) (Atezo-Bev) and a single priming dose of tremelimumab (anti-CTLA4) with durvalumab (anti-PD-L1) (STRIDE regimen) are currently the first-choice treatments in first-line, as they improve survival compared to sorafenib [125,130,129]. Based on the IMBrave-150 trial, the benefit of Atezo-Bev is in patients with preserved liver function and absence of high-risk stigmata for bleeding (properly treated esophageal varices and no history of variceal bleeding), cardiovascular disorders, autoimmune conditions (including post-transplant patients), and uncontrolled arterial hypertension. More recently, data from the phase 3 HIMALAYA trial showed that STRIDE regimen provided a significant survival benefit vs. sorafenib, and durvalumab as monotherapy was not inferior to sorafenib in first-line treatment in advanced HCC, but excluding those with tumoral invasion of the main portal vein [129]. Up-to now, atezolizumab + bevacizumab has been approved in most Latin American countries, expect for some Caribbean, countries in which TKIs are still the first-line treatment of choice. In contrast, the STRIDE regimen has been approved in limited number of countries in the region. The access and high-related health costs of these treatments require further discussion, not the scope of this review.
Phase III clinical trials evaluating systemic treatment options in which efficacy was demonstrated following the hypothesis main test of their design.
| Study | Population | Design/Intervention | Results |
|---|---|---|---|
| First-line | |||
| SHARP*[122] | n = 602Child Pugh AECOG 0–2BCLC B-C | Randomized 1:1.Double-blindedPhase III• Sorafenib 800 mg• Placebo | 88 % Europe. 29 % HCV+Median OS 10.7 vs. 7.9 monthsHR 0.69 (95 % CI 0.55;0.87)ORR 2% vs. 1 % (RECIST)Median TTP 5.5 vs. 2.8 monthsHR 0.58 (CI 0.45;0.74) |
| REFLECT[124] | n = 954Child Pugh AECOG 0–1BCLC B-CNo main portal vein tumor thrombosis,no biliary involvement and no >50 % of liver volume compromised. | Randomized 1:1.Open-labeledPhase III,Non-inferiority• Lenvatinib 8/12 mg• Sorafenib 800 mg | 67 % Asia. 50 % HBV+Median OS 13.6 vs. 12.3 monthsHR 0.92 (95 % CI 0.79;1.06)ORR 24% vs 9 % (RECIST 1.1)Median PFS 7.3 vs. 3.6 monthsHR 0.60 (CI 0.51;0.71)Grade 3–4 SAEs 43% vs. 30 % |
| IMbrave-150 [125,130] | n = 501Child Pugh AECOG 0–1BCLC B-CControlled esophageal varices, no immune active disease. | Randomized 2:1.Open-labeledPhase III• Atezolizumab 1200 mg + bevacizumab 15 mg/kg Q3W• Sorafenib 800 mg | 40 % Asia. 47 % HBV+Median OS 19.2 vs. 13.4 monthsHR 0.66 (95 % CI 0.52;0.85)ORR 30% vs. 11 % (RECIST 1.1)Median PFS 6.9 vs. 4.3 monthsHR 0.65 (CI 0.53;0.81)Grade 3–4 TRAEs 43% vs. 46 %imAE 21% vs. 12 %Bleeding 7.5% vs. 4.5 % |
| HIMALAYA***[129] | n = 1171**Child Pugh AECOG 0–1BCLC B-CNo main portal vein tumor thrombosis, no immune active disease | Randomized 1:1.Open-labeledPhase III• Durvalumab 1200 mg Q4W + Tremelimumab 300 mg single dose (STRIDE)• Durvalumab 1200 mg Q4W(Non-inferiority)• Sorafenib 800 mg | 41 % Asia. 42 % Non-viralMedian OS STRIDE 16.4 vs.Durvalumab 16.6 vsSorafenib 13.8 monthsHR STRIDE 0.78(95 % CI 0.65;0.93)HR Durvalumab 0.86(CI 0.73;1.03)ORR 20 %, 17 % and 5 % (RECIST 1.1)Median PFS 3.8, 3.6 and 4.1 months, respectivelyHR 0.90 (CI 0.77;1.05)and 1.02 (CI 0.88;1.19)Grade 3–4 TRAEs 26% vs 37 %imAE 36% vs. 1.6 %Bleeding 1.8% vs. 4.8 % |
| RATIONALE-301[131] | n = 750Child Pugh AECOG 0–1BCLC B-CNo main portal vein tumor thrombosis, no immune active disease | Randomized 1:1.Open-labeledPhase IIINon-inferiority• Tislelizumab 200 mg Q3W• Sorafenib 800 mg | Median OS 15.9 vs. 14.1 monthsHR 0.85 (95 % CI 0.71;1.02)HR Durvalumab 0.86(CI 0.73;1.03)ORR 14 %, vs. 5 % (RECIST 1.1)Median PFS 2.1 and 3.4 monthsHR 1.11 (CI 0.92;1.33)Grade 3–4 TRAEs 22.2% vs. 53.4 %imAE 17.2% vs. 3.1 % |
| CARES-310[132] | n = 543Child Pugh AECOG 0–1BCLC B-C | Randomized 1:1.Open-labeledPhase III• Camrelizumab 200 mg Q2W + rivoceranib 250 mg• Sorafenib 800 mg | 83 % Asia. 74 % HBV+Median OS 22.1 vs. 15.2 monthsHR 0.62 (95 % CI 0.49;0.80)ORR 25 %, vs. 6 % (RECIST 1.1)Median PFS 5.6 and 3.7 monthsHR 0.52 (CI 0.41;0.65)Grade 3–4 TRAEs 80.5% vs. 52 %Bleeding 32% vs. 3.3 % |
| Second-line | |||
| RESORCE[126] | n = 553Child Pugh AECOG 0–1BCLC B-CTolerant post Sorafenib under progression | Randomized 2:1.Double-blindedPhase III• Regorafenib 160 mg QD(ONOFF)• Placebo(ONOFF) | 62 % Asia. 38 % HBV+Median OS 10.6 vs. 7.8 monthsHR 0.63 (95 % CI 0.50;0.79)ORR 11 %, vs. 4 % (RECIST 1.1)Median PFS 3.1 and 1.5 monthsHR 0.46 (CI 0.37;0.56)Grade 3–4 TRAEs 50% vs. 17 % |
| CELESTIAL[127] | n = 747Child Pugh AECOG 0–1BCLC B-CTolerant or intolerant post Sorafenib under progressionUp-to 2 prior-lines (one should be sorafenib) | Randomized 2:1.Double-blindedPhase III• Cabozantinib60 mg QD• Placebo | 25 % Asia. 38 % HBV+Median OS 10.2 vs. 8.0 monthsHR 0.76 (95 % CI 0.63;0.93)ORR 4 %, vs. 1 % (RECIST 1.1)Median PFS 5.2 and 1.9 monthsHR 0.44 (CI 0.36;0.52)Grade 3–4 TRAEs NAGrade 3–4 AEs 68% vs. 37 % |
| REACH-2[128] | n = 747Child Pugh AECOG 0–1BCLC B-CTolerant or intolerant post Sorafenib under progressionAFP ≥400 ng/ml | Randomized 2:1.Double-blindedPhase III• Ramucirumab8 mg/kg Q2W• Placebo | 53 % Americas. 38 % HBV+Median OS 8.1 vs. 5.0 monthsHR 0.69 (95 % CI 0.57;0.84)ORR 5 %, vs. 1 % (RECIST 1.1)Median PFS 3.0 and 1.6 monthsHR 0.44 (CI 0.31;0.58)Grade 3–4 TRAEs NAGrade 3–4 AEs 4% vs. 3 % |
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NA, non-assessed; RECIST, response evaluation criteria for solid tumors; OS, overall survival; PFS, progression free survival; ORR, objective response rate; SAE, serious adverse events; TTP, time-to-progression; TRAEs, treatment-related adverse events.
Note: *Similar results in ASIA-PACIFIC trial **A fourth arm exploring Tremelimumab 75 mg + Durvalumab was closed over the study period (n = 153) [129].***Similar results in the ORIENT trial with the combination of Sintilimab plus bevacizumab biosmilar (Chinese population) [161].
The CARES-310 trial assessing the combination of camrelizumab (anti-PD-L1) and rivoceranib (oral TKi anti-VEGF) demonstrated benefits in OS and PFS compared to sorafenib [132]. However, it is worth noting that approximately 83 % of the enrolled patients were from Asia, with a prevalence of over 85 % with chronic liver disease related to hepatotropic viruses, primarily HBV (more than 70 %). This demographic composition makes it challenging to generalize the results to Latin America. In the CARES-310 study, adverse events related to treatment of grade 3 or higher were observed in 81 % of patients receiving camrelizumab-rivoceranib, with discontinuation of at least one medication in 24 % of cases. This indicates that camrelizumab-rivoceranib therapy was associated with a higher risk of toxicity than sorafenib therapy [132].
Other first-line treatment options are based on non-inferior results against sorafenib, including lenvatinib [134], durvalumab [129], and tislelizumab (RATIONALE-301 trial, NCT03412773). Lenvatinib has been approved in Latin America, but durvalumab or tislelizumab, as well as the combination explored in CARES-310, has not yet been approved.
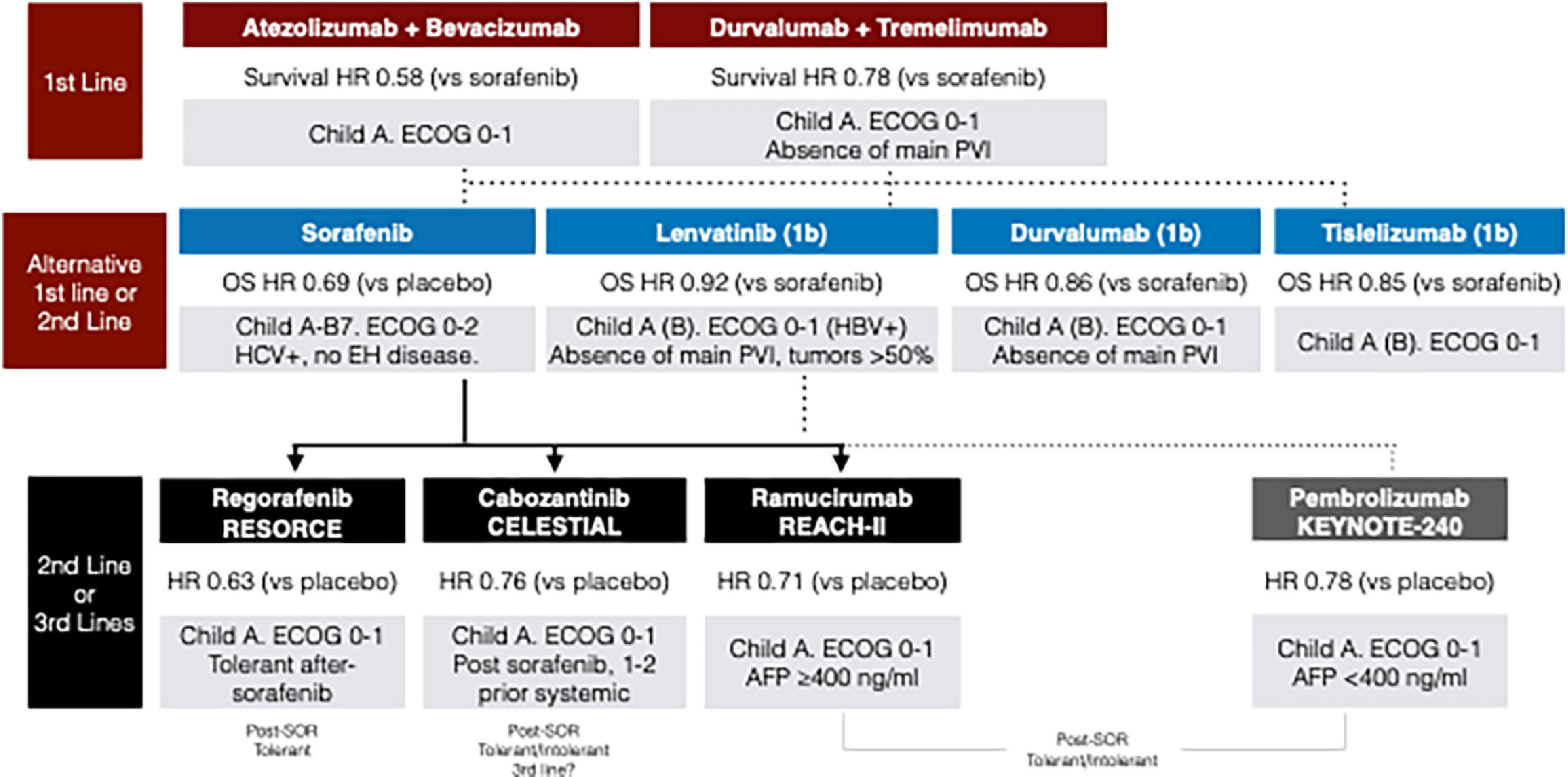
The choice of initial treatment should be based on the patient's clinical characteristics, including comorbidities, bleeding risk, and presence of tumoral portal thrombosis. This selection should be aligned with the inclusion criteria of studies supporting each treatment option. Presently, two treatment regimens have demonstrated superiority over sorafenib; however, there is insufficient evidence to recommend one over the other. The decision should consider the patient's unique clinical profile, eligibility criteria from the trials, treatment availability within the region, and patient preference. For instance, in cases where patients have a high risk of bleeding or contraindications to bevacizumab, dual therapy with immune checkpoint inhibitors (ICIs), such as tremelimumab with durvalumab, may be considered. Similarly, it is mandatory to note that patients with tumor invasion of the main portal vein were excluded from the HILAMAYA [129] and REFLECT [134] trials and therefore, the efficacy of Tremelimumab-Durvalumab or Lenvatinib in this population is not proven. Lastly, for patients with autoimmune diseases or prior organ transplantation, with a substantial risk of immunotherapy-related adverse effects, TKIs such as sorafenib or lenvatinib, will continue to be first-line treatment options. In summary, given the array of available first-line treatment choices, a thorough clinical assessment of each patient is essential to determine the most appropriate treatment based on the individual clinical situation.
Among second-line treatments, there are those with robust data in patients who have progressed on sorafenib (regorafenib, cabozantinib, and ramucirumab) (Table 3) [126–128]. Currently, we only have real-world data to support the selection of second-line regimens after the progression of immunotherapy-based treatments [135–140]. In this context, the best option clearly results in enrolling patients in clinical trials, but availability and access to these are at least scarce in Latin America [141,142].
Finally, there are two contentious issues in the realm of systemic HCC treatment. The first pertains to the impact of immunotherapy on a subgroup of patients with a non-viral etiology of liver disease. Although it was initially postulated that immunotherapy might have reduced efficacy in this patient subgroup, these conclusions were refuted by a recent meta-analysis, shedding light on the impact of immunotherapy in patients with non-viral etiology [143]. In this setting, the etiology of the liver disease does not determine the selection of systemic treatment. Similarly, a critical point of discussion revolves around the impact of immunotherapy on patients excluded from clinical trials. This includes patients with some degree of liver function deterioration, such as Child-Pugh B. Although acceptable tolerability has been demonstrated in this subgroup with nivolumab or atezolizumab-bevacizumab, substantial uncertainty remains regarding the clinical benefit in terms of survival when applying these treatments [144].
8.2CholangiocarcinomaSystemic chemotherapy is the standard treatment for patients with advanced-stage CCA [145,146]. Until recently, the only option that demonstrated a survival benefit in first-line treatment was the combination of gemcitabine and cisplatin (GemCis) (Fig. 4). In the ABC-02 trial, the GemCis combination showed a median overall survival of 11.7 months compared to 8.1 months for gemcitabine alone [147]. Two recent clinical trials, TOPAZ-1 [148] and KEYNOTE-966 [149], have shown promising results in the treatment of metastatic and advanced CCA. The addition of either durvalumab or pembrolizumab to GemCis has been found to improve patient survival, leading to the widespread adoption of triple therapy comprising an anti-PD-1/anti-PD-L1 agent and GemCis as the current first-line treatment for CCA [145,146].
In the TOPAZ-1 trial, the combination of GemCis and Durvalumab demonstrated a median OS of 12.8 (11.1–14.0) months, with a median PFS of 7.2 (6.7–7.4) months and an ORR of 26.7 %. This combination was well-tolerated. Updated OS and safety data after an additional 6.5 months of follow-up, were recently reported in ESMO 2022 [150]. The addition of Durvalumab to GemCis resulted in a longer median OS of 12.9 (11.6–14.1) months, HR: 0.76 (95 % CI 0.64–0.91), and manageable safety [150]. Additionally, a real-life Italian multicenter study recently confirmed these positive results [151]. In the KEYNOTE-966 study, the combination of GemCis plus pembrolizumab demonstrated a median OS of 12.7 (11.5–13.6) months, with a median PFS of 6.5 (5.7–6.9) months, an ORR of 29 %, and manageable safety. These results are encouraging, and as a result, durvalumab plus gemcitabine and pembrolizumab plus gemcitabine have become the new standard first-line systemic therapy options for advanced CCA.
Targeted therapies represent one of the most significant therapeutic advances in CCA [145,146]. Several targeted therapies in phase 2 studies have shown promising results, with some gaining FDA approval as second-line therapies. Examples of these advances include pemigatinib [152], infigratinib [153], and futibatinib [154] for patients with FGFR2 fusion/rearrangement; ivosidenib for patients with IDH1 mutation [155]; pembrolizumab for patients with metastatic solid tumors with deficient mismatch repair/high microsatellite instability (dMMR/MSI-H) [156]; and dabrafenib-trametinib for patients with BRAF V600E mutated metastatic solid tumors [157]. These results have reshaped the treatment approaches for patients with iCCA, even in the context of effective immunotherapy, depending on the presence of specific mutations (Fig. 2). Currently, performing molecular profiling in iCCA patients considered for first-line systemic therapy is recommended, not only to maximize treatment benefits but also to advocate for personalized treatments in the near future [145,146].
Systemic treatments for cholangiocarcinoma following molecular profiling. GEM-CIS: Gemcitabine-cisplatin;.DURVA: druvalumab; PEMBRO: pembrolizumab; FGRi: Fibroblast growth factor receptor inhibitor; GBC: gall bladder cancer; IDH mutations: isocitrate dehydrogenase mutations; mFOLFOX: modified: 5-fluorouracil-oxaliplatin-folinic acid; CA: cholangiocarcinoma; dCA: distal cholangiocarcinoma, pCA: perihiliar cholangiocarcinoma; iCA: intrahepatic cholangiocarcinoma.
Systemic treatments for advanced hepatocellular carcinoma. AFP: Alpha-fetoprotein; HR: hazard ratio; HBV: hepatitis B virus; HCV: hepatitis C virus; OS: overall survival; SOR: sorafenib; PVI: portal vein tumor invasion.
Note: In the second-line, strong data is only available after sorafenib failure (continuous arrows). Treatment recommendations after immunotherapy-based treatments are based only on real-world data (dotted arrows). Up to this year, there has been no approval of STRIDE regimen (durvalumab-tremelimumab), or durvalumab, or tislelizumab in Latin America. Pembrolizumab has been approved in some Latin American countries, even showing negative statistical results in the KEYNOTE-240 trial [162].
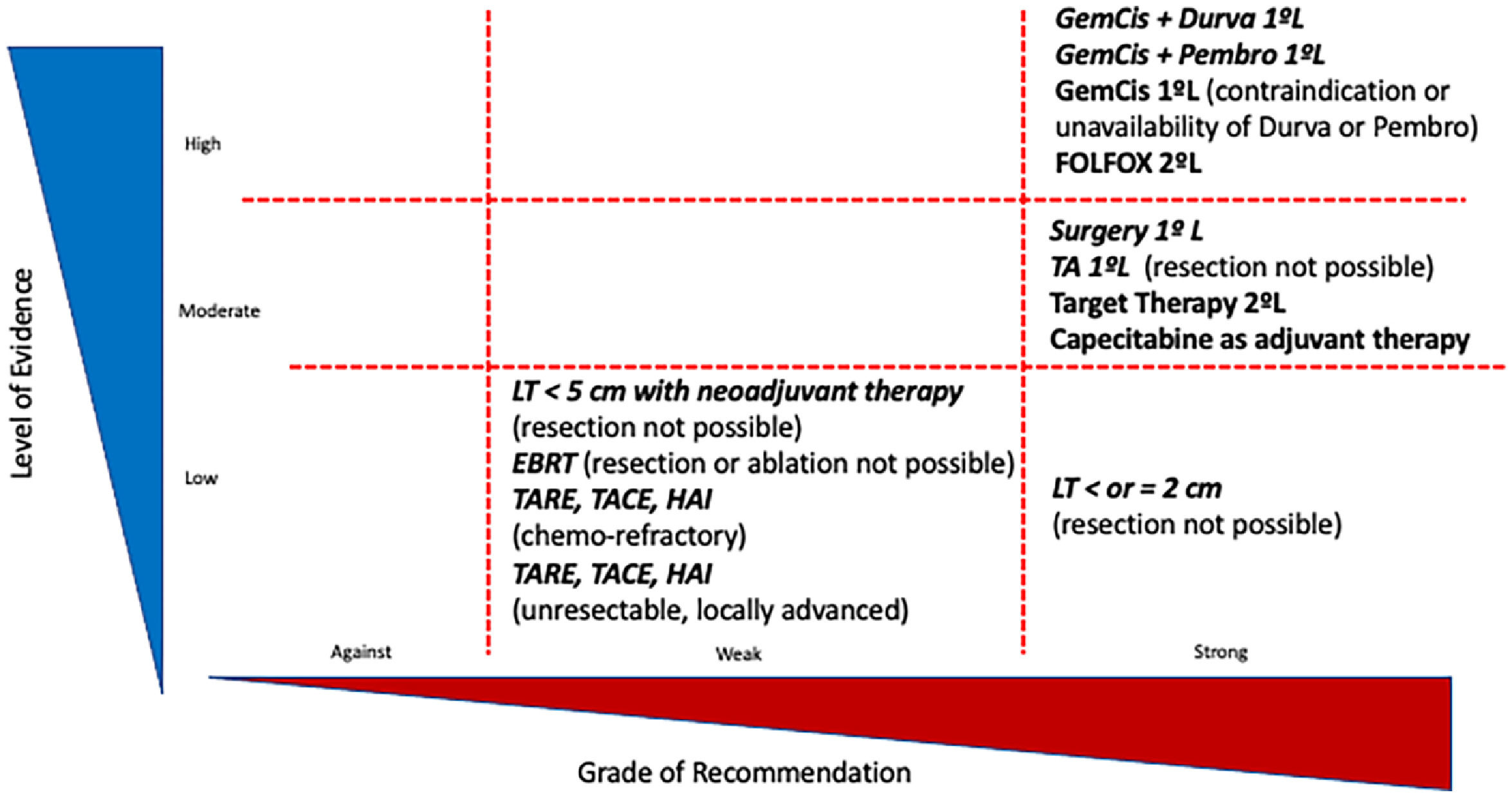
Level of the evidence and grade of recommendation for the treatment of cholangiocarcinoma. GEM-CIS: Gemcitabine-cisplatin;.DURVA: druvalumab; PEMBRO: pembrolizumab; FGRi: Fibroblast growth factor receptor inhibitor; GBC: gall bladder cancer; IDH mutations: isocitrate dehydrogenase mutations; mFOLFOX: modified: 5-fluorouracil-oxaliplatin-folinic acid; SIRT: Selective Internal Radiotherapy; TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization; HAI: hepatic arterial infusion.
The landscape of knowledge and availability of effective interventions in the realm of liver tumors have experienced rapid escalation. This intricate panorama should not underscore the importance of multidisciplinary care and meticulous management of individual cases in clinical decision making.
In HCC, novel scenarios will be shaped by ongoing phase 3 trials examining the efficacy of adjuvant therapies anchored in immunotherapy regimens, combinations of immune checkpoint inhibitors with locoregional treatments, and the development of second-line systemic therapies. With the advent of new treatments, it is imperative to sustain surveillance efforts to maximize the detection of patients at early disease stages. In addition, there is a pressing need for treatment response biomarkers to fine-tune the patient selection.
Similarly, the CCA landscape is in urgent need of advancements in prognostic staging tools and integration of personalized therapies and locoregional treatments. This will not only enhance and reorder the therapeutic benefits according to disease stage but will also lead to a better selection of patients who could potentially benefit from LT. Finally, as in HCC, the future outlook will be punctuated by the need to identify treatment response biomarkers that will facilitate therapeutic decision-making.







![The latest Barcelona Clinic Liver Cancer staging system [35]. AFP: Alpha-fetoprotein; ALBI: Albumin to Bilirubin ratio; BCLC: Barcelona Clinic Liver Cancer; LT: Liver Transplantation; PS: performance status; SIRT: Selective Internal Radiotherapy; TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization. The latest Barcelona Clinic Liver Cancer staging system [35]. AFP: Alpha-fetoprotein; ALBI: Albumin to Bilirubin ratio; BCLC: Barcelona Clinic Liver Cancer; LT: Liver Transplantation; PS: performance status; SIRT: Selective Internal Radiotherapy; TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization.](https://static.elsevier.es/multimedia/16652681/0000002900000003/v1_202404281202/S1665268123002880/v1_202404281202/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

![Systemic treatments for advanced hepatocellular carcinoma. AFP: Alpha-fetoprotein; HR: hazard ratio; HBV: hepatitis B virus; HCV: hepatitis C virus; OS: overall survival; SOR: sorafenib; PVI: portal vein tumor invasion. Note: In the second-line, strong data is only available after sorafenib failure (continuous arrows). Treatment recommendations after immunotherapy-based treatments are based only on real-world data (dotted arrows). Up to this year, there has been no approval of STRIDE regimen (durvalumab-tremelimumab), or durvalumab, or tislelizumab in Latin America. Pembrolizumab has been approved in some Latin American countries, even showing negative statistical results in the KEYNOTE-240 trial [162]. Systemic treatments for advanced hepatocellular carcinoma. AFP: Alpha-fetoprotein; HR: hazard ratio; HBV: hepatitis B virus; HCV: hepatitis C virus; OS: overall survival; SOR: sorafenib; PVI: portal vein tumor invasion. Note: In the second-line, strong data is only available after sorafenib failure (continuous arrows). Treatment recommendations after immunotherapy-based treatments are based only on real-world data (dotted arrows). Up to this year, there has been no approval of STRIDE regimen (durvalumab-tremelimumab), or durvalumab, or tislelizumab in Latin America. Pembrolizumab has been approved in some Latin American countries, even showing negative statistical results in the KEYNOTE-240 trial [162].](https://static.elsevier.es/multimedia/16652681/0000002900000003/v1_202404281202/S1665268123002880/v1_202404281202/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)






