Differentiating biliary atresia from other causes of neonatal cholestasis is challenging, particularly when cytomegalovirus (CMV) and biliary atresia occur simultaneously. We aimed to elucidate whether CMV infection would affect the differential diagnosis of biliary atresia and intrahepatic cholestasis.
Patients and methodsThis retrospective study was conducted among patients with neonatal cholestasis admitted to three tertiary hospitals between January 2010 and August 2019. The clinical characteristics, laboratory, and imaging findings were recorded. On the basis of the CMV serology results, the infants were classified into CMV-IgM (+) and CMV-IgM (−) groups. The clinical differences and diagnostic performances of routine predictors between biliary atresia and intrahepatic cholestasis were analyzed in each group. Finally, we compared the diagnostic performances of various tests in the two groups.
ResultsA total of 705 patients with neonatal cholestasis were enrolled: 215 (30.5%) patients were positive for CMV-IgM, among whom 97 had biliary atresia and 118 had CMV hepatitis; 490 infants were CMV-IgM (−), among whom 240 had biliary atresia and 250 had intrahepatic cholestasis. The diagnostic performances of stool color, direct bilirubin level, γ-glutamyl transpeptidase level, abnormal gallbladder, triangular cord sign, and hepatobiliary scintigraphy between CMV hepatitis and CMV-IgM (+) biliary atresia were similar to those between CMV-IgM (−) biliary atresia and CMV-IgM (−) intrahepatic cholestasis groups.
ConclusionsOur large-scale study showed a high prevalence of CMV infection in patients with neonatal cholestasis in China. The presence of CMV infection did not affect the routine predictors to discriminate biliary atresia and intrahepatic cholestasis.
Biliary atresia (BA) is one of the most common causes of neonatal cholestasis (NC) [1]. It is a progressive life-threatening condition that can affect the quality of life. More importantly, the prognosis of BA depends directly on timely Kasai hepatic portoenterostomy (ideally within the first 45 days of life) [2]. Therefore, early identification of BA can help prevent liver dysfunction or cirrhosis, thus saving lives.
Unfortunately, BA can be difficult to distinguish from other causes of neonatal cholestasis because of similar manifestations [3]. Cytomegalovirus (CMV) infection may be one of the most confounding factors. Previous studies have focused on the etiologic association between CMV and BA; however, the suggestion that CMV infection may trigger BA is still controversial [4]. Despite this, BA and CMV infections can coexist, and clinicians are more likely to treat neonatal cholestasis as an isolated CMV infection, because the incidence of CMV (0.2%–2.2%) is much higher than that of BA (1:15,000–18,000) [5,6]. In addition, patients with CMV-associated BA might have a delay in the referral and the optimal window of Kasai procedure compared to that of BA IgM (−) patients [7,8]. To avoid this situation, we need to differentiate BA from other causes of cholestasis early, even in the presence of CMV infection.
Current studies have described the clinical differences and surgical outcomes between patients with CMV IgM (+) BA and CMV IgM (−) BA [9,10]. However, no study has shown the clinical differences between BA and non-BA cholestasis in the presence or absence of CMV. Moreover, a series of investigations, such as ultrasound and hepatobiliary scintigraphy (HBS), have been performed to differentiate BA from other causes of neonatal cholestasis. We hypothesized that CMV infection could pose further challenges for clinicians to differentiate BA from other cholestatic conditions. In addition, few studies have described the clinical features of CMV-associated cholestasis in China and globally; however, these studies had limitations, such as small sample size or single-center survey [8,9].
In this study, we reviewed the demographic, clinical, laboratory, and imaging data of NC infants from three tertiary hospitals in Shanghai, China. First, we described the prevalence of CMV infection in this large Chinese cohort. Second, we investigated the clinical and laboratory characteristics of patients with neonatal cholestasis according to the presence of CMV infection. Third, we determined whether the presence of CMV infection affected the differential diagnosis between BA and intrahepatic cholestasis (IHC). To achieve this, infants were classified on the basis of the presence or absence of CMV infection, and the clinical differences and diagnostic performances of routine predictors between BA and IHC were analyzed and compared in each group. On the basis of these findings, pediatricians might better understand the concurrence of BA and CMV infection in neonatal cholestasis so that the best possible outcomes could be attained in infants with underlying life-threatening conditions.
2Patients and methods2.1Study population and ethical considerationsThe medical records of NC patients at three institutions, namely Xinhua Hospital (January 2010–August 2019), Shanghai Children’s Medical Center (January 2016–August 2019), and Children’s Hospital of Shanghai (June 2014–August 2019), were retrospectively reviewed. This study was approved by the institutional review boards of each participating institution. The requirement for written informed consent was waived because of the retrospective nature of this study.
Patients were enrolled in the study if they met the following eligibility criteria: age at first hospital admission of <100 days, gestational age of >34 weeks or birth weight of >2000 g, and direct bilirubin (DBIL) >20% of the total bilirubin (TBIL) for a TBIL ≥ 85 μmol/L or DBIL > 17 μmol/L for a TBIL < 85 μmol/L [11]. The exclusion criteria were: multiple congenital malformations, missing the detection of CMV serological testing, and inability to undertake the required number of investigations or follow-up visits to establish the etiology of the NC.
2.2Investigations and data collectionUpon admission, a relatively rapid series of investigations were performed to establish the etiology of NC. Routine investigations included demographic information, medical history, physical examination, stool color, liver function test, complete blood count, coagulation studies, blood/urine culture, viral antibodies (such as IgM and IgG of CMV and Epstein-Barr virus), hepatitis B surface antigen, blood tandem mass spectrometry, urine gas chromatography inspection, abdominal ultrasonography, and HBS. In infants who were suspected of having congenital disorders, next-generation sequencing or whole-exome sequencing was also performed. If BA could not be ruled out by the aforementioned investigations, intraoperative cholangiography (IOC) and liver biopsy were performed.
The diagnosis of BA was confirmed by IOC in combination with the histological characteristics of intraoperative liver biopsy, which showed an abnormal biliary tree, bile duct proliferation, bile plugs, moderate-to-marked ductular reaction, portal stromal edema, and periportal fibrosis [12–14]. IHC was confirmed by IOC, showing a patent biliary tree or normalized/significantly reduced bilirubin level during hospitalization or at follow-up. Infectious hepatitis was diagnosed using different viral antibodies or positive cultures. CMV hepatitis was defined as intrahepatic cholestasis with a positive CMV IgM antibody in the absence of other etiologies [15]. Metabolic or genetic disorders were confirmed by blood tandem mass spectrometry, urine gas chromatography inspection, or gene sequencing. Parenteral nutrition-associated cholestasis was defined as cholestatic patients who received parenteral nutrition for ≥2 weeks [16]. An infant was considered to have idiopathic neonatal hepatitis by excluding other causes of IHC after thorough history review, physical examinations, and laboratory investigations [15].
Data collection included demographic information (age at admission, birth weight, weight at admission, etc.) and clinical features (stool color, hepatomegaly, and splenomegaly). Data on serum TBIL, DBIL, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (AKP), γ-glutamyl transpeptidase (GGT), albumin, serum CMV IgM results, and parameters of conventional ultrasound (US) were also obtained from hospital records. The gallbladder was considered abnormal when absent or if it had a size <1.5 cm [17]. The triangular cord (TC) sign was defined as the presence of an abnormal triangular or tubular echogenic area in the region of the porta hepatis [18]. A positive HBS was defined as the absence of isotopes in the intestines up to 24 h [17].
2.3Statistical analysesFor descriptive analysis, we summarized the etiologies of cholestasis. For comparison between BA and IHC groups in terms of positive and negative serology for CMV, the categorical variables were analyzed using the χ2 or Fisher exact test, and the continuous variables were compared using Student’s t-test or the Wilcoxon rank-sum test. The diagnostic performance of different predictors for differentiating BA from IHC with or without CMV infection was expressed in terms of the area under the curve (AUC), sensitivity, specificity, predictive values, and negative predictive value. All statistical analysis was performed using SAS 9.2 statistical software (SAS Institute, Inc., Cary, North Carolina). A P-value <0.05 was considered significant.
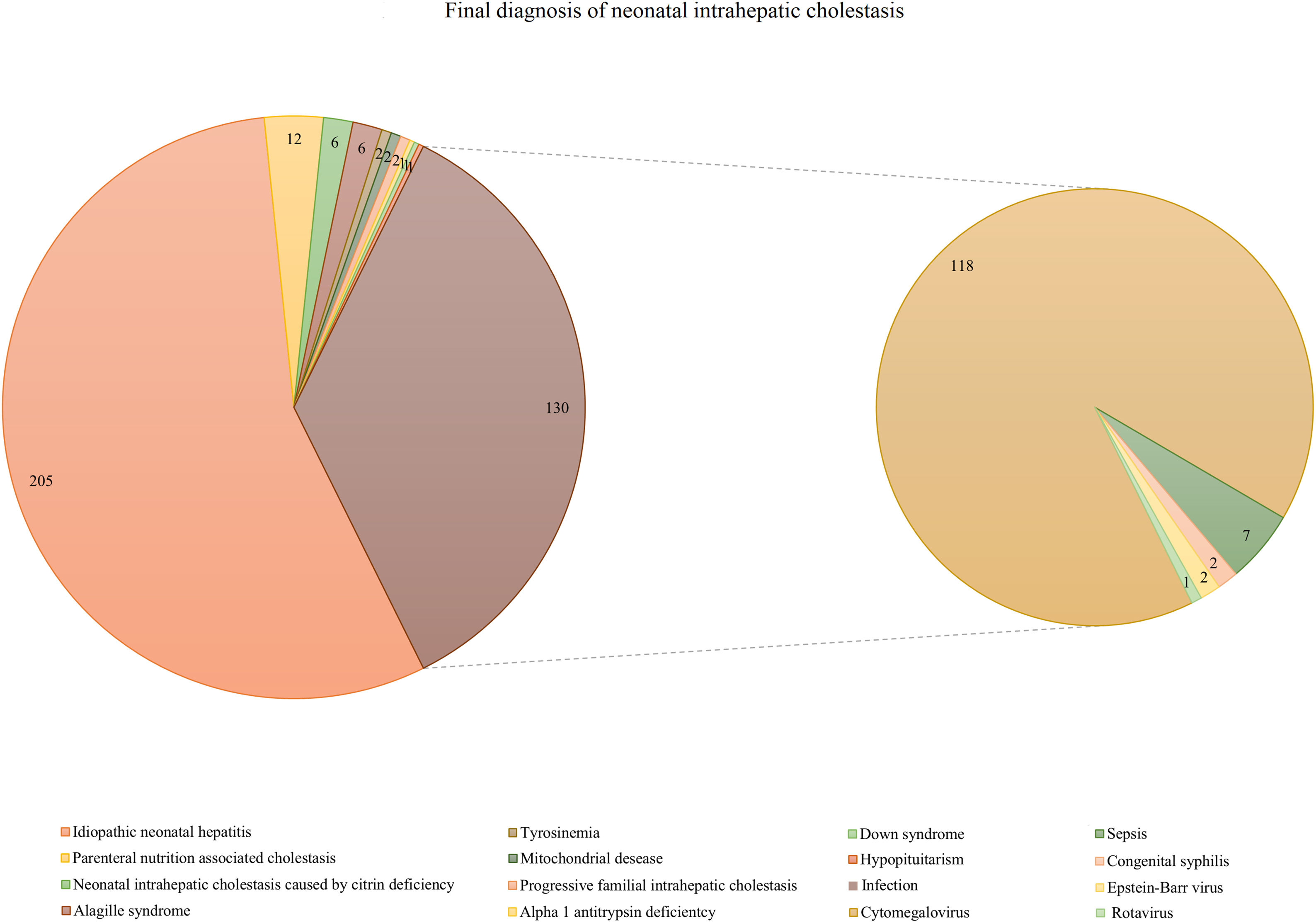
3Results3.1Etiologies of neonatal cholestasis and the prevalence of CMVDuring the study period, a total of 705 patients fulfilled the inclusion criteria for NC. A total of 337 (47.8%) infants were diagnosed as having BA, and 368 were diagnosed as having IHC. In the IHC group, 67 infants underwent diagnostic IOC that showed a patent biliary tree, while 301 recovered from cholestasis or had significantly reduced bilirubin levels during hospitalization or at follow-up. Among the 368 infants with IHC, idiopathic neonatal hepatitis was reported in 205 (55.7%) cases, and infection-associated cholestasis was identified in 130 cases (35.3%). The other IHC included parenteral nutrition-associated cholestasis (n = 12), neonatal intrahepatic cholestasis caused by citrin deficiency (n = 6), Alagille syndrome (n = 6), tyrosinemia (n = 2), mitochondrial disease (n = 2), progressive familial intrahepatic cholestasis (n = 2), Down syndrome (n = 1), alpha 1 antitrypsin deficiency (n = 1), and hypopituitarism (n = 1). Among the 130 patients with NC of infectious origin, 118 had CMV infection, accounting for 32.1% of IHC patients and 90.8% of infection-associated cholestasis. Sepsis (n = 7), congenital syphilis (n = 2), Epstein-Barr virus (n = 2), and rotavirus (n = 1) were other causes of infection-associated cholestasis (Fig. 1).
Final diagnosis of neonatal intrahepatic cholestasis (n = 368). Idiopathic neonatal hepatitis (n = 205, 55.7%) and infection-associated cholestasis (n = 130, 35.3%) were the most two common etiologies of neonatal intrahepatic cholestasis. In addition, among the 130 subjects patients with neonatal cholestasis of infectious etiologies origin, 118 infants had CMV infection, accounting for 32.1% of intrahepatic cholestasis patients and 90.8% of infection-associated cholestasis.
In total, 215 (30.5%) patients were positive for CMV-IgM, including 118 patients with CMV hepatitis and 97 with BA. A total of 490 infants had CMV-IgM (−), among whom 240 cases were BA and 250 were IHC. CMV hepatitis accounted for 32.1% of IHC infants, and positive CMV-IgM was detected in 28.8% of BA infants.
3.2Comparison of BA and IHC groups with positive and negative serology for CMVIn this study, we further classified infants with NC into four groups to compare the clinical differences among them, namely CMV hepatitis (n = 118), IHC with CMV-IgM (−) (n = 250), BA with CMV-IgM (+) (n = 97), and BA with CMV-IgM (−) (n = 240).
Table 1 presents the differences in the clinical characteristics between patients with CMV hepatitis and those with IHC with CMV-IgM (−). We found that patients with CMV hepatitis had a higher age and weight at admission and the presence of hepatomegaly than those with IHC with CMV-IgM (−) (p < 0.05, all). There were almost no significant differences in the laboratory and imaging findings between the two groups, except for the levels of TBIL and DBIL (p < 0.001 and p = 0.039, respectively).
Clinical comparison between CMV hepatitis and intrahepatic cholestasis with CMV-IgM(–).
| Parameters | CMV hepatitis | IHC with CMV-IgM(–) | P |
|---|---|---|---|
| n = 118 | n = 250 | ||
| Birth weight (g) | 3152 ± 514 | 3086 ± 534 | 0.276 |
| Age at admission (day) | 57 ± 16 | 45 ± 23 | <0.001 |
| Weight at admission (g) | 4769 ± 1060 | 4159 ± 1145 | <0.001 |
| Preterm (%) | 16 (13.6%) | 38 (15.2%) | 0.678 |
| Male (%) | 76 (64.4%) | 157 (62.8%) | 0.765 |
| Clinical measures | |||
| Stool color (%) | 0.699 | ||
| Clay stool | 20 (16.9%) | 36 (14.4%) | |
| Yellow pale | 31 (26.3%) | 61 (24.4%) | |
| Yellow | 67 (56.8%) | 153 (61.2%) | |
| Hepatomegaly (%) | 55 (46.6%) | 89 (35.6%) | 0.043 |
| Splenomegaly (%) | 26 (22.0%) | 38 (15.2%) | 0.106 |
| Liver function test | |||
| TBA (μmol/L) | 108 (72, 142) | 106 (71, 152) | 0.819 |
| ALT (U/L) | 113 (64, 197) | 114 (47, 191) | 0.142 |
| AST (U/L) | 169 (101, 271) | 186 (97, 297) | 0.535 |
| AKP (U/L) | 532 (382, 674) | 497 (332, 657) | 0.184 |
| TBIL (μmol/L) | 122 (82, 166) | 157 (112, 213) | <0.001 |
| DBIL (μmol/L) | 72 (45, 101) | 78 (52, 113) | 0.039 |
| GGT (U/L) | 117 (79, 200) | 139 (81, 282) | 0.160 |
| ALB (g/L) | 38 (35, 40) | 38 (35, 40) | 0.164 |
| Ultrasonography findings | |||
| Abnormal gallbladder (%) | 27 (22.9%) | 57 (22.8%) | 0.986 |
| Triangular cord sign (%) | 2 (1.7%) | 8 (3.2%) | 0.407 |
| Hepatobiliary scintigraphya | |||
| Positive (%) | 31 (53.4%) | 64 (66.7%) | 0.102 |
BA: biliary atresia; IHC: intrahepatic cholestasis; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate transaminase; AKP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; ALB, albumin.
Data are presented as mean ± S.D., N (%), or median (IQR).
In Table 2, we have enumerated the differences in the findings of BA between CMV-IgM (+) and CMV-IgM (−) infants. The mean age and weight at admission were higher for CMV-IgM (+) patients than that for CMV-IgM (−) ones (p < 0.01). The median levels of ALT, AST, and AKP were significantly higher in CMV-IgM (+) infants than in CMV-IgM (−) BA infants (p < 0.05, all). However, there was no significant difference in the ultrasonography and HBS findings between the two groups (p > 0.05, all).
Clinical comparison between biliary atresia with CMV-IgM(+) and CMV-IgM(-) groups.
| Parameters | BA with CMV-IgM(+) | BA with CMV-IgM(-) | P |
|---|---|---|---|
| n = 97 | n = 240 | ||
| Birth weight (g) | 3173 ± 517 | 3261 ± 468 | 0.132 |
| Age at admission (day) | 60 ± 13 | 50 ± 17 | <0.001 |
| Weight at admission (g) | 4990 ± 915 | 4668 ± 883 | 0.003 |
| Preterm(days) | 6 (6.2%) | 11 (4.6%) | 0.585 |
| Male (%) | 53 (54.6%) | 121 (50.4%) | 0.483 |
| Clinical measures | |||
| Stool color (%) | |||
| Clay stool | 46 (47.4%) | 110 (45.8%) | 0.663 |
| Yellow pale | 44 (45.4%) | 105 (43.8%) | |
| Yellow | 7 (7.2%) | 25 (10.4%) | |
| Hepatomegaly (%) | 43 (44.3%) | 105 (43.8 %) | 0.923 |
| Splenomegaly (%) | 19 (19.6%) | 25 (10.4%) | 0.024 |
| Liver function test | |||
| TBA (μmol/L) | 122 (97, 150) | 110 (87, 136) | 0.052 |
| ALT (U/L) | 140 (101, 224) | 120 (77, 192) | 0.014 |
| AST (U/L) | 245 (171, 327) | 198 (137, 278) | 0.001 |
| AKP (U/L) | 606 (481, 746) | 506 (407, 621) | <0.001 |
| TBIL (μmol/L) | 164 (143, 191) | 161 (134, 196) | 0.784 |
| DBIL (μmol/L) | 104 (84, 130) | 99 (82, 125) | 0.310 |
| GGT (U/L) | 377 (225, 749) | 526 (283, 755) | 0.402 |
| ALB (g/L) | 39 (37, 42) | 39 (36, 41) | 0.181 |
| Ultrasonography findings | |||
| Abnormal gallbladder (%) | 63 (64.9%) | 157 (65.4%) | 0.935 |
| Triangular cord sign (%) | 34 (35.1%) | 91(37.9%) | 0.622 |
| Hepatobiliary scintigraphya | |||
| Positive (%) | 35 (97.2%) | 113 (98.3%) | 0.561 |
BA: biliary atresia; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate transaminase; AKP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; ALB, albumin.
Data are presented as mean ± S.D., N (%), or median (IQR).
To clarify whether CMV infection could affect the differential diagnosis between BA and IHC groups, we further compared the clinical differences between these two groups stratified according to the CMV-IgM results (Tables 3 and 4). In the CMV-IgM positive group, clay stool was seen more often in patients with CMV-IgM (+) BA than in those with CMV hepatitis. As for liver function, the median levels of ALT, AST, AKP, TBIL, DBIL, GGT, and ALB were significantly higher in CMV-IgM (+) BA infants than those in the CMV hepatitis infants (p < 0.05, all). Additionally, the frequencies of TC sign, abnormal gallbladder, and positive findings in the HBS were significantly higher in CMV-IgM (+) BA than in those with CMV hepatitis (p < 0.01, all) (Table 3).
Clinical comparison between CMV hepatitis and biliary atresia with CMV-IgM(+).
| Parameters | CMV hepatitis | BA with CMV-IgM(+) | P |
|---|---|---|---|
| n = 118 | n = 97 | ||
| Birth weight (g) | 3152 ± 514 | 3173 ± 517 | 0.769 |
| Age at admission (day) | 57 ± 16 | 60 ± 13 | 0.131 |
| Weight at admission (g) | 4769 ± 1060 | 4990 ± 915 | 0.107 |
| Preterm (%) | 16 (13.6%) | 6 (6.2%) | 0.076 |
| Male (%) | 76 (64.4%) | 53 (54.6%) | 0.146 |
| Clinical measures | |||
| Stool color (%) | <0.001 | ||
| Clay stool | 20 (16.9%) | 46 (47.4%) | |
| Yellow pale | 31 (26.3%) | 44 (45.4%) | |
| Yellow | 67 (56.8%) | 7 (7.2%) | |
| Hepatomegaly (%) | 55 (46.6%) | 43 (44.3%) | 0.738 |
| Splenomegaly (%) | 26 (22.0%) | 19 (19.6%) | 0.661 |
| Liver function test | |||
| TBA (μmol/L) | 108 (72, 142) | 122 (97, 150) | 0.067 |
| ALT (U/L) | 113 (64, 197) | 140 (101, 224) | 0.023 |
| AST (U/L) | 169 (101, 271) | 245 (171, 327) | <0.001 |
| AKP (U/L) | 532 (382, 674) | 606 (481, 746) | 0.005 |
| TBIL (μmol/L) | 122 (82, 166) | 164 (143, 191) | <0.001 |
| DBIL (μmol/L) | 72 (45, 101) | 104 (84, 130) | <0.001 |
| GGT (U/L) | 117 (79, 200) | 377 (225, 749) | <0.001 |
| ALB (g/L) | 38 (35, 40) | 39 (37, 42) | 0.016 |
| Ultrasonography findings | |||
| Abnormal gallbladder (%) | 27 (22.9%) | 63 (64.9%) | <0.001 |
| Triangular cord sign (%) | 2 (1.7%) | 34 (35.1%) | <0.001 |
| Hepatobiliary scintigraphya | |||
| Positive (%) | 31 (53.4%) | 35 (97.2%) | <0.001 |
BA: biliary atresia; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate transaminase; AKP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; ALB, albumin.
Data are presented as mean ± S.D., N (%), or median (IQR).
Clinical comparison between biliary atresia and intrahepatic cholestasis with CMV-IgM(−).
| Parameters | BA with CMV-IgM(-) | IHC with CMV-IgM(-) | P |
|---|---|---|---|
| n = 240 | n = 250 | ||
| Birth weight (g) | 3261 ± 468 | 3086 ± 534 | <0.001 |
| Age at admission (day) | 50 ± 17 | 45 ± 23 | 0.008 |
| Weight at admission (g) | 4668 ± 883 | 4159 ± 1145 | <0.001 |
| Preterm (%) | 11 (4.6%) | 38 (15.2%) | <0.001 |
| Male (%) | 121 (50.4%) | 157 (62.8%) | 0.006 |
| Clinical measures | |||
| Stool color (%) | <0.001 | ||
| Clay stool | 110 (45.8%) | 36 (14.4%) | |
| Yellow pale | 105 (43.8%) | 61 (24.4%) | |
| Yellow | 25 (10.4%) | 153 (61.2%) | |
| Hepatomegaly (%) | 105 (43.8 %) | 89 (35.6%) | 0.065 |
| Splenomegaly (%) | 25 (10.4%) | 38 (15.2%) | 0.114 |
| Liver function test | |||
| TBA (μmol/L) | 110 (87, 136) | 106 (71, 152) | 0.285 |
| ALT (U/L) | 120 (77, 192) | 114 (47, 191) | 0.050 |
| AST (U/L) | 198 (137, 278) | 186 (97, 297) | 0.097 |
| AKP (U/L) | 506 (407, 621) | 497 (332, 657) | 0.486 |
| TBIL (μmol/L) | 161 (134, 196) | 157 (112, 213) | 0.217 |
| DBIL (μmol/L) | 99 (82, 125) | 78 (52, 113) | <0.001 |
| GGT (U/L) | 526 (283, 755) | 139 (81, 282) | <0.001 |
| ALB (g/L) | 39 (36, 41) | 38 (35, 40) | 0.009 |
| Ultrasonography findings | |||
| Abnormal gallbladder (%) | 157 (65.4%) | 57 (22.8%) | <0.001 |
| Triangular cord sign (%) | 91(37.9%) | 8 (3.2%) | <0.001 |
| Hepatobiliary scintigraphya | |||
| Positive (%) | 113 (98.3%) | 64 (66.7%) | <0.001 |
BA: biliary atresia; IHC: intrahepatic cholestasis; TBA, total bile acid; ALT, alanine transaminase; AST, aspartate transaminase; AKP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase, ALB, albumin.
Data are presented as mean ± S.D., N (%), or median (IQR).
We found that patients with BA and CMV-IgM (−) had higher birth weight, weight at admission, age, and lower proportion of preterm and male infants than IHC with CMV-IgM (−). Additionally, we found differences in stool colour, serum DBIL, GGT, ALB levels, presence of abnormal gallbladder, TC sign, and positive hepatobiliary scintigraphy between the two groups (p < 0.05, all), which were similar to those between patients with CMV hepatitis and those with BA with CMV-IgM (+) (Table 4).
3.3Diagnostic performances of various methods in distinguishing biliary atresia from intrahepatic cholestasis with or without CMVAs mentioned above, the differences between the CMV hepatitis and BA with CMV-IgM (+) groups were quite similar to those between the BA with CMV-IgM (−) and IHC with CMV-IgM (−) groups. The parameters with a p-value of <0.01 in both comparisons were selected to evaluate their diagnostic performances of BA, including stool color, DBIL, GGT levels, abnormal gallbladder, TC sign, and HBS.
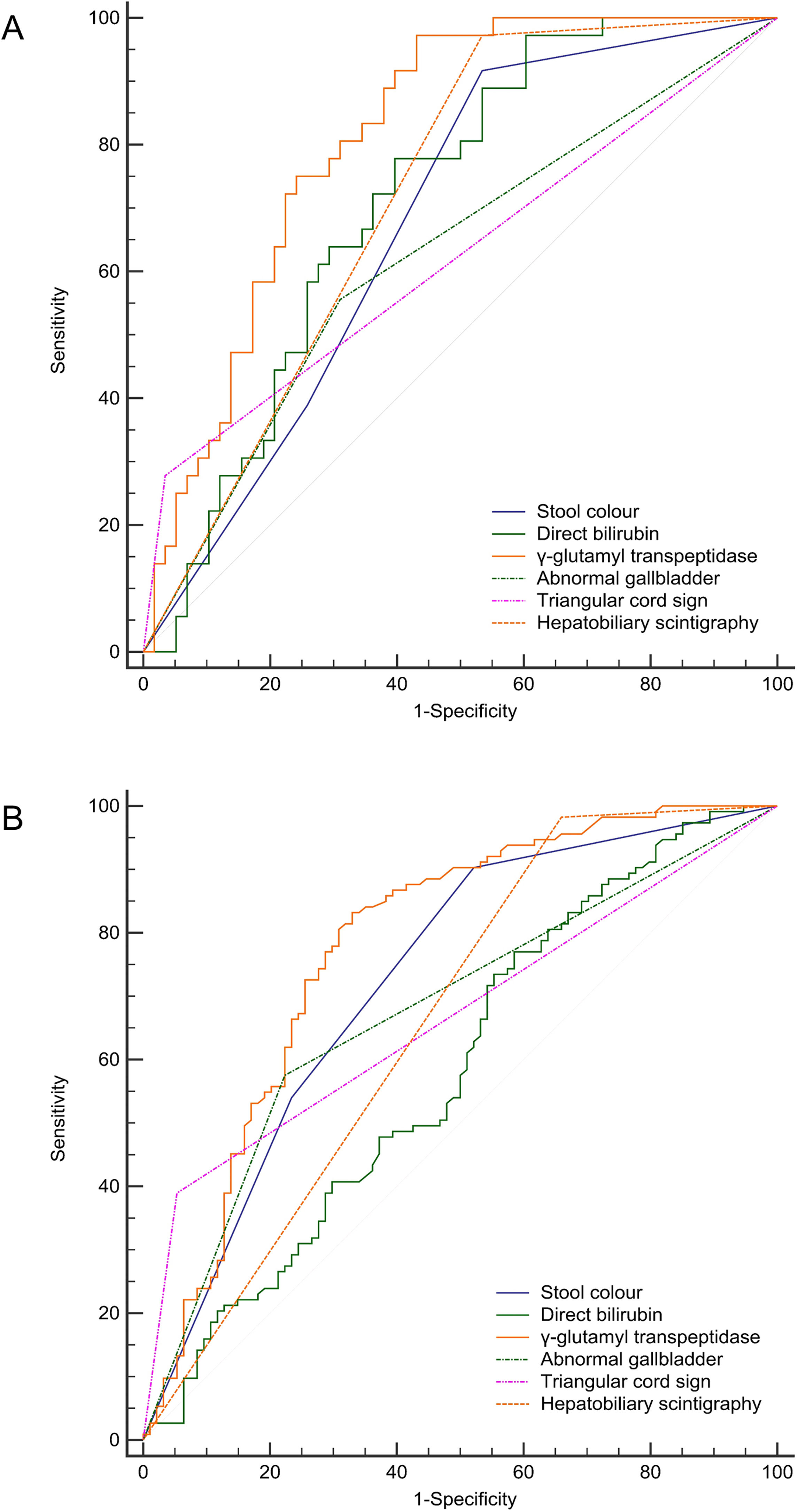
The receiver operating characteristic curve analysis revealed that GGT was the best diagnostic indicator of BA in either the CMV-IgM positive or negative group, with an AUC of 0.817–0.845. A positive HBS finding had the highest sensitivity (97.2–98.3%) for BA, and the presence of the TC sign had the highest specificity (96.8–98.3%) in both comparisons (Fig. 2 and Table 5). Overall, the diagnostic performance of these predictors between CMV hepatitis and CMV-IgM (+) BA was quite similar between CMV-IgM (−) BA and CMV-IgM (−) IHC groups (Table 5). We determined that the presence of CMV infection did not affect the routine predictors to discriminate BA and IHC.
Diagnostic performances of stool color, direct bilirubin level, γ-glutamyl transpeptidase level, abnormal gallbladder, triangular cord sign, and hepatobiliary scintigraphy in infants with neonatal cholestasis with or without CMV infection. A) AUC for stool color, direct bilirubin level, γ-glutamyl transpeptidase, abnormal gallbladder, triangular cord sign, and hepatobiliary scintigraphy were 0.772, 0.749, 0.845, 0.710, 0.667, and 0.719 respectively as compared between CMV hepatitis and biliary atresia with CMV-IgM positive groups. B) AUC for stool color, direct bilirubin level, γ-glutamyl transpeptidase, abnormal gallbladder, triangular cord sign, and hepatobiliary scintigraphy were 0.778, 0.643, 0.817, 0.713, 0.674, and 0.658 respectively as compared between intrahepatic cholestasis and biliary atresia with CMV-IgM negative groups.
Diagnostic performances of various predictors to distinguish biliary atresia from intrahepatic cholestasis stratified by CMV-IgM.
| AUC | 95%CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| CMV-IgM positive (BA and CMV hepatitis) | ||||||
| Stool color | 0.772 | 0.710 - 0.826 | 92.8 | 56.8 | 63.8 | 90.5 |
| Direct bilirubin | 0.749 | 0.685 - 0.805 | 91.8 | 52.5 | 61.4 | 88.6 |
| γ-Glutamyl transpeptidase | 0.845 | 0.789 - 0.890 | 81.4 | 75.4 | 73.1 | 83.2 |
| Abnormal gallbladder | 0.710 | 0.645 - 0.770 | 65.0 | 77.1 | 70.0 | 72.8 |
| Triangular sign | 0.667 | 0.599 - 0.729 | 35.1 | 98.3 | 94.4 | 64.8 |
| Hepatobiliary scintigraphya | 0.719 | 0.617 - 0.807 | 97.2 | 46.6 | 53 | 96.4 |
| CMV-IgM negative (BA and IHC) | ||||||
| Stool color | 0.778 | 0.739 - 0.814 | 89.6 | 61.2 | 68.9 | 86.0 |
| Direct bilirubin | 0.643 | 0.599 - 0.686 | 75.0 | 54.4 | 61.2 | 69.4 |
| γ-Glutamyl transpeptidase | 0.817 | 0.780 - 0.851 | 81.9 | 69.7 | 72.5 | 79.8 |
| Abnormal gallbladder | 0.713 | 0.671 - 0.753 | 65.4 | 77.2 | 73.4 | 69.9 |
| Triangular sign | 0.674 | 0.630 - 0.715 | 37.9 | 96.8 | 91.9 | 61.9 |
| Hepatobiliary scintigraphyb | 0.658 | 0.590 - 0.722 | 98.3 | 33.3 | 63.8 | 94.1 |
BA: biliary atresia; IHC: intrahepatic cholestasis.
In this large population-scale study, positive CMV serology was found in 32.1% of IHC infants and 28.8% of BA infants. CMV infection accounted for an overwhelming proportion (90.8%) of infection-associated cholestasis. We evaluated the different clinical features of BA and IHC infants with or without CMV infection. The clinical differences and diagnostic performances of routine predictors between the two disorders in the CMV-IgM (+) group were similar to those in the CMV-IgM (−) group. The presence of CMV infection should not eliminate BA from the differential diagnosis of neonatal cholestasis.
Currently, CMV infection has been proposed as a possible etiologic agent of NC [19]. Stormon et al. and Jain et al. found 7 and 10 CMV infections in 207 and 168 NC infants in an Australian and Indian NC cohort, respectively, and CMV accounted for 38.9% and 29.4% of all infection-induced cholestasis, respectively [20,21]. In our study, we found a high prevalence of CMV infection, especially in infection-associated cholestasis. Although we tested the IgM and IgG of CMV, Epstein-Barr virus, hepatitis B surface antigen, and other pathogens, we scarcely found other viral infections other than CMV, suggesting that there is a different spectrum of infection-associated cholestasis in China. Additionally, the detection rate of CMV in BA infants was about 10% in British and German groups, which was also lower than the detection rate in our study [9,22]. A much higher rate of CMV-IgM or CMV DNA positivity of up to 48–60% in BA infants was shown in other Chinese cohorts [23–25]. It is speculated that perinatal infection with CMV might be one of the etiologies of BA in China [23]. Wang et al. assumed that a high maternal CMV seroprevalence might be ubiquitous across China, and the prevalence of congenital CMV infection was generally higher in populations with higher maternal seroprevalence [26]. Thus, the detection rate of CMV could be increased in Chinese NC and BA infants.
Our results also showed more infants with CMV-IgM (−) BA than infants with CMV hepatitis, even though CMV hepatitis is much more common among all infants [27]. Our study was a not population-based one; the enrolled institutions were tertiary care hospitals located in Shanghai, and most of the infants with CMV infection were treated in their local hospitals. Only those infants in whom BA was suspected were referred to hospitals in Shanghai for further examination. Nevertheless, our study is still useful for estimating CMV prevalence owing to the enrollment of a sizeable number of NC patients. In addition, we determined the relationship between CMV and BA on the basis of these large populations of patients with CMV infection.
By comparing CMV positive and negative patients in IHC and BA groups, we found some statistical differences in the demographic characteristics. Patients with CMV hepatitis seemed to have a higher age and weight at admission than those with IHC and CMV-IgM (−). According to a previous study, some CMV infections were acquired in the neonatal or infantile period [28]. Thus, these patients might develop clinical symptoms later in their lives and have a later referral age compared to those without CMV infection. Moreover, our results also showed that infants with CMV-IgM (+) BA were older at admission and had higher ALT and AST levels than those with CMV-IgM (−) BA. This result demonstrated a delayed referral for the subgroup with CMV-IgM (+) BA, and the elevated enzymes indicated a more serious liver cell injury and abnormal liver function in the CMV-IgM (+) BA group. Although Fischler et al. found that ongoing CMV infection had no association with survival rate with native liver after ten years of follow-up [29], Zani et al. reported that CMV IgM (+) BA showed a greater degree of inflammation, fibrosis, and a poorer outcome with reduced clearance of jaundice, native liver survival, and increased mortality [9]. Therefore, we should realize that the procedures used to differentiate BA from IHC should not be interrupted even in the presence of a positive IgM serology for CMV.
Since up to 28.8% of BA patients demonstrated serum IgM CMV positivity in this study, we had enough samples to determine whether there were any differences in identifying BA from IHC stratified by CMV infection. By comparing CMV hepatitis and BA with positive CMV-IgM, we noted higher GGT and DBIL levels, clay stool, abnormal gallbladder, TC sign, and positive finding of HBS in BA with CMV-IgM (+) than in the CMV hepatitis group. These results could also be found in patients with BA and IHC in the negative CMV-IgM group. Further analysis also demonstrated that the diagnostic performance of these predictors between CMV hepatitis and CMV-IgM (+) BA was quite similar to that in the CMV-IgM (−) BA and CMV-IgM (−) IHC groups. Thus, we speculated that the presence of CMV infection did not affect routine examinations to differentiate BA from IHC.
Moreover, to differentiate BA from IHC in the presence or absence of CMV infection, GGT had the best AUC among the diagnostic methods, and the result was similar to that of the study by Lertudomphonwanit et al. [30]. However, its specificity (75.4–69.7%) and sensitivity (81.4–81.9%) were not acceptable for clinicians to identify BA. The positive findings of the TC sign or HBS showed good specificity (96.8–98.3%) or sensitivity (97.2–98.3%), while their low sensitivity (35.1–37.9%) or specificity (46.6–33.3%) limited their use in discriminating BA. No test is currently 100% accurate; thus, all of these results should be considered compositely to solve this diagnostic challenge.
There are limitations to this study that must be considered during the interpretation of the findings. First, besides CMV antibody detection, CMV DNA using the PCR method is also regarded as a standard method for diagnosing CMV infection. Some reports assumed that CMV infection by CMV-IgM alone appeared insufficient because of the considerable number of false-negative cases [31]. Zhao et al. found that the overall positive rate of CMV DNA was 29.08%, while the positive rate of CMV-IgM was 10.40% in patients with HIV/AIDS [32]. Our reports might underestimate the exact number of CMV infections, and the universal inspection of CMV PCR testing would be proposed in later clinical practice. Second, our study was conducted in three different hospitals over a long period and hence some information bias could not be excluded. For example, various US and HBS scanners with various protocols were used by multiple radiologists. These conditions might introduce inherent disparities in the evaluation.
5ConclusionsIn conclusion, this study is one of the large-scale descriptive studies on NC infants in China. We reinforced the different spectra of BA and CMV infections in China. The presence of CMV could not exclude the diagnosis of BA. In addition, no method was found to have adequate sensitivity and specificity to differentiate BA from IHC with or without CMV. Therefore, in the clinical approach for an infant with cholestasis, extensive investigations are still recommended for timely diagnosis and appropriate management, even if CMV-IgM is detected.AbbreviationsCMVCytomegalovirusBABiliary atresiaNCNeonatal cholestasisHBSHepatobiliary scintigraphyDBILDirect bilirubinTBILTotal bilirubinIOCIntraoperative cholangiographyIHCIntrahepatic cholestasisALTAlanine transaminaseASTAspartate transaminaseGGTγ-Glutamyl transpeptidaseTCTriangular cordAUCArea under the curve
AbbreviationsCMV Cytomegalovirus Biliary atresia Neonatal cholestasis Hepatobiliary scintigraphy Direct bilirubin Total bilirubin Intraoperative cholangiography Intrahepatic cholestasis Alanine transaminase Aspartate transaminase γ-Glutamyl transpeptidase Triangular cord Area under the curve
This work was supported by Shanghai Municipal Commission of Health and Family Planning (2016ZB0103).
Conflicts of interestThe authors have no conflicts of interest to declare.